The incorporation of new guidelines or strategies as part of good practices in the use of muscle relaxants is not a requirement at present in the practice of anesthesia. There are only action recommendations designed to persuade clinicians of the fact that neuromuscular monitoring is a very useful tool for the rational use of muscle relaxants.
MethodologyComplications occur, and residual paralysis is a significant event. For this reason, the authors advocate that monitoring neuromuscular block may be a determining factor in improving patient care and reducing morbidity and mortality. This review and its methodology based on the experience of the authors is designed to present, in a simple format, the knowledge that is considered fundamental for the systematic use of neuromuscular monitoring in every day practice.
Results and conclusionsThis update describes the fundamental principles of the methods available at present, emphasizing quantitative recording measurements. It then describes the different ways in which muscles respond to the effect of neuromuscular blockade, as these are critical fundamental principles that have to be known. Neuromuscular monitoring is a practice that should be implemented every time a neuromuscular block is required. We are aware of the difficulty of generating an explicit recommendation, but our enthusiasm is derived from the benefits we have personally experienced when applying these methods that have been known for a long time. Due to the potential morbidity associated with residual muscle relaxation, perioperative monitoring of neuromuscular function is essential.
La necesidad por incorporar nuevas guías o estrategias en la buena práctica de uso de los bloqueantes neuromusculares no es un hecho de obligado cumplimento en la actualidad dentro de la anestesiología. Solo existen recomendaciones de actuación con el propósito de convencer que la monitorización neuromuscular es una herramienta muy útil para el buen uso racional de los bloqueantes neuromusculares.
MetodologíaLas complicaciones surgen, y la parálisis residual es un evento destacado. Por esta razón, los autores propugnamos que la monitorización del bloqueo neuromuscular puede ser un factor determinante en la mejora del cuidado de nuestros pacientes, disminuyendo tanto la morbilidad como la mortalidad. Esta revisión y su metodología en base a la experiencia de los autores solo pretende exponer de forma sencilla conocimientos que consideramos básicos para su utilización sistemática en nuestra práctica rutinaria.
Resultados y conclusionesEsta actualización describe los principios fundamentales de los métodos que disponemos en la actualidad, priorizando las medidas cuantitativas de registro. Y también demuestra el diferente comportamiento de la musculatura al efecto de los bloqueantes neuromusculares, fundamentos relevantes que es preciso conocer. La monitorización neuromuscular es una práctica que debe utilizarse siempre que un bloqueo neuromuscular sea necesario. Somos conscientes que generar una recomendación explícita es difícil. Pero nuestro entusiasmo parte del beneficio de una experiencia personal con estos métodos que son conocidos desde antiguo. Debido a la potencial morbilidad asociada con bloqueos neuromusculares residuales, la monitorización perioperatoria de la función neuromuscular es esencial.
It was as a result of the work published by Beecher and Todd in 19541 about the toxicity of d-tubocurarine (dTc) and the mortality associated with its use when compared to patients who did not receive it, that a group of authors like Christie and Churchill-Davidson in 1958 suggested the use of the nerve stimulator as a diagnostic tool for prolonged apnea after the use of a muscle relaxant. These researchers popularized the observation of the adductor pollicis (AP) response, stimulated on the ulnar nerve at the wrist.2 These practices, just like “old editorials”, need to be remembered. “The only satisfactory means for determining the degree of neuromuscular blockade is to stimulate a motor nerve with electrical current and observe the degree of contraction of the muscles innervated by that nerve”.3 The reason why neuromuscular monitoring (NMM) has not been introduced into clinical practice reflects the discrepancy between what the literature recommends and what we clinicians are able to measure. Many anesthetists do not monitor neuromuscular function, or do not know how to interpret results correctly. Clinicians are not really convinced of the benefits provided by NMM.4 If we add to this that we are still looking for a monitor that is easy to use, inexpensive and safe, it is not surprising that the use of the nerve stimulator is more of an exception than the rule in anesthesia services. We may assert that “residual paralysis” is a difficult lesson still to be learned and to which we deny the value it deserves.5
Literature review methodologyBased on the personal experience of the authors. A PubMed search using the key phrases: “neuromuscular monitoring/neuromuscular block/degree o muscle relaxation/residual paralysis/adductor pollicis muscle/corrugator supercilii muscle/mechanomyography/Acceleromyography/electromyography/fade/twitch stimulation/train of four TOF/train of four ratio/Post-Tetanic Count (PTC)/tetanus/normalization of the TOF/TOF- Watch/intubation/guidelines”.
Consensus guidelines must prove their usefulness in those situations in which they are necessary, and the fundamental goal is to improve outcomes. Today, even after more than 50 years since the introduction of a peripheral nerve stimulator, its usefulness is still under debate. According to the “ASA Task Force on Post-anesthetic Care” the assessment of neuromuscular function includes NMM only occasionally.6 The scientific community tries to persuade, but we are only on the road towards a simple recommendation, and there is no mandatory standard. NMM requires training and a learning curve, and there are inherent technical factors that need to be known. Anesthetists working in this area must communicate the true meaning of this method from a critical perspective, and it is important to recognize that evidence in this field is limited. Meta-analyses of randomized controlled trials are crucial in order to create scientific evidence, but expert opinions are also important as an alternate road to decision-making.7 This review attempts to communicate the personal experience of the authors. First we describe the stimulation patterns (supramaximal stimulus), but with greater emphasis on recording methods such as acceleromyography, the most widely used at the present time because it is easy to set up. We then refer to the practical aspects of visualization, where a key factor is to stabilize the signal in order for the muscle contraction to be constant and easy to interpret. Finally, we refer to the clinical relevance of residual paralysis (RP), its diagnosis and treatment. NMM is an evidence-based practice that must be used whenever muscle relaxation is required.8
Why use muscle function monitoringNMM is good guidance whenever there is a need to use neuromuscular blockade to significantly improve the quality of intubation and reduce airway injury.9 NMM is useful for maintaining adequate neuromuscular blockade, but also for the diagnosis of residual paralysis (RP). Residual paralysis occurs even with the use of intermediate-acting non-depolarizing muscle relaxants (NDP).10 Only through the use of objective monitoring is it possible to avoid RP.11 Evoked responses do not require patient cooperation.
NMM reports the degree of neuromuscular blockade only in the paralyzed muscle, and there are substantial differences between different muscle groups. The adductor pollicis (AP) muscle does not reflect the neuromuscular block of laryngeal muscles. In cases of thoracic or abdominal surgery, where profound relaxation is required, a second option is to monitor a muscle that behaves similarly to diaphragmatic and laryngeal muscles, as is the case of the corrugator supercilii (CSC) muscle.12 In contrast, it is better to monitor the AP for extubation, considering that it is a more sensitive muscle. Complete AP recovery rules out any issue of residual paralysis.13
Methods for assessing neuromuscular functionNMM is a quick and simple maneuver, but in order for it to be reliable, it is important to bear in mind certain aspects.
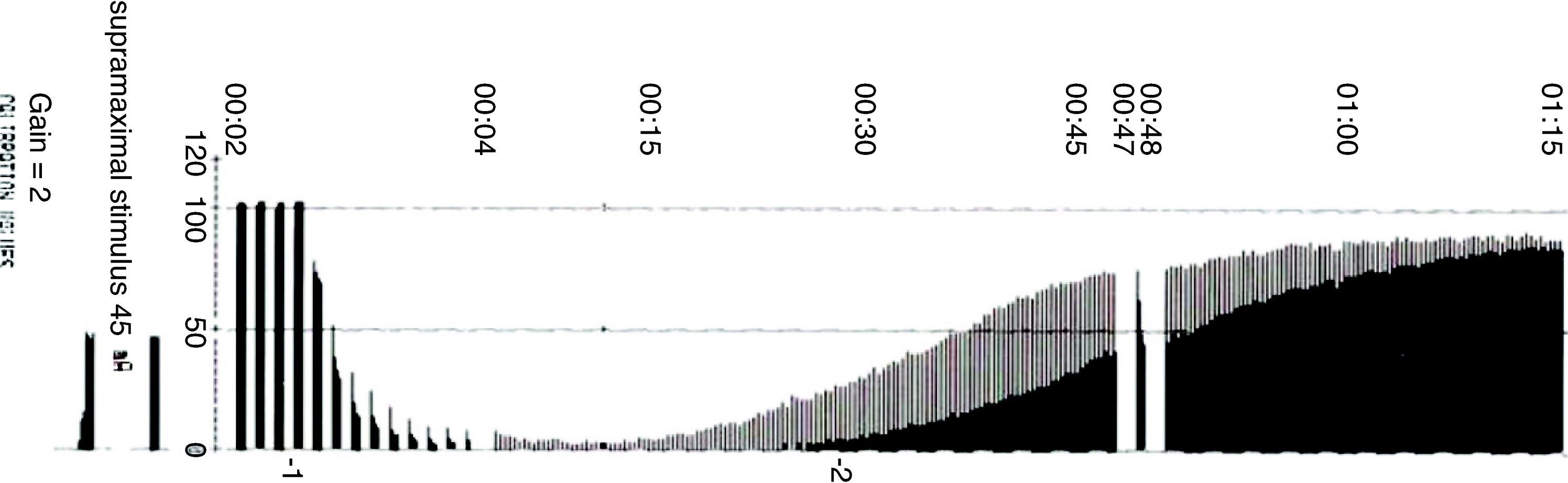
Principles of nerve stimulation (supramaximal current)In assessing muscle activity, nerve stimulation amplitude is key. The reaction of the neuromuscular junction to an electrical stimulus is an “all-or-nothing” kind of thing. This means that it may or may not contract but, when it does, there is maximum contraction. During nerve stimulation, the muscle contraction force increases as the intensity of the stimulus grows, until a plateau is reached where the stimulus is sufficiently intense as to activate all axons. That is when supramaximal intensity is achieved, and muscle response does not increase beyond that point even if the intensity of the stimulus increases (this intensity varies depending on the nerves and the individual patient). Supramaximal stimulation is a pre-requisite to ensure that the recorded muscle response depends exclusively on the degree of neuromuscular blockade.
Sites for neuromuscular monitoringThe ideal site for stimulation is the one most readily accessible during surgery and where muscle response may be identified clearly and unmistakably. The best-studied muscle is the adductor pollicis, which serves as a useful marker of the most important aspects of neuromuscular function, as is the case of recovery from relaxation.
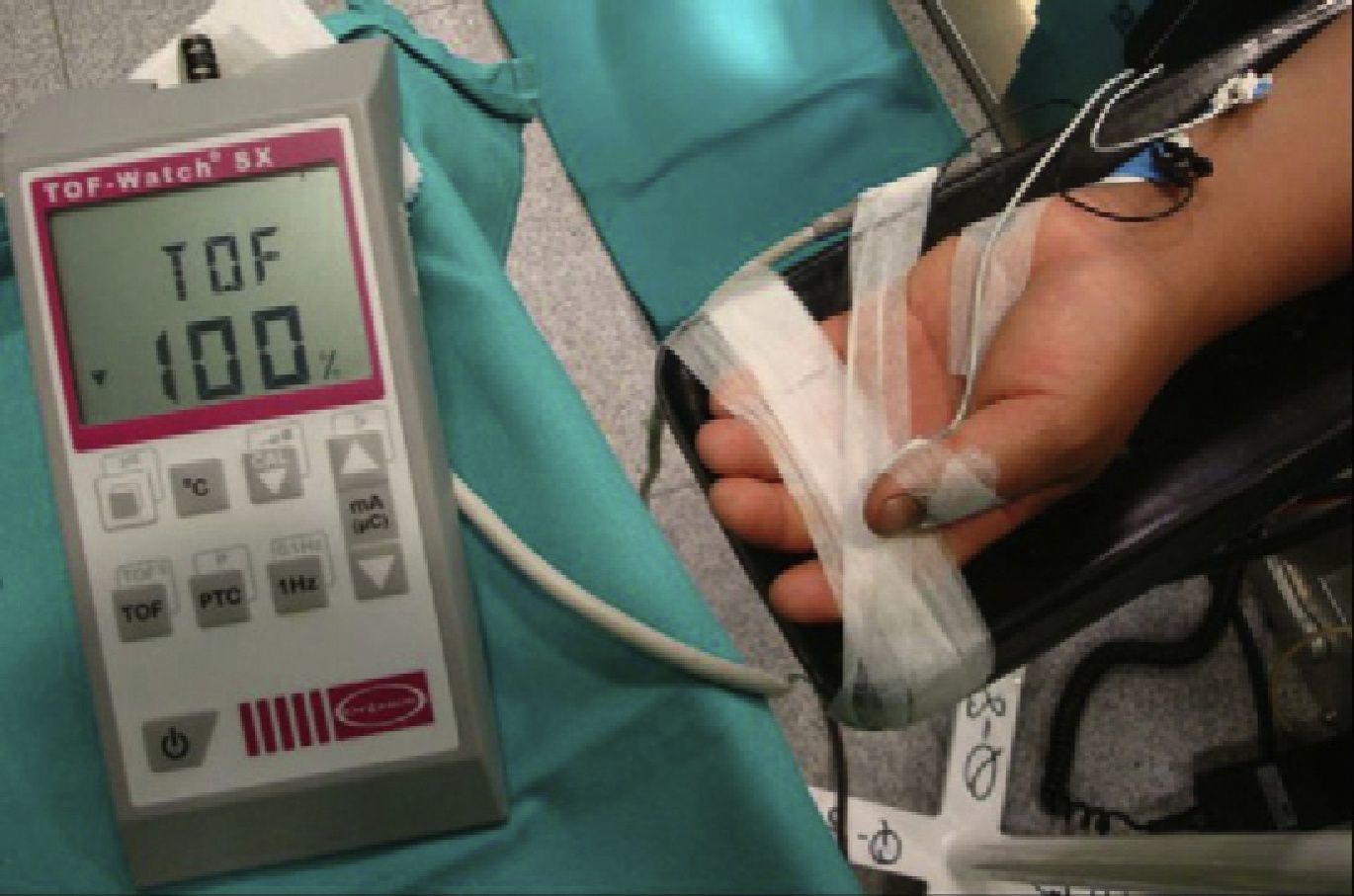
The degree of paralysis may be quantified appropriately by assessing the AP response (Fig. 1). In order to monitor de AP muscle, the ulnar nerve has to be stimulated. The electrodes are attached on the internal aspect of the wrist on the surface of the skin, along the course of the ulnar nerve. The contact area of the stimulating electrodes must not be greater than 7–11mm. Most of the knowledge pertaining to the pharmacology of NDP neuromuscular blockers comes from this “nerve-muscle group”. When there is no access to the upper limbs, muscle response may be monitored by means of facial nerve stimulation.
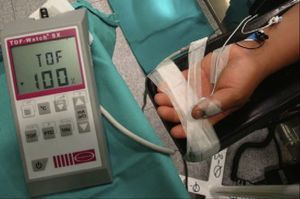
Quantitative monitoring of the adductor pollicis (AP) muscle, with the electrodes stimulating the ulnar nerve. Negative distal black electrode. Acceleration transducer fixed with tape on the internal distal aspect of the thumb. The model is a TOF-Watch®-SX monitor, Organon Ireland Ltd., a division of MSD, Swords, Co., Dublin, Ireland. A 100% TOFR is seen on the monitor screen.
Source: Authors.
In daily practice, many anesthetists use tactile and visual assessment to determine the degree of neuromuscular blockade by means of peripheral nerve stimulation. Although it is a simple procedure, responses are observed with the naked eye and counted, making it an imprecise method, given the subjective interpretation of the response. Even though we can count and feel muscle weakening, there is some inability to estimate with certainty the difference in the force of the contraction between successive responses. Even when the patient is conscious, the accuracy of the clinical tests is limited and they do not rule out RP with certainty.14
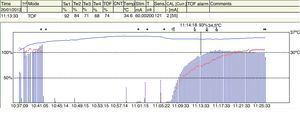
Muscle response recording, quantitative monitoringResponses may be measured using quantitative recording methods such as mechanomyography (MMG), which measures the isometric contraction of the AP in response to ulnar nerve stimulation.15Electromyography (EMG) records muscle action potentials generated by the electrical stimulation of a peripheral motor nerve. An example of EMG is the Relaxograph NMT Monitor (Datex Instrumentarium Corp., Helsinki, Finland), which measures T1, TOF and TOFR (Figs. 2 and 3).
Acceleromyography (Fig. 1) is a good solution for the technical and commercial difficulties of the traditional methods: it records the isotonic acceleration of a muscle (e.g., the thumb), in response to peripheral nerve stimulation. It was described by Viby-Mogensen et al.,16 and it may be applied on all muscles whose movement or acceleration may be evoked by electrical stimulation. AMG is based on Newton's second law that force is equal to mass multiplied by acceleration (F: M×A). If the mass of the thumb remains constant, acceleration will be directly proportional to force. When the thumb responds to stimulation with a twitch, the electrical signal produced is proportional to the acceleration generated. The advantages of this method are many, including a real-time, objective measurement of the neuromuscular function, rapid calibration, low cost, and no need for preloading or special immobilization of the hand. The acceleration sensor is fixed with tape on the internal distal aspect of the thumb, which needs to be free to move unimpeded. The TOF-Watch®-SX (TOF-Watch®-SX monitoring software (Organon Ireland Ltd., a division of Merck and Co., Inc., Swords, Co., Dublin, Ireland) allows to capture evoked responses with the use of a computer, a fiber optic cable and an excellent software package (TOF-Watch®-SX Monitor, version 2.2 INT, Organon) that assess data in real time.
“Monitoring Practice”: interpretation of a non-depolarizing neuromuscular block (NDPNMB)The fundamental characteristic of NDP neuromuscular blockade is “fade”. As indicated below, the graphic representation is the TOFR, which consists of a gradual fading of the response with repetitive stimulation in the curarized patient. Visually, it is seen as a reduction of the muscle contraction force. From the physiological point of view, it is important to know the mechanism of action at the level of the motor plate receptors, since the two phenomena are the representation of different mechanisms of action depending on the site. Inhibition or blockade of the first TOF twitch depends on a competitive antagonist action on the post-synaptic nicotinic receptor (αβδ¿). Weakening or fade reflects the blockade mediated mainly by the pre-synaptic nicotinic receptor (α3β2).17
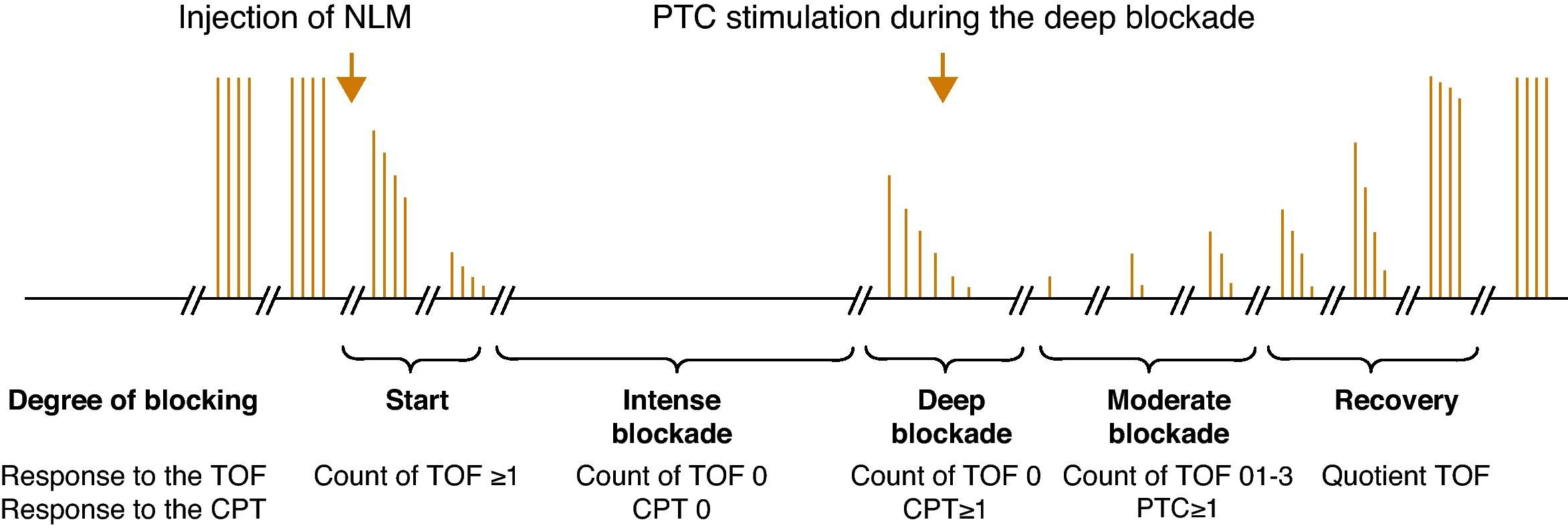
Degrees of neuromuscular blockade18Intense blockNeuromuscular block induced immediately after the administration of a non-depolarizing muscle relaxant (intubation dose) where there is no response evoked after tetanic stimulation (Fig. 4).
Deep blockThe fundamental characterictic of (NDPNMB). This is the phase following intense blockade where there is no TOF response. It begins with responses to single stimuli after tetanic stimulation (post-tetanic count) and ends with the first TOF twitch.
Moderate blockIt is defined as the time period between the first and the fourth TOF twitch.
Recovery phaseThis is when the fourth TOF twitch occurs and the TOFR is determined.
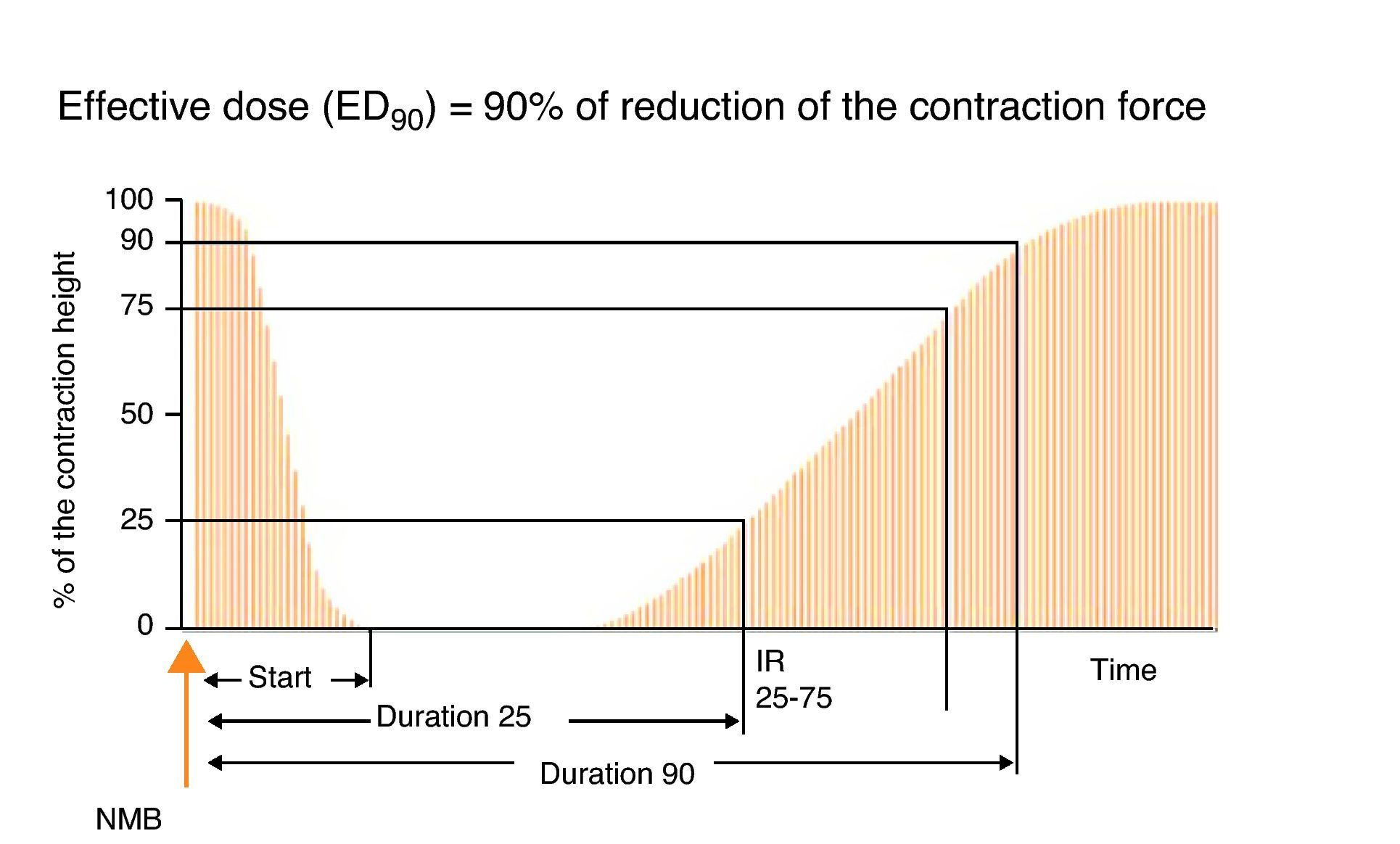
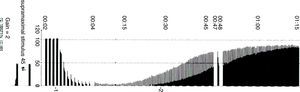
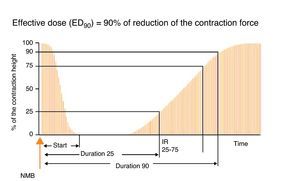
Types of stimuliSingle stimulusIt consists of the application of supramaximal stimuli on a peripheral motor nerve at a frequency ranging between 1Hz (one stimulus per second) and 0.1Hz (one stimulus every 10s) (Fig. 5). A single stimulus is a good tool to study the pharmacodynamics of muscle relaxants. If after giving 0.3mg/kg of a non-depolarizing neuromuscular blocker the height of the single stimulus is reduced by 90% over a control value in a specific patient, at a frequency of 0.10Hz, it can be said that the administered dose is ED90 (the effective dose to inhibit muscle contraction by 90%). It is important to make a measurement before administering a muscle relaxant in order to make a correct calibration of the response so that any changes with regard to the control value determine the onset of action of the neuromuscular block. On occasions, the baseline control level is not available and it is not possible to compare or assess muscle weakness in the post-operative period. With superficial curarization, or when assessing residual effects, the muscle response to the single stimulus is hardly significant, because it may produce contractions of similar amplitude as those observed during the control period. Ideally, we should be able to make a quantitative estimate of the degree of neuromuscular blockade without the need for a control twitch, in particular if RP is suspected.
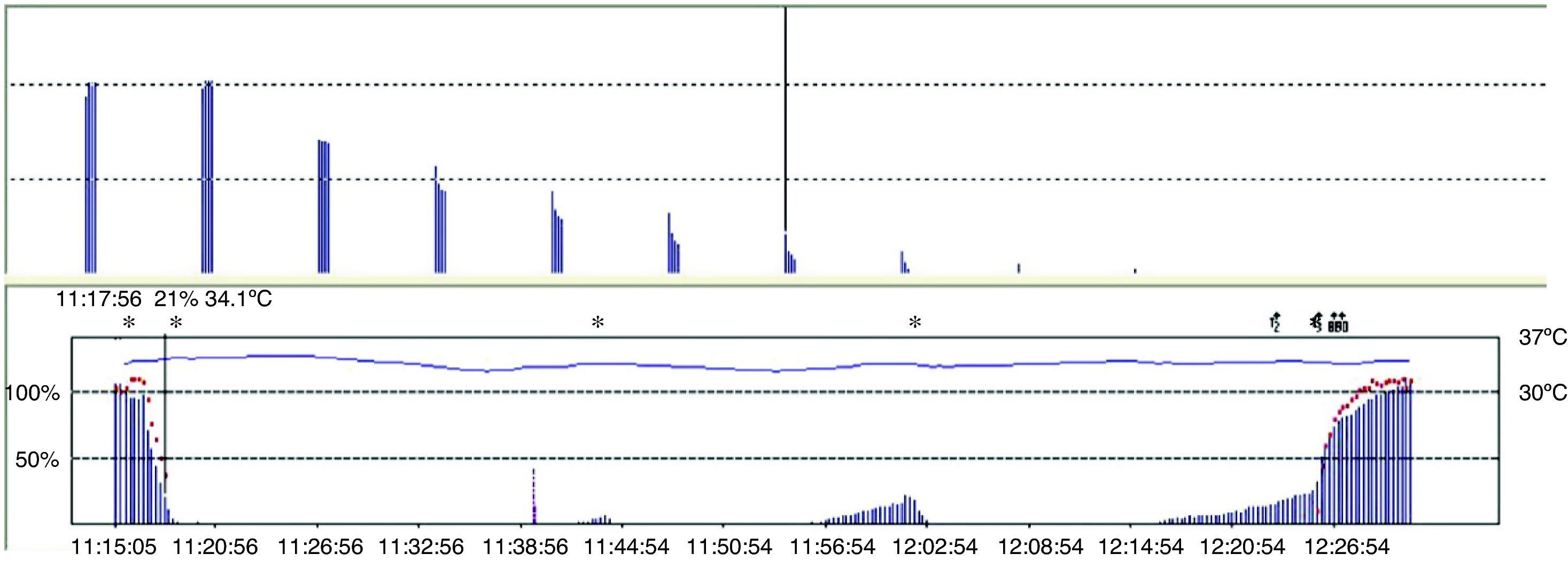
Train of four (TOF)TOF is the standard method for NMM (Fig. 6). In 1971, Ali et al.19 published that when four stimuli were produced at 0.5-s intervals there was progressive weakening of the subsequent twitches in curarized patients, and that the magnitude of the fade was dependent on the degree of curarization. The TOF technique has remained the most useful method for assessing neuromuscular function for more than 40 years because it is simple and easy to assess. It is based on the observation that increased stimulation frequency produces muscle fatigue or fade. TOF frequency is sufficiently slow to distinguish individual contractions, and sufficiently fast to show fade. The proportion that results from dividing the fourth by the first evoked twitch (T4/T1) is the train-of-four ratio (TOFR). TOF has been recommended in clinical practice because it is the test that measures neuromuscular function exclusively and is capable of providing information even when no prior value has been obtained. Moreover, it is easy to use and may be used repeatedly.20 The following is the TOF rule for the AP: the onset of twitches 1, 2, 3, and 4 is approximately consistent with the height over the control value of 5, 15, 25 and 35%, respectively.21 Consequently, the TOF count is an excellent guide considering that it reports not only the degree of neuromuscular block but also the state of recovery from it. It predicts recovery from neuromuscular blockade (balance between anticholinesterase activity and spontaneous recovery from neuromuscular blockade).22
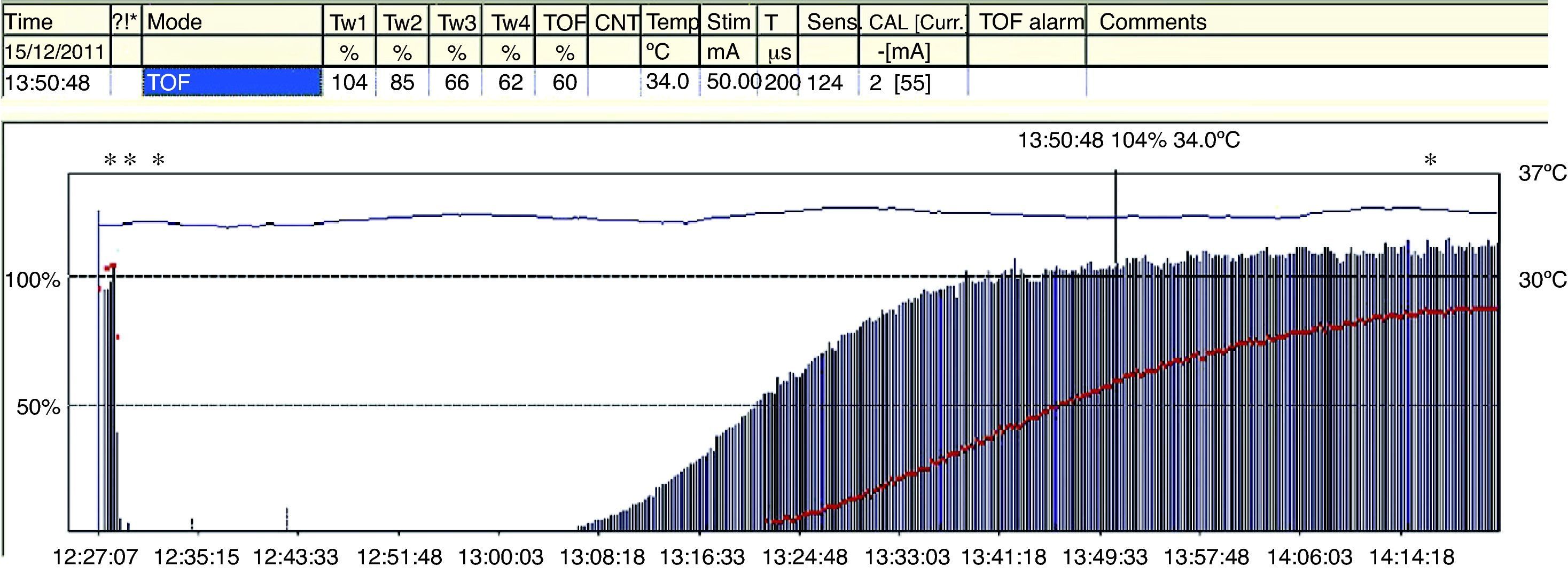
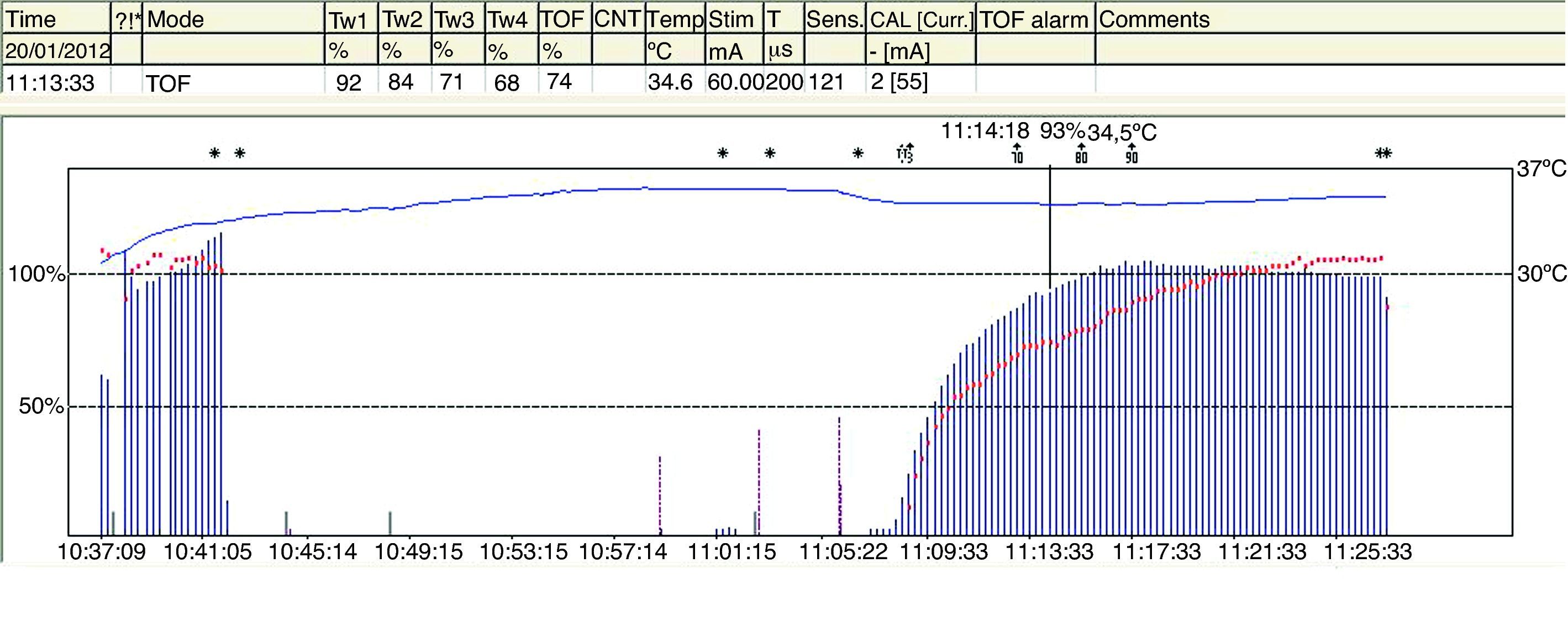
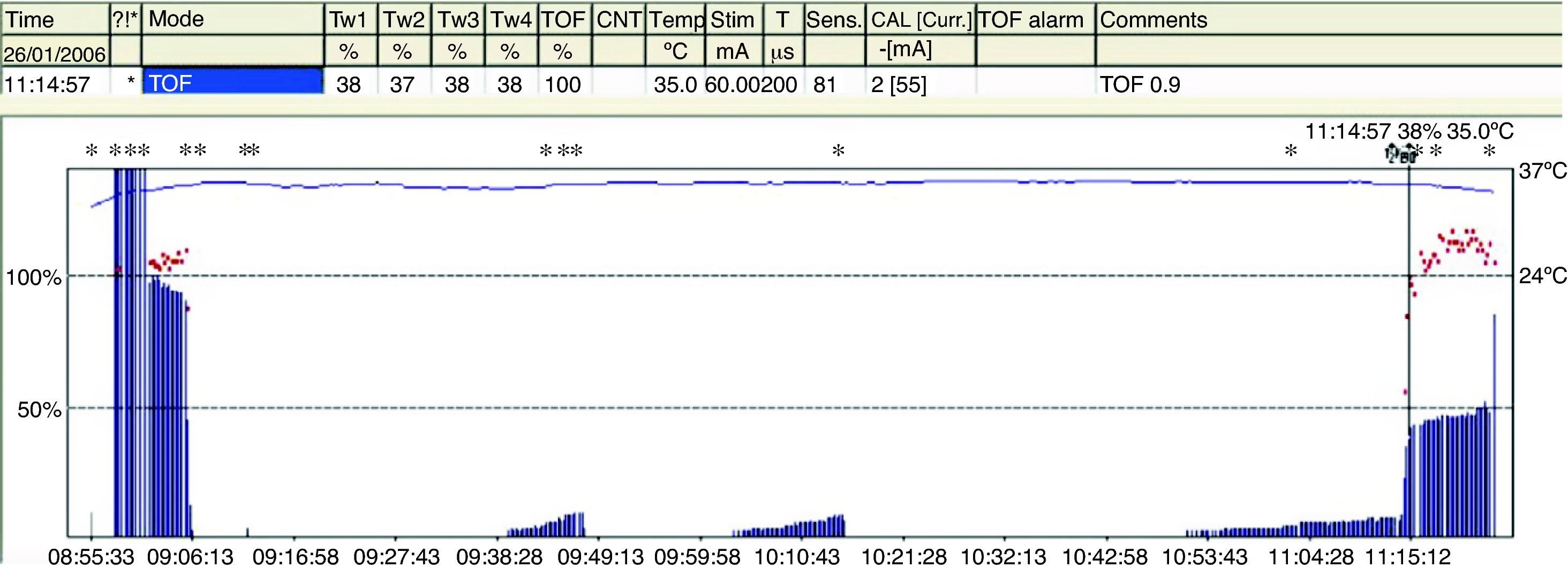
The TOFR is the graphic quantitative representation of the typical fade phenomenon of a NDPNMB.23 The ratio reflects the effects of a NDPNMB at a pre-synaptic level. During spontaneous recovery from a NDPNMB, as well as during reversal with a anticholinesterases, when the first TOF response reaches the control value (100%) the TOFR reaches values ranging between 64% and 80%24 (Figs. 7 and 8). This can be considered as a typical pattern in both circumstances where the first TOF twitch always precedes the TOFR because TOFR fade phenomenon persists for a longer period of time (red dotted line in Figs. 7 and 8). The time relationship between the first twitch and the TOFR after reversal with sugammadex following rocuronium-induced muscle relaxation is different. Staals et al. were the first authors to demonstrate this finding. TOFR recovery of 0.9 precedes the recovery of the height of the first twitch (Fig. 9). The real meaning of this finding in unknown and probably does not have significant clinical repercussions. However, these researchers concluded that the TOFR, if it is to be used as an adequate measure of reversal, needs to be accompanied by complete recovery of the first twitch.25
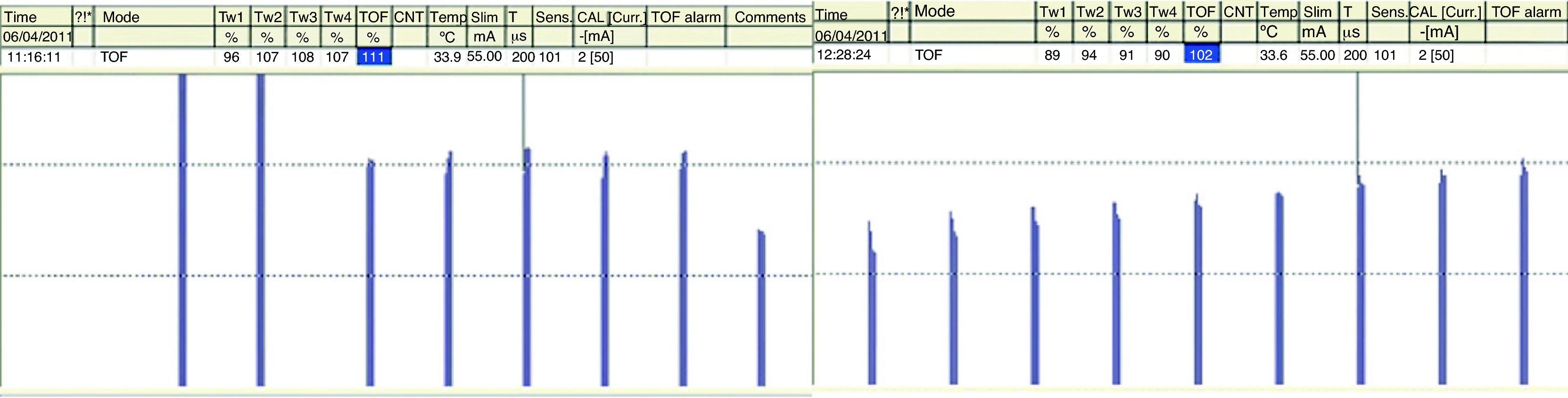
In a recent publication, AMG was shown to have some variability in terms of prior calibration; this might challenge the accuracy of recovery from neuromuscular blockade when compared with MMG and EMG.26 Unlike MMG and EMG, where before giving a muscle relaxant the baseline TORF value is 1 (T4 is 100% of T1), the TORF control value of AMG tends to be higher than 100%. In order to avoid this finding, the signal has to be stabilized (baseline calibration). This effect, commonly seen with AMG, suggests that the TORF has to be normalized or corrected. Correction implies comparing values at the end of monitoring with prior or baseline values. For example, if the control TORF is as high as 111%, with a recorded value of 102% at the end of monitoring, it would be consistent with a recording of 0.91 (102/111) over the baseline value (Fig. 10). AMG is a technique for neuromuscular monitoring that has shown to be useful in daily practice as well as in research programs. However, initial calibration before giving the muscle relaxant in order to obtain a T4/T1 value as close as possible to 100%, modulates stimulation amplitude, which favors interpretation and information to avoid the potential danger of an incomplete neuromuscular blockade. Normalization of a TOFR of 1.027 in relation to the baseline value is considered to ensure adequate recovery of the neuromuscular blockade and to improve detection of RP.28
Acceleromyography recording. A baseline control value, previously calibrated, is displayed first on the left together with a control TORF of 111% before the administration of a NDPNMB. To the right, after recovery from neuromuscular blockade, a TORF recorded value of 102% at the end of monitoring must be normalized. It corresponds with a recorded value of 0.91 (102/111) over the baseline value.
Source: Authors.
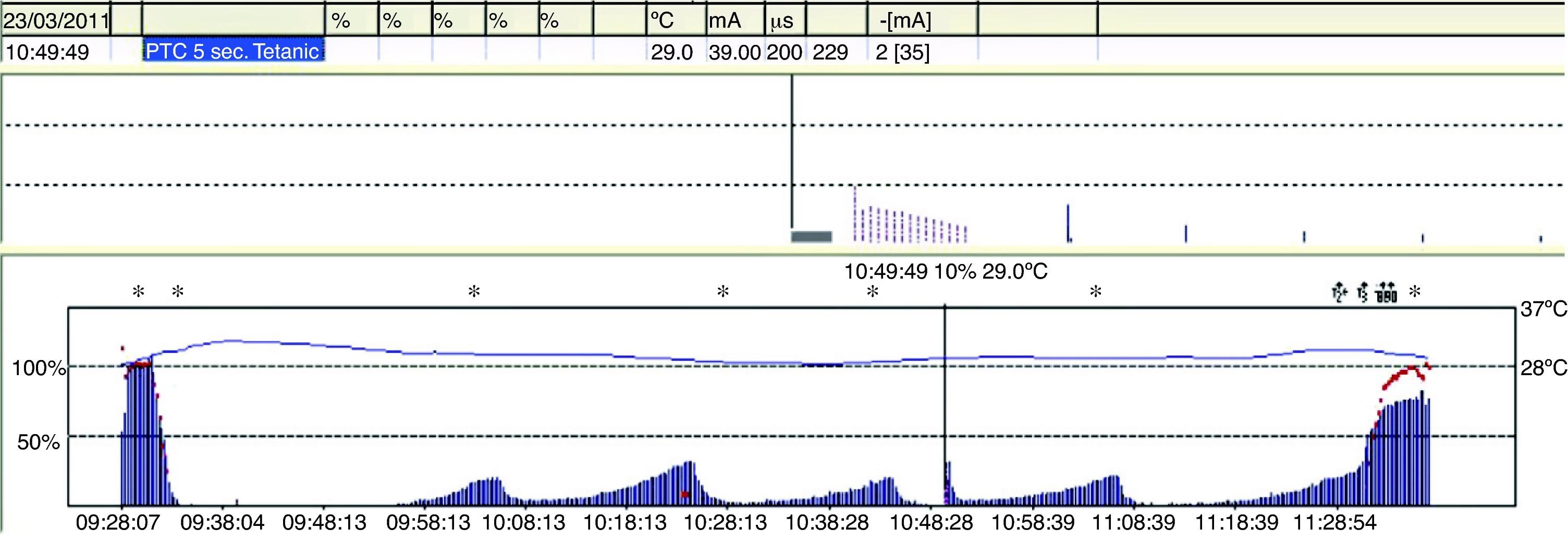
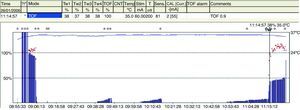
TOF is of limited use during deep blockade. The clinician is unable to assess the degree of muscle paralysis with certainty. The answer to this problem was found by Viby-Mogensen et al.,29 who showed that post-tetanic potentiation is a useful tool for the accurate assessment of the degree of NDPNMB. This means that the clinician may observe, after tetanic stimulation, responses to single stimuli where they did not exist previously. They suggested the following sequence: tetanic stimulus at 50Hz for 5s, stop for 3s and follow with 20 single stimuli at 1-s intervals. In patients with a deep block, only one post-tetanic contraction is detectable at first (PTC: 1). As recovery of the NDP muscle blockade progresses, the PTC increases. These authors were able to show that the PTC is a very practical indicator of how the NDP neuromuscular block evolves. Post-tetanic count is also a valid method for predicting time of onset of the first TOF twitch30 (Fig. 11). The physiological explanation is the following: high-frequency tetanic stimulation produces a transient release of huge quantities of A-c at the pre-synaptic level, creating a state that favors the A-c neurotransmitter at the end plate. Clinically, this manifests as a transient increase in the height of the successive contractions evoked by single stimuli, the so-called post-tetanic facilitation or potentiation phenomenon.31
Different muscle behaviors (practical applications)The AP muscle is used for practical and convenience reasons. However, there are important differences between the muscles. Consequently, the AP response does not represent the state of relaxation of the entire body, although it may be interpreted based on the knowledge of the differences between the muscles. During deep neuromuscular blockade or when it is difficult to access the AP, it is possible to monitor other stronger muscles that will guide the clinician in those situations and will be better predictors of the timing for intubation.
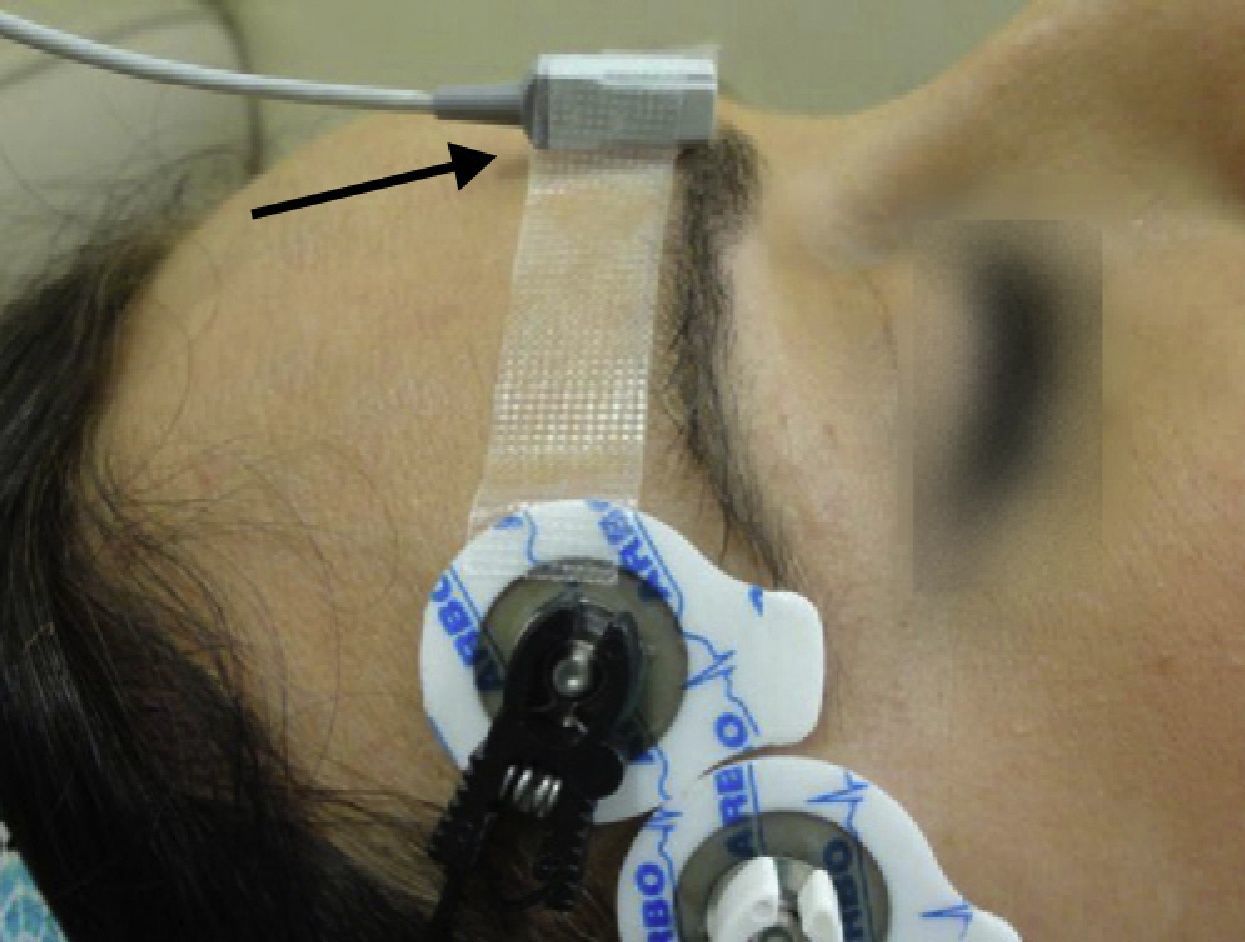
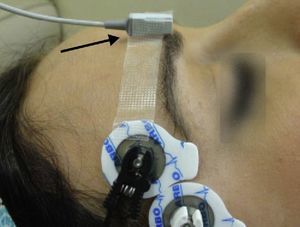
Monitoring of the CSC muscle to guide tracheal intubationGood intubation conditions may be predicted using the CSC muscle as a guide. Its neuromuscular behavior profile is the same as that of the laryngeal muscles.32 Neuromuscular blockade at the CSC muscle ensures optimal timing for intubation. If, after administering a NDPNMB we wait until the muscles of the hand are completely paralyzed, the waiting time may result in an overestimation of the time required for relaxation of the laryngeal muscles. The CSC is a small muscle localized in the medial portion of the eyebrow and it acts by pulling the brow towards the nose. CSC monitoring may be achieved using AMG, but attention has to be paid to several aspects if it is to be done correctly. The VIIth cranial nerve (facial nerve) may be stimulated on the lateral aspect of the superciliary arch, with a supramaximal current intensity of only 20mA. The transducer is placed on the medial half of the superciliary arch, at a 90° angle perpendicular to the direction of the CSC contraction33 (Fig. 12).
CSC muscle: sensitivity profileMuscle sensitivity to NDP neuromuscular blockers is related to the number of muscle receptors on the motor end plate and the size of the muscle fiber. The morphological composition of the muscle fibers has been determined on the basis of histological studies.34 Receptor density in central muscles is higher than in peripheral muscles. Therefore, the relationship between the number of A-c receptors and the thickness of the muscle fiber is a morphological predictor of the various muscle responses (sensitivity) to NDP muscle relaxants. Sensitivity increases with the size and diameter of the fiber and drops in relation to the number of A-c receptors. This important anatomical factor explains the similarity between the sensitivity profiles of the laryngeal muscles and facial muscles (higher resistance). For this reason, monitoring the CSC response is a guide to a more adequate prediction of the optimal timing for intubation35 when compared to the peripheral muscles (AP).
Residual paralysis: clinical relevanceThe adverse effects of RP are a cause of morbidity and mortality during the immediate post-operative period.36 The following are the main factors that help reduce the problem, based on the systematic use of NMM and the administration of a reversal agent.
Diagnosis and incidence of residual paralysisTOF ratio (T4/T1)The absence of RP means that neuromuscular transmission is sufficiently recovered, but it is important to be able to measure it effectively. In the absence of neuromuscular blockade, the height of the four twitches is the same, and the TOF equals 1. The TOFR is a very sensitive test that correlates well with the clinical tests used to assess the degree of recovery from the neuromuscular blockade.
With the introduction of TOF in 1970, it was determined that the TOFR37 in the adductor pollicis (AP) correlated well with the clinical signs of recovery, and it was considered a basic parameter in neuromuscular monitoring. Values of <0.6 were associated with significant muscle weakness. In 1979, Viby-Mogensen et al. were the first to show that, despite apparent clinical recovery, 42% of the patients had a TOFR<0.7.38 At present, after numerous studies, researchers have agreed on a definition of TOFR that represents a safe level of recovery. Using AMG, it has been accepted that the safest TOFR must be 0.9. This endpoint ensures complete control of the pharyngeal muscles and normal ventilator response to hypoxia.39,40 When assessing all the studies that refer to the wide incidence of RP in the post-anesthetic care units, it is important to consider a series of details pertaining to the intra-operative management, which are not always available. Was a nerve stimulator used? Was reversal used for residual blockade? We may suggest that RP is infrequent when NMM is used. Considering that the AP is one of the last muscles to recover, it is better to assess recovery by means of AP monitoring.41
Mandatory strategies for the reduction of residual paralysisThe following are the principles that should be followed in order to avoid RP42–44:
- 1.
Intra-operative neuromuscular monitoring. AMG, as a quantitative method, is better than visual assessment for diagnosing RP.
- 2.
Avoiding total TOF inhibition.
- 3.
Use of intermediate-acting NDP muscle relaxant.
- 4.
The administration of anticholinesterases with a certain degree of spontaneous recovery of neuromuscular transmission is a critical step in reducing or eliminating RP.
- 5.
Delaying extubation until a TOF ratio of 0.9 is achieved.
- 6.
A proposal developed recently is to use the new molecule sugammadex (abbreviation of sugar and γ-cyclodextrin), specifically designed to bind to rocuronium selectively. At the present time, it is approved and marketed in Europe and Australia, but not in North America. Its main advantage is rapid action with minimal variation between individuals, after an adequate dose that can achieve a TOFR of 0.9 (3–5min).45
Research carried out during the immediate post-operative period at the time of extubation has shown RP with a higher possibility of morbidity. Murphy et al.46 conducted a study of TOFR quantified using AMG, immediately before tracheal extubation. It was concluded that RP was present in most patients at the time of extubation. Despite a protocol designed for monitoring and reversal, and despite the use of an intermediate-acting NDPNMB, there was some degree of RP at the end of surgery, while the patient was still in the operating room. These authors recommend NMM to ensure that recovery is complete and that respiratory and pharyngeal function is normal.
Prevention of residual paralysisPerhaps one of the most convincing pieces of evidence regarding RP assessment came from the studies by Baillard et al. These researchers showed that intra-operative NMM using AMG as an objective method, together with an effort at educating the clinicians, led to a reduction of RP from 62% down to very low levels.47,48 This finding contributed to the provision of quantitative neuromuscular function assessment devices in all the operating rooms.
Conclusions: what is the final lesson?Despite the landmark introduction in 1942 of curare in clinical practice and the important advances in the understanding of the physiology of neuromuscular transmission, it is surprising to see that NMM continues to be considered only as an option. The purpose of this review is to convey practical and academic reasons to convince clinicians that NMM should be used in daily practice. Published surveys show a low rate of use of NMM, as revealed by a survey conducted recently in the United Kingdom among 715 anesthetists. Of them, 28% used a monitor occasionally, 10% used it routinely, and up to 62% never used it.49 We are aware that NMM is no absolute guarantee of perfect intubation conditions, optimal intra-operative muscle relaxation, or complete recovery. AP monitoring may be used for the duration of anesthesia, alternating if needed, with CPT. The information provided by NMM must be interpreted on the basis of the individual patient, the drugs used, and the clinical signs. NMM is very useful provided it is interpreted correctly. AMG and a TOFR of 1 at the end of surgery help rule out RP. In an editorial,50 Eriksson concluded that, “it is time to act and give priority to the introduction of neuromuscular transmission monitors in all operating rooms and use them every time we give NDP muscle relaxants, instead of using them exclusively for research. It is our duty as researchers interested in this matter to call the attention on the advances of the art and the science, and to highlight that muscles relaxants must be used with good judgment in order to maintain an adequate degree of relaxation according based on timing and need”.
FundingOwn resources.
Conflicts of interestNone declared in relation with the article.
We thank Belén Mingorance Diaz, the nurse at our Recovery Unit, for her help with CSC muscle monitoring image.
Please cite this article as: Fabregat López J, et al. La monitorización neuromuscular y su importancia en el uso de los bloqueantes neuromusculares. Rev Colomb Anestesiol. 2012;40:293–303.