The following report on the perioperative anesthetic management of severe cardiomyopathy and resection of pheochromocytoma tumors offers a clinical and pharmaceutical experience with a good outcome for a high-risk pathology with little available world literature. The female patient accesses emergency services in distress with tachycardia, labile blood pressure, dyspnea, and severe abdominal pain. Clinical studies reveal heart failure, an adrenal mass, and derivates of high levels of catecholamines in the blood, which leads to the diagnosis of severe cardiomyopathy induced by pheochromocytoma. The medical management for the acute crisis is performed with therapy in the intensive care unit, antihypertensives and magnesium sulfate. Once stabilized, a laparoscopic tumor resection followed. Her postoperative progress was adequate with a progressive resolution of symptoms. Cardiomyopathy secondary to pheochromocytoma is a pathology with high morbimortality and low frequency and is produced by the action of great quantities of catecholamines released subacutely due to hemorrhagic tumor necrosis or manipulation of the pheochromocytoma. It requires strict care in its acute crises and during surgery for its definitive resection. This report shows our experience with the usefulness of magnesium sulfate as a contributory drug in the control of this pathology throughout the perioperative period due to its mechanism of action and pharmacodynamics. Its easy availability in hospitals, the good clinical results it produces, and its scientific backing are important factors that make it a pharmacological option for pheochromocytoma.
El siguiente reporte de manejo anestésico perioperatorio de cardiomiopatía severa y resección de tumor de feocromocitoma, ofrece una experiencia clínica y farmacológica con buen resultado, de una patología de alto riesgo con poca literatura mundial. La paciente ingresa al servicio de urgencias con angustia, taquicardia, tensión arterial lábil, disnea y dolor abdominal severos. Sus estudios clínicos revelan insuficiencia cardiaca, masa suprarenal y derivados de catecolaminas elevados en sangre, que hacen diagnóstico de cardiomiopatía severa inducida por Feocromocitoma; se realiza el manejo médico de la crisis aguda con terapia en unidad de cuidado intensivo, antihipertensivos y sulfato de magnesio y una vez estabilizada se lleva a resección tumoral laparoscopica. Su evolución postoperatoria fue adecuada, con resolución progresiva de los síntomas. La cardiomiopatía secundaria a feocromocitoma es una patología de alta morbimortalidad e inusual frecuencia, producida por la acción de grandes cantidades de catecolaminas liberadas de modo subagudo por necrosis tumoral hemorrágica o manipulación de feocromocitoma, que requiere manejo estricto en su crisis aguda y en la cirugía de resección definitiva. Este reporte muestra la experiencia de la utilidad del sulfato de magnesio, como fármaco coadyuvante en el control de esta patología durante todo el periodo perioperatorio, por su mecanismo de acción y farmacodinamia. Su fácil accesibilidad hospitalaria, buen resultado clínico y soporte científico son factores importantes para ser considerado una opción farmacológica en Feocromocitoma.
Pheochromocytoma refers to a tumor originating in the catecholamine producing chromaffin cells, located mainly in the adrenal medulla.1 The main clinical signs of the acute effect of catecholamines secreted in high concentrations are: hypertension, palpitations, headache, anxiety, sweating, and paleness. Potentially lethal complications, like arrhythmia, heart or peripheral ischemia, cardiomyopathy, and cerebrovascular disease, can occur due to the acute and uncontrolled release of catecholamines during the induction of anesthesia or tumor resection surgery. An unusual preoperative presentation is severe cardiomyopathy with hypertension, hypertrophic or dilated myocardial compromise, pulmonary edema, and arrhythmias.2 Adequate handling of the acute crisis is based on the control of cardiac contractility with stabilization of rhythm, rate, and arterial pressure.3
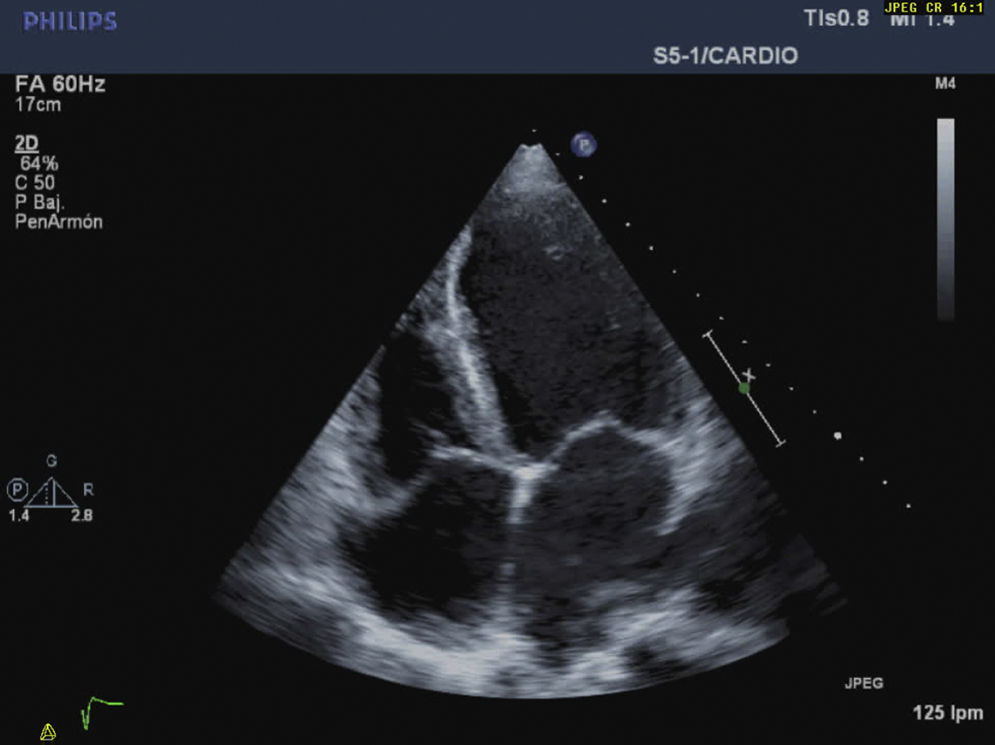
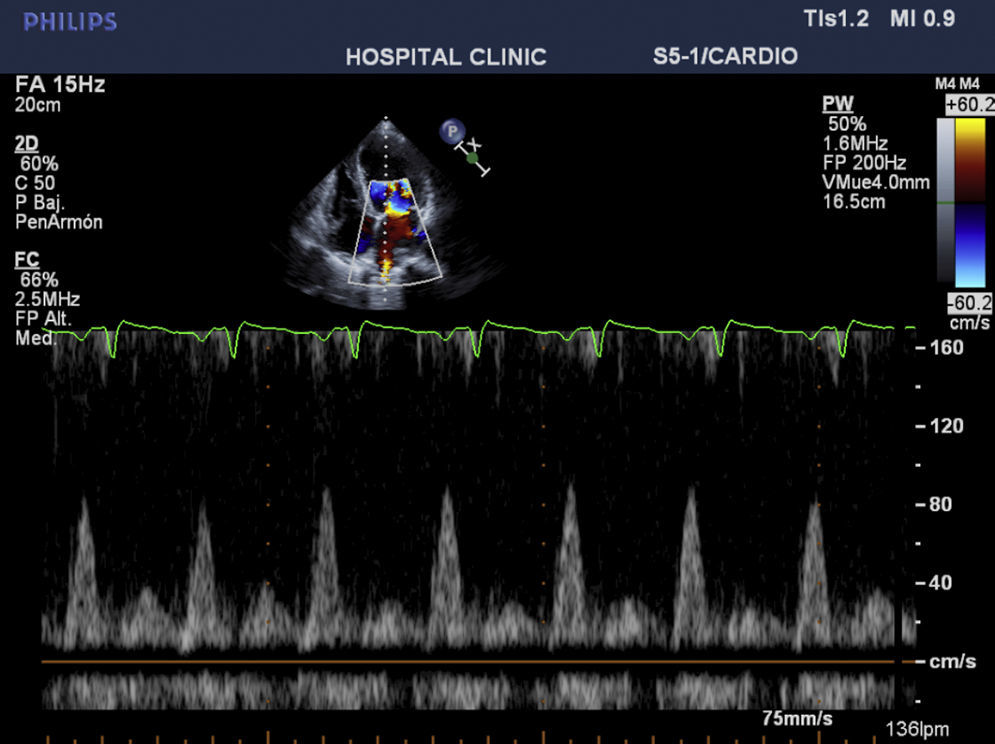
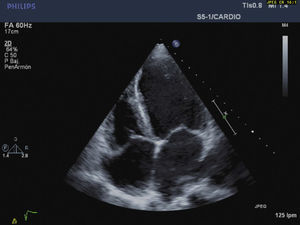
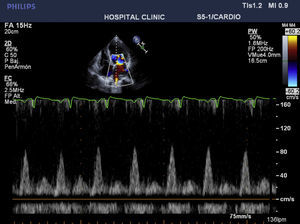
CaseWe report the case of a 42-year-old female patient, without important pathological antecedents, who was admitted to the coronary unit with symptoms of anxiety, polypnea, sweating, and abdominal pain and distension. Upon admission, she presented a labile arterial pressure between 180/120 and 95/60mmHg, a heart rate of 115bpm, a mitral systolic murmur, and jugular engorgement. The electrocardiogram showed evidence of sinus tachycardia, and the transthoracic echocardiogram showed left ventricular dilation, severe global hypokinesis, a restrictive diastolic pattern with an E/A ration of 3.8cm/s, an ejection fraction of 20%, systolic pressure of the pulmonary artery of 85mmHg, and severe tricuspid and mitral insufficiency (Figs. 1 and 2). Cardiac enzyme levels were normal, as was the coronary arteriography.
Before the abdominal scanography that reported an image of a right suprarenal tumor with necrosis, pheochromocytoma was suspected and the administration of doxazosin at 4mg/day and bisoprolol at 1.5mg/day. The plasma levels of catecholamine derivates (metanephrine at 2063pg/ml (normal<90) and normetanephrine at 1291 (normal<200)) and the function studies with images confirmed the diagnosis. Despite the treatment, for the first two weeks the instability of the arterial pressure and the same compromise of contractility persisted. Therefore, the alpha-blocker phenoxybenzamine was modified and the beta-blocker was suspended. The echocardiogram in the following week revealed only minimal improvement of the ejection fraction to 25%. It was decided that intravenous magnesium sulfate would be initiated to improve contractility, diastolic function, and to reduce toxic effects of the intracellular calcium in the myocardium with a bolus injection of 40mg/kg and an infusion of 15mg/kg/h. One week later, the day before the surgery, though the ejection fraction continued at 25%, a great improvement was observed in the diastolic function, the pulmonary systolic pressure was at 29mmHg, the E/A ration was 1.2cm/s, and the mitral and tricuspid insufficiency was slight.
On the day of the surgery, the physicians proceeded to prepare for the laparoscopic resection of the pheochromocytoma. Under sedation, invasive arterial pressure monitoring was performed, finding a mean arterial pressure of 105mmHg and a heart rate of 100bpm. Induction was performed with 0.2mg/kg etomidate, 3mcg/kg fentanyl, 0.6mg/kg rocuronium, and orotracheal intubation without cardiovascular complications. The maintenance of anesthesia was with 3mcg/kg of fentanyl, 10mcg/kg/min rocuronium, and desflurane at 4–5% MAC, as well as the infusion of magnesium at 15mg/kg/h continued up to the moment of complete tumor resection.
Cardiac function was monitored with a transesophageal echocardiogram and a modified Swan-Ganz pulmonary artery catheter (Edwards CCO/SVO2 746HF8), which allowed for a continuous estimation of cardiac output, end-diastolic volume (EDV), and right ejection fraction. The initial profile was: cardiac output at 3.4l/min, EDV of 240ml, ejection fraction of 25%, central venous pressure at 22mmHg, systemic vascular resistance at 2850dyn.s/cm5, pulmonary vascular resistance of 340dyn.s/cm5. As such, dobutamine was initiated at 5mcg/kg/min. The invasive monitoring permitted us to progressively determine the improvement in contractility until a final ejection fraction of 42% was reached with a reduction of EDV to 160ml and we could perform a titrated increase in blood volume while monitoring the central venous pressure, which went from values of 22 to 6mmHg. Two moments of hypertensive crisis occurred at minutes 30 and 50 of the surgery (mean arterial pressure of 122 and 160mmHg, respectively) and were controlled with phentolamine in bolus injections of 2mg each. The surgical result was satisfactory with a surgical time of 120min and bleeding of 350cc. Extubation was performed without cardiovascular or neuromuscular problems with adequate postsurgical progress and inotropic support that could be withdrawn after 24h.
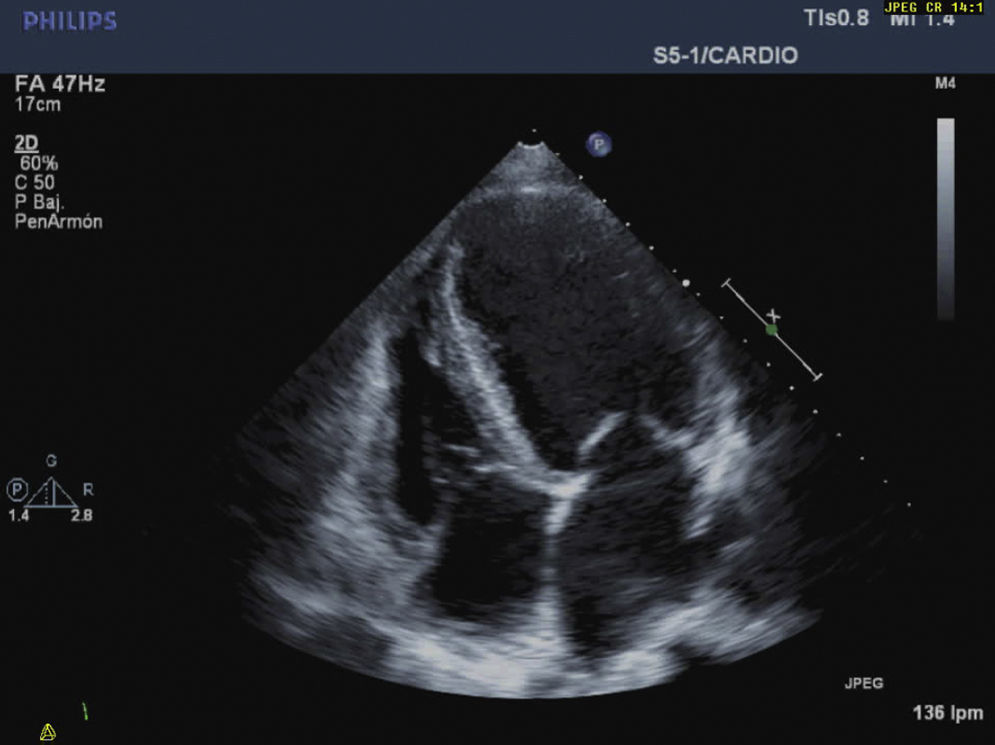
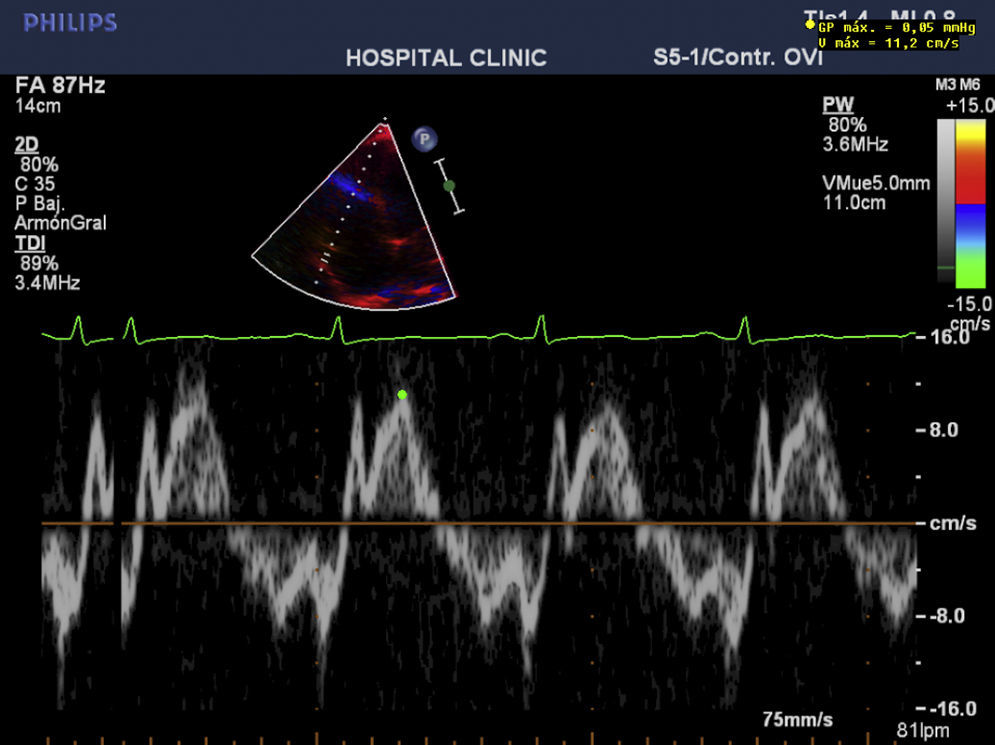
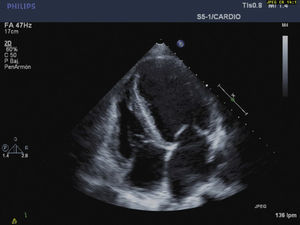
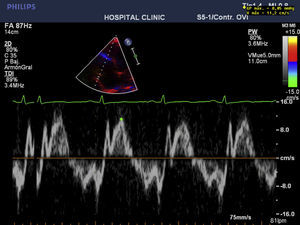
To summarize, this is the case of a female patient with a sub-acute presentation of severe heart failure, lability in blood pressure values, and severe abdominal pain with negative studies for coronary pathologies. The abdominal scanography and elevated levels of catecholamine metabolites clarified the diagnosis that guided the slow improvement initiated with alpha-blockers and the introduction of magnesium sulfate. The cardiovascular response to this drug was essential: it improved cardiac contractility and arterial pressure thereby obtaining optimal physiological conditions that permitted the tumor resection with adequate results (Figs. 3 and 4).
DiscussionThis patient with a pheochromocytoma tumor started with a crisis of severe dilated cardiomyopathy induced by catecholamines, with a clinical presentation of left heart failure, and abdominal symptoms secondary to acute tumor necrosis. This is an infrequent presentation that is difficult to diagnosis. As a result, it has high morbimortality due to a late initiation of care. The diagnostic bases were the absence of a history of cardiovascular disease with normal cardiac enzymes and coronary arteriography. Cardiomyopathy in pheochromocytoma is produced by excessive levels of catecholamines, with an overload of intracellular calcium as the main injury, an ischemia-reperfusion type lesion with free radicals, deterioration of the myocardial fiber,4 microvascular alteration, and vasospasm. Finally, an increase in myocardial oxygen demand presents with a decrease in its contribution through coronary vasoconstriction. The patient can present clinical signs of hypertrophic or dilated heart failure, cardiogenic or non-cardiogenic pulmonary edema, fatal arrhythmia and sudden death.5
This pathophysiology of the cells and adrenergic receptors has suggested magnesium sulfate as a possible therapy for the management of acute crises and surgery.6 Extensive literature exists telling of its efficacy in the management of hypertension and arrhythmias throughout the perioperative period to achieve an appropriate optimization of the patient. In some cases, it has been useful in situations where labetalol or sodium nitroprusside have not been beneficial.7 The optimization of this patient begins with alpha-adrenergic blockers (doxazosin and phenoxybenzamine), but faced with little improvement in myocardial contractility and instability of arterial pressure, magnesium sulfate is introduced starting in the acute crisis and during the surgical process up until the complete removal of the flow from the pheochromocytoma, ensuring a maximum level of magnesemia of 4.5mmol/l. This drug has been reported clinically in this context in case series in adults,8 pediatric patients,9 and pregnant patients.10 Magnesium is an ion that participates in essential enzymatic processes related to energy synthesis and metabolism. It acts as a modulator and stabilizer of Na/K currents in the plasmatic membrane by antagonizing calcium at the intracellular and vascular smooth muscle levels. It also reduces the release of catecholamines from the adrenal medulla and in nerve endings and directly blocks their receptors. As a consequence, it blocks the systemic hemodynamic repercussions of catecholamines.11 In addition, it preserves myocardial cells during the ischemia-reperfusion process and is an anti-arrhythmic in the context of high concentrations of catecholamines.12 The clinical result is an improvement in myocardial, systolic, and diastolic function, the stabilization of the heart rate, progressive vasodilation of the vascular bed (mainly arterial and minimal venous) of target and peripheral organs. In perioperative care, it also decreases the adrenergic response to all stimuli such as orotracheal intubation and the surgical stimulus, thereby achieving better perioperative hemodynamic control.6
Echocardiographic monitoring during the crisis allowed us to give evidence of the response to the magnesium with sustained improvement of the restrictive pattern of the diastolic dysfunction (change in E/A ration from 3.8 to 1.2cm/s; increase in ejection fraction from 20 to 25%; and reduction in systolic pressure of the pulmonary artery from 80 to 29mmHg), which, in association with the dobutamine, facilitated intraoperative hemodynamic management. Intraoperatively, the best monitor is transesophageal echography to establish overall cardiac compromise, contractility, and blood volume, using the pulmonary artery catheter only in cases of severe cardiac compromise.
Recently, cases of Takotsubo cardiomyopathy have been reported. This is a syndrome characterized by transitory left ventricular dysfunction with apical ballooning, and basal hyperkinesis simulating symptoms of myocardial infarction without significant coronary disease. In cases of pheochromocytoma and Takotsubo cardiomyopathy, the most frequent pattern is inverted with basal and midventricular akinesis and a hyperkinetic apex. This syndrome is associated with emotional or physical stress augmented by catecholamines, damage to cardiomyocytes, and stunned heart, which suggests a similarity in the pathophysiology of the two conditions—adrenergic overload—with Takotsubo syndrome having a more favorable prognosis than that of pheochromocytoma. There are probably other neurohumoral agents secreted by the tumor chromaffin cells, such as neuropeptide Y, that can act synergistically with the catecholamines and worsen the myocardial injury and the patient's progress.13
Magnesium sulfate has a good safety profile with easily managed toxicity and reversal. It provides hemodynamic stability and facilitates cardiovascular control during the cardiomyopathy crisis and tumor removal,6,13 representing a first-line option for reducing morbimortality in the management of pheochromocytoma.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingNone
Please cite this article as: Sanabria C, Vendrell M. Cardiomiopatia severa secundaria a feocromocitoma: utilidad del sulfato de magnesio. Reporte de un caso. Rev Colomb Anestesiol. 2016;44:58–62.