The development of percutaneous dilatational tracheostomy techniques (PDT) has facilitated the procedure in Intensive Care Units (ICU).
ObjectiveTo describe the early intra and post-operative complications in ICU patients requiring percutaneous dilatational tracheostomy using the Ciaglia Blue Rhino technique, without fiber optic bronchoscopy.
Patients and methodsWe collected data from eighty ICU patients during three years. The demographic variables were recorded, in addition to severity, number of days in mechanical ventilation prior to the procedure and intraoperative as well as early postoperative complications.
ResultsEighty patients were included, with the mean age of 61.5 (15–89) years old (29 females). The mean APACHE II score was 17.9. In average, the patients required 11.6 days of mechanical ventilation prior to the PDT. 11.6% had intraoperative complications and 9.1% experienced early postoperative complications. In two patients the endotracheal tube was accidentally punctured and three patients had self-limiting bleeding at the tracheostomy site. None of the complications was life threatening to the patients.
ConclusionsPDT using the Ciaglia Blue Rhino technique, without fiber optic bronchoscope is a procedure with low incidence of complications.
El desarrollo de técnicas para la realización de una traqueostomía percutánea por dilatación (TPD) ha facilitado su implementación en las unidades de cuidados intensivos (UCI).
ObjetivoDescribir las complicaciones tempranas intra y postoperatorias en pacientes de cuidados intensivos que requirieron traqueostomía percutánea por dilatación utilizando la técnica Ciaglia Blue Rhino y que se realizaron sin la asistencia de fibrobroncoscopio.
Pacientes y métodosSe revisaron de forma retrospectiva los registros cínicos de 80 pacientes durante un periodo de 3 años. Se recolectaron 80 pacientes consecutivos durante 3 años en cuidado intensivo. Se registraron las variables demográficas, de severidad, días de ventilación mecánica antes del procedimiento y las complicaciones intraoperatorias y postoperatorias tempranas.
ResultadosSe incluyeron 80 pacientes con una edad media de 61,5 (15-89) años (29 mujeres). La media de puntuación APACHEII fue 17,9. Los pacientes requirieron en promedio 11,6 días de ventilación mecánica antes de la TPD. El 11,6% presentó complicaciones intraoperatorias y el 9,1% complicaciones postoperatorias tempranas. En 2 pacientes se puncionó accidentalmente el tubo endotraqueal y 3 pacientes presentaron sangrado autolimitado del sitio de traqueostomía. Ninguna de las complicaciones representó un riesgo vital para los pacientes.
ConclusionesLa TPD mediante la técnica Ciaglia Blue Rhino sin la asistencia de fibrobroncoscopio es un procedimiento con baja incidencia de complicaciones.
Tracheostomy is a frequent procedure in the ICU, indicated for the treatment of critically ill patients who require extended mechanical ventilation.1
PDT was introduced in 1985 and since then has undergone several modifications. Currently, three techniques are described: Ciaglia (and its Blue Rhino modification); Griggs, and modified Howard Kelly.2–7
In the last few years, PDT has gained wide acceptance. It is a minimally invasive procedure with numerous advantages including high level of safety, easy to do and learn, smaller skin incision that limits tissue injury, low risk of bleeding, short surgical time, low cost and does not require transferring the patient to the OR and consequently reduces the morbidity from transport to the OR (in case of open tracheostomy).3,8–11
PDT has some limitations and risks, including decannulation and airway obstruction, blind tracheal intubation, perioperative bleeding, infections, tracheal stenosis, pneumothorax, esophageal perforation, subcutaneous emphysema and tracheal ring fracture.12
The purpose of the study was to determine the intraoperative and early postoperative complications in intensive care patients requiring PDT using the Ciaglia Blue Rhino technique and performed without fiber optic bronchoscope assistance.
Patients and methodsA descriptive, observational trial was completed that consecutively included all medical or surgical patients admitted to the ICU of the la Estancia Clinic in Popayan (Colombia) undergoing PDT during a three-year period (December 2006 to December 2009). The exclusion criteria were patients less than 18 years old, patients with cervical anatomy defects or infection of the cervical tissues. This group of patients was referred for conventional open tracheostomy.
All PDTs were elective procedures and were done at the ICU following a standardized approach, using the Ciaglia Blue Rhino Percutaneous Tracheostomy Introducer set (Cook Critical Care, Bloomington, IN, USA).
Surgical techniqueFirst, all the instruments needed for the procedure were checked. The vital signs were measured and the patient's position was optimized for better exposure of the cervical anatomy. The neck was cleansed, the surgical drapes were put into place and sedation and analgesia was administered (fentanyl, remifentanl, midazolam and/or propofol).
Following the identification by palpation of the second tracheal ring, the underlying skin was infiltrated with 2% lidocaine without epinephrine. The orotracheal tube was carefully removed under the laryngoscope until the tracheal balloon was visualized in the glottal stricture. The catheter needle guide was assembled in a 10ml syringe containing the local anesthetic and was punctured between the first and the second tracheal ring or between the second and the third ring, according to the anatomical structure in each case and perpendicularly until the resistance faded away. The syringe aspiration confirmed the extraction of air that is indicative of the tip placement on the tracheal lumen. Once this position was secured, the polyvinyl catheter was advanced at a 45° caudal angle and the syringe and the metallic needle were removed. Then the “J” tip wire-guide was introduced through the catheter and the catheter was then removed leaving the guide in position.
A 0.5cm long cross incision was made on each side of the metallic guide involving the skin and the subcutaneous cell tissue (this is a modification introduced by our service and we have observed less incision trauma for the insertion; first, a puncture is made and then the incision). Then, the first short dilator was passed until it glided smoothly and was then removed leaving the wire guide behind. Then the white catheter guide was advanced over the wire guide until the two ends met the distal and proximal markings. The single dilator was then passed over the two guides, until the proximal end coincided with the catheter guide marking at a 45° angle with respect to the skin and caudal. The dilator was introduced firmly and advanced one step at a time, until the black marking line of the dilator was reached. The mechanical ventilation was disabled as soon as the dilator reached the tracheal lumen.
At this point, the dilator was removed and the lubricated tracheostomy tube was introduced, assembled with the balloon deflated and the holder over the guide catheters. Finally, the catheter holder and the two guides (the catheter's and the wire guide) were removed, the cannula was sutured to the skin and fixed with cloth tape around the neck and the ventilation process was reinitiated. A control chest X-ray was obtained from every patient.
The detailed technique can be seen on line at “Traqueostomía Percutánea en Cuidado Intensivo”.13
Gathering of information and analysisA special form was used to collect the patient's information: age, sex, diagnosis at admission to the ICU, APACHE II, diagnostic for prescribing PDT, mechanical ventilation mode and parameters at the time of the procedure; length of time in MV prior to PDT, drugs used for sedation, medical staff who performed the procedure, intraoperative and postoperative complications. The timing of the DPT procedure was classified according to the reports of other authors as early (less than 4 days before the orotracheal intubation) and late (more than 10 days).14
The data were analyzed using the statistical package SPSS version 15.0 with descriptive statistics (Statistical Package for the Social Sciences, Chicago, IL, USA). The categorical variables are expressed as proportions and frequencies. The continuous variables are summarized as medians or means. The data scatter was quantified using standard deviation or the interquartile range (IQR) depending on each distribution and the proximity to normality. In order to explore the independent nature of some variables and the length of time of the procedure, the Mann–Whitney and χ2 tests were used for independent samples for categorical and continuous variables, respectively. p values less than 0.05 were considered significant.
ResultsDuring the study period, the DPT procedure was performed in 80 patients in total and 64% were males (n=51) and 36% females (n=29). The average age was 61.5 ranging between 15 and 89 years.
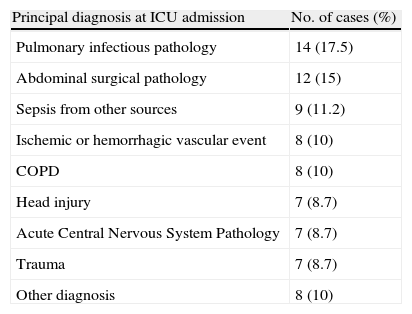
Table 1 shows the pathologies for which the patients were admitted to the ICU. The mean severity APACHE II score at the time of admission was 17.9 (SD=6.4, IQR=14–21, trajectory=3–37). The indication for PDT was prolonged mechanical ventilation in 72% of the patients and secondary to central or peripheral nervous system disease in 28%. The patients remained with orotracheal intubation prior to the procedure 11.6 days in average (SD=7.1; IQR=7.9–11).
Principal diagnosis at ICU admission of patients requiring PDT (n=80).
| Principal diagnosis at ICU admission | No. of cases (%) |
| Pulmonary infectious pathology | 14 (17.5) |
| Abdominal surgical pathology | 12 (15) |
| Sepsis from other sources | 9 (11.2) |
| Ischemic or hemorrhagic vascular event | 8 (10) |
| COPD | 8 (10) |
| Head injury | 7 (8.7) |
| Acute Central Nervous System Pathology | 7 (8.7) |
| Trauma | 7 (8.7) |
| Other diagnosis | 8 (10) |
Source: author.
The PDT was performed early (less than 4 days) in 15% (n=12) of the cases and late (over 10 days) in 67.5% (n=54). In 15% (n=12) of the patients the procedure was done 18 days after orotracheal intubation.
The most frequently used drugs for the procedure were fentanyl 62%, remifentanil 18%, midazolam 86% and rocuronium 29%.
49% of PDTs were performed by an anesthesiologist resident or general surgery under the direct guidance of an intesivist; 40% of PDTs were performed by an intensivist and the remaining 10% by other specialists.
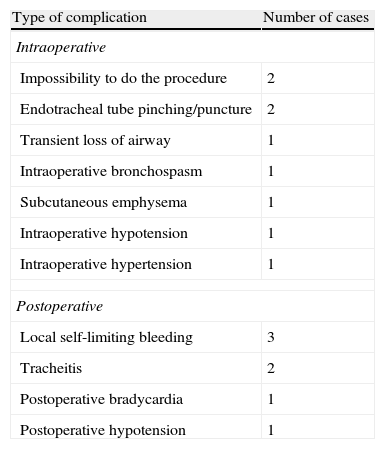
Nine patients exhibited intraoperative complications (11.6%) and seven (9.1%) patients had postoperative complications (Table 2). None of the complications was life threatening.
Intraoperative and early postoperative DPT complications (n=80).
| Type of complication | Number of cases |
| Intraoperative | |
| Impossibility to do the procedure | 2 |
| Endotracheal tube pinching/puncture | 2 |
| Transient loss of airway | 1 |
| Intraoperative bronchospasm | 1 |
| Subcutaneous emphysema | 1 |
| Intraoperative hypotension | 1 |
| Intraoperative hypertension | 1 |
| Postoperative | |
| Local self-limiting bleeding | 3 |
| Tracheitis | 2 |
| Postoperative bradycardia | 1 |
| Postoperative hypotension | 1 |
Source: author.
Table 3 shows a comparison between the length of time of the PDT in all patients and the frequency of complications. Statistical differences were identified in the occurrence of global and intraoperative complications in the early vs the late procedures. The differences among other variables such as age, sex, APACHE II score at admission showed no statistical significance.
DiscussionPDT is a widely accepted procedure and is increasingly being used in the last few years.15,16 One of the modifications to the technique is the fiber optic bronchoscope – assisted procedure for direct endotracheal visualization. However, this requires the availability of the equipment, skilled personnel and may increase the overall costs of the procedure.17
Some authors have documented that the fiber optic bronchoscopy affects the gas exchange retaining carbon dioxide. Reilly et al., showed increased intracranial pressure with the use of the fiber optic bronchoscope due to a rise in the carbon dioxide pressure.18 Nawaz et al. reported that not using the bronchoscope does not increase the morbidity rates but it does increase the length of time of the procedure.19 Routine use of the fiber optic bronchoscope has proven to reduce the incidence of complications and it is actually recommended20–22; however, it is not yet generally available.
Our study compares its results against those reported by Romero et al., who used fiber optic bronchoscopy in 100 patients.23 The authors reported an incidence of overall early complications of 12% (8% intraoperative and 4% postoperative). Similarly to our results, none of the complications resulted in fatal outcomes for the patient.
In our daily practice the DPT fiber optic bronchoscope-assisted procedure is not available to us; however, this study illustrates that there is basically little difference in the incidence of overall early complications without the use of the bronchoscope as compared to the study by Romero et al.23 Moreover, our results are supported by the results recently published by Jackson et al. in 2011. This latter retrospective study showed that the incidence of early or late complications of the procedure were no different with or without the use of the fiber optic bronchoscope.24 It must be noted however, that two of our early complications were due to pinching or puncture of the endotracheal tube, despite it being dislodged up to the glottal stricture during the puncture. These complications may have been eventually prevented under direct endotracheal visualization. Evidently, this does not preclude the possibility of puncturing the fiber optic bronchoscope, a complication reported by Ramirez et al.12
The American College of Chest Physicians recommends doing a tracheostomy in patients expected to need an artificial line for over 21 days.25 Though the exact day for performing the tracheostomy is yet to be determined, we know that endotracheal mechanical ventilation for less than 7 days has a low incidence of sequels, which are usually reversible. A 7–10-day time interval is a transition that requires careful evaluation since it has a 12% incidence of laryngotracheal stenosis.26 Whited et al. report that the risk of long-term airway complications rises considerably after ten days of endotracheal intubation.9 In our study, the average time elapsed before the PDT procedure was 11.6 days. Romero et al. reported in his study an average of 16.7 days. The type of patients treated in each group may account for this difference, their particular indication for doing the tracheostomy and the protocols followed in each institution.
The complications of the procedure vary in accordance with the place of the study, the surgical team and the severity of the patient's condition. Several studies report complications ranging from 2% to 60%,27,28 with associate mortality between 0% and 5%.29,30 Bleeding at the puncture site has been reported at a frequency of 1.2%, and tracheal stenosis has been reported at 0.8% and the occurrence of pneumothorax at 0.4%.3,31 Hill et al. report 0.3% mortality and an overall rate of complications of 19%.32
In our retrospective study we found an incidence of early complications of 11%, which is lower than the figures reported by other studies.33,34 One of the most frequent intraoperative complications was the inability to perform a PDT. These patients required open tracheostomy.
We have documented significant differences in the percentage of complications with regard to the timing for a PDT. The ideal moment for a tracheostomy remains controversial. Recent evidence suggests that the decision to do a tracheostomy should be individualized, based on the future potential for extubation and on a risk–benefit evaluation. In patients with severe head injury a potential benefit of early PDT has been considered (3–4 days after the orotracheal intubation).35 The randomized clinical trial TracMan (Tracheostomy Management in Critical Care) was designed to evaluate the differences between patients undergoing the procedure before 4 days or after 10 days of the orotracheal intubation (early “vs” late).14 Though the technique for the tracheostomy was not standardized in the study protocol, most of the procedures were percutaneous at by the patient's bedside. The authors have reported the inclusion of over 900 patients in multiple ICUs throughout the UK. Additionally, there have very cases of intraoperative complications and no deaths related to the procedure. The early tracheostomy group showed shorter sedation times. However, there was no difference in length of stay in the ICU or the hospital between the groups. The final report of the trial is yet to be published. The differences with our study in terms of the time elapsed till the procedure was done should be carefully analyzed because of the size of the sample and the possibilities for Type I error. There were other complications similar to those reported by other authors.
The study was designed to evaluate only intra- and early postoperative complications of the tracheostomy in our center using the multiple dilators technique. Evidently, this estimate is directly dependent on the complications considered in the definition of this compounded outcome, which is not standard for different studies. One of the limitations is the short-term patient evaluation, not taking into account other important outcomes related to tracheostomies in critical patients. For his retrospective methodological characteristics, this study is subject to potential bias of information. However, his results are an important source of hypothesis for our group and the medical community as a whole. Moreover, recent evidence strongly suggests that the single dilation technique affords enhanced safety and higher success rates.36,37
In conclusion, this study shows that our group has developed a learning curve without the assistance of the fiber optic bronchoscope leading to a low incidence of early complications, similar to that reported in the literature.
Sources of fundingThe authors’ own resources.
Conflict of interestThe authors declare not to have any conflicts of interest.
Please cite this article as: Calvache JA, et al. Traqueostomía percutánea por dilatación sin fibrobroncoscopio. Evaluación de 80 casos en cuidados intensivos. Rev Colomb Anestesiol. 2012. http://dx.doi.org/10.1016/j.rca.2012.05.016.
This paper was awarded the second place in the Jorge Colmenares Contest, 39th Colombian Congress of Anesthesiology and Resuscitation, 2011.