Hypotension after spinal anesthesia in cesarean section should be minimized. The use of vasopressors is an effective measure to treat hypotension. The objective of this paper is to compare the safety and effectiveness of etilefrine vs. phenylephrine in the management of this condition.
MethodsThis multicenter, double-blind trial between August 2009 and November 2010 included 196 patients with hypotension during spinal anesthesia for cesarean delivery; the patients were randomized to receive etilefrine or phenylephrine as vasopressor. The primary outcome was the fetal umbilical arterial pH. The secondary outcomes were: fetal acidosis (umbilical arterial pH<7.20), Apgar score at 1 and 5min, need for intubation and admission to the neonatal intensive care unit for newborns, and time of hypotension, total dose of vasopressor, atropine requirement, intravenous fluids volume and incidence of nausea and vomiting in mothers.
Results98 patients received etilefrine and 98 phenylephrine. There were no differences in umbilical arterial pH (7.27 vs. 7.28, respectively, P=0.493). The total dose of vasopressor (5.66 vs. 6.51ml, respectively, P=0.024) and total time of hypotension (2.78 vs. 3.25min, respectively, P=0.021) were lower in the etilefrine group. Other outcomes studied showed no statistically significant differences.
ConclusionEtilefrine and phenylephrine are equally effective for the treatment of hypotension during spinal anesthesia for cesarean delivery. This study found no difference in the maternal or fetal outcomes.
La hipotensión que ocurre luego de anestesia espinal para cesárea debe minimizarse. El uso de vasopresores es una medida eficaz para su tratamiento. El objetivo de este trabajo es comparar la seguridad y efectividad de etilefrina vs fenilefrina para manejo de esta condición.
MétodosEn este estudio multicéntrico y doble ciego, entre agosto de 2009 y noviembre de 2010, 196 pacientes con hipotensión durante anestesia espinal para cesárea, fueron asignadas aleatoriamente para recibir etilefrina o fenilefrina como vasopresor. El resultado primario fue el pH arterial umbilical fetal. Los resultados secundarios fueron: acidosis fetal (pH arterial umbilical<7,20), puntaje Apgar al 1 y 5 minutos, necesidad de intubación e ingreso a la unidad de cuidados intensivos neonatal para los recién nacidos; y tiempo de hipotensión, dosis total de vasopresor, necesidad de uso de atropina, líquidos endovenosos totales e incidencia de nausea y vómito para las madres.
Resultados98 pacientes recibieron etilefrina y 98 fenilefrina. No se encontraron diferencias en el pH arterial umbilical (7,27 vs 7,28 respectivamente; p=0,493). La dosis total de vasopresor (5,66 vs. 6,6ml, respectivamente; P=0,024) y el tiempo total de hipotensión (2,78 vs. 3,25min, respectivamente; p=0,021), fueron menores en el grupo de etilefrina. Los demás desenlaces estudiados no presentaron diferencia estadísticamente significativa.
ConclusiónLa etilefrina y la fenilefrina son igualmente efectivas para el tratamiento de la hipotensión por anestesia espinal para cesárea. Este estudio no encontró diferencia en los resultados fetales ni maternos.
In the last few years there has been an increasing incidence of cesarean sections worldwide, with rates ranging from 25 to 30%.1 Regional anesthesia is considered superior to general anesthesia for cesarean delivery because it reduces maternal morbidity, although mortality and neonatal outcomes are similar as compared to general anesthesia.2,3 Spinal anesthesia has become the technique of choice for this procedure because it is safer and simpler to use, is administer in a shorter time, has a quick onset of action, and is more comfortable for the patient.
Maternal hypotension is an unwanted consequence of spinal block. Its incidence ranges from 55 to 90%,4–6 and is more frequent in patients scheduled for elective cesarean section and no labor.7
Hypotension during spinal anesthesia – regardless of how mild or short duration – results in deleterious effects for both the mother and the fetus. There is decreased utero-placental blood flow (UBF) causing hypoxia and fetal acidosis, as well as neonatal depression.1,8 The mother experiences low cardiac output symptoms, including nausea, vomiting, dizziness, and decreased consciousness. Several interventions have been studied and implemented to reduce the incidence of hypotension; i.e., uterine displacement, intravascular volume expansion with intravenous fluids, and the use of vasopressors.9,4
Despite the use of pre-load or co-load of intravenous fluids, there is still a high frequency of hypotension and vasopressors are required in a high proportion of patients.10
Though most studies have shown that the incidence of fetal acidosis following spinal anesthesia is secondary to hypotension, others feel that acidosis may be associated to the transfer of the vasopressor across the placenta.8,11–14
Phenylephrine is one of the most studied vasopressors and is the drug of choice in obstetrics because of its high transfer rate across the placental barrier, increased fetal metabolism due to direct stimulation of the α and β receptors, and because it increases catecholamines and PaCO2. Phenylephrine has shown less transfer across the placenta with enhanced utero-placental blood flow and improved acid–base fetal status.12–15
Etilefrine is the most frequently used vasopressor in Colombia for the treatment of hypotension from spinal anesthesia during cesarean section.16 Etilefrine is a direct action sympathomimetic agent that stimulates the α-1 and β-2 receptors.17 We have not been unable to find any studies comparing these two vasopressors.
The purpose of this study is to compare the fetal outcomes as evidenced by the pH of the umbilical artery and the Apgar score, and maternal outcomes, measured in terms of the dose of vasopressor, hypotension time and the incidence of nausea and vomiting, following the administration of etilefrine vs. phenylephrine for the management of hypotension secondary to the administration of spinal anesthesia for cesarean section.
Materials and methodsA randomized, double blind, controlled clinical trial was undertaken in two clinics in Medellin: Clínica Universitaria Bolivariana and Clínica del Prado. The trial was approved by the Institute of Ethics and Bioethics of the Pontificia Bolivariana University.
A random allocation in blocks of three was performed, classified in accordance with the institution, using RALLOC version 3.5.2 statistical software. The trial was initially planned for three institutions but when data collection was initiated, only two participated. The blinding process was done in opaque envelopes containing the study group to which the patient was allocated. The envelopes for the third institution were distributed between the other two.
All pregnant women who underwent elective or programmed cesarean section according to the Lucas and Yentis classification18, under spinal anesthesia, with a gestational age of ≥36 and <42 weeks, aged over 18 years old, single pregnancy and ASA physical condition 1–219 who accepted to participate in the study were included. Patients with congenital and clinical fetal abnormalities – including nonreassuring fetal status or episodes of resolved fetal bradycardia, patients with pregnancy-associated hypertensive disorders, diabetes mellitus, gestational diabetes, and patients with known allergy to any of the vasopressors, were excluded.
The patients were included upon understanding, accepting and signing of the informed consent. Once the patient was accepted, a head nurse from each department who was not a member of the research group and was not in charge of the patient's care, consecutively opened an opaque envelope from the study group and prepared the corresponding mixture. The medications were prepared in 10ml of 0.9% saline solution in identical syringes; there were no differential characteristics to indicate the medication used. The syringe labeled with each patient's random number was then delivered to the anesthetist in charge of the procedure and the anesthetist was not aware of the medication prepared.
The anesthetic technique was identical for every patient. Spinal anesthesia was administered in the sitting position with a pencil tip needle. A 7.5mg hyperbaric bupivacaine solution was used, in addition to fentanyl 25μg plus morphine 100μg, with the aim of blocking up to T4 level. Simultaneously with the drug, a co-load of 500ml of 0.9% saline solution was administered. After the spinal anesthesia was administered, the patients were placed in supine position with the uterus deviated to the left and a wedge under the right pelvis.
The patients joined the study only after presenting an episode of hypotension following spinal anesthesia, identified by serial non-invasive blood pressure measurements using an automatic blood pressure device, at one-minute intervals for the first 10min. The patients who did not become hypotensive at the end of the 10min were excluded.
Hypotension was defined as a systolic blood pressure (SBP) below 100mmHg and/or a mean blood pressure (MBP) of less than 60mmHg. When hypotension developed, 2ml boluses of vasopressor were administered every minute, corresponding to 2mg of etilefrine or 50μg of phenylephrine, until the SBP and/or MBP values were above the limits established to join the trial.
In the case of non-responders that required over 10ml of the vasopressor's solution, the patient was unblinded so that the anesthesiologist could continue managing the patient appropriately to avoid unfavorable maternal or fetal consequences.
At the time of birth, a 15cm double clamped cord segment was obtained before the baby's first breath. Before the next 60min an arterial blood sample was taken and processed to measure the pH value, using an i-STAT® (The i-STAT® System- Point of Care Testing) blood gas analyzer supplied by Arrow Laboratories.
The anesthetist entered the data into a form designed by the research team. The initial protocol was registered with the Latin American Trial Registry “Latin American Ongoing Clinical Trials Register – LATINREC-” COL101. The study was presented as an abstract at the annual meeting of the “Society for Obstetric Anesthesia and Perinatology (SOAP)” in 2012. Abstract Number: TW-2.
Statistical analysisAs primary outcome we assessed the average pH difference of the fetuses’ umbilical vein. A total of 98 patients were programmed for each group's sample in order to identify a 0.03 difference in pH among the groups, with a 0.05 standard deviation considering an alpha error of 0.05, a beta error of 0.2 and a 10% loss in each group. These numbers were based on a previous study by Cooper et al., which found a 2.08% incidence of fetal acidosis with phenylephrine.12
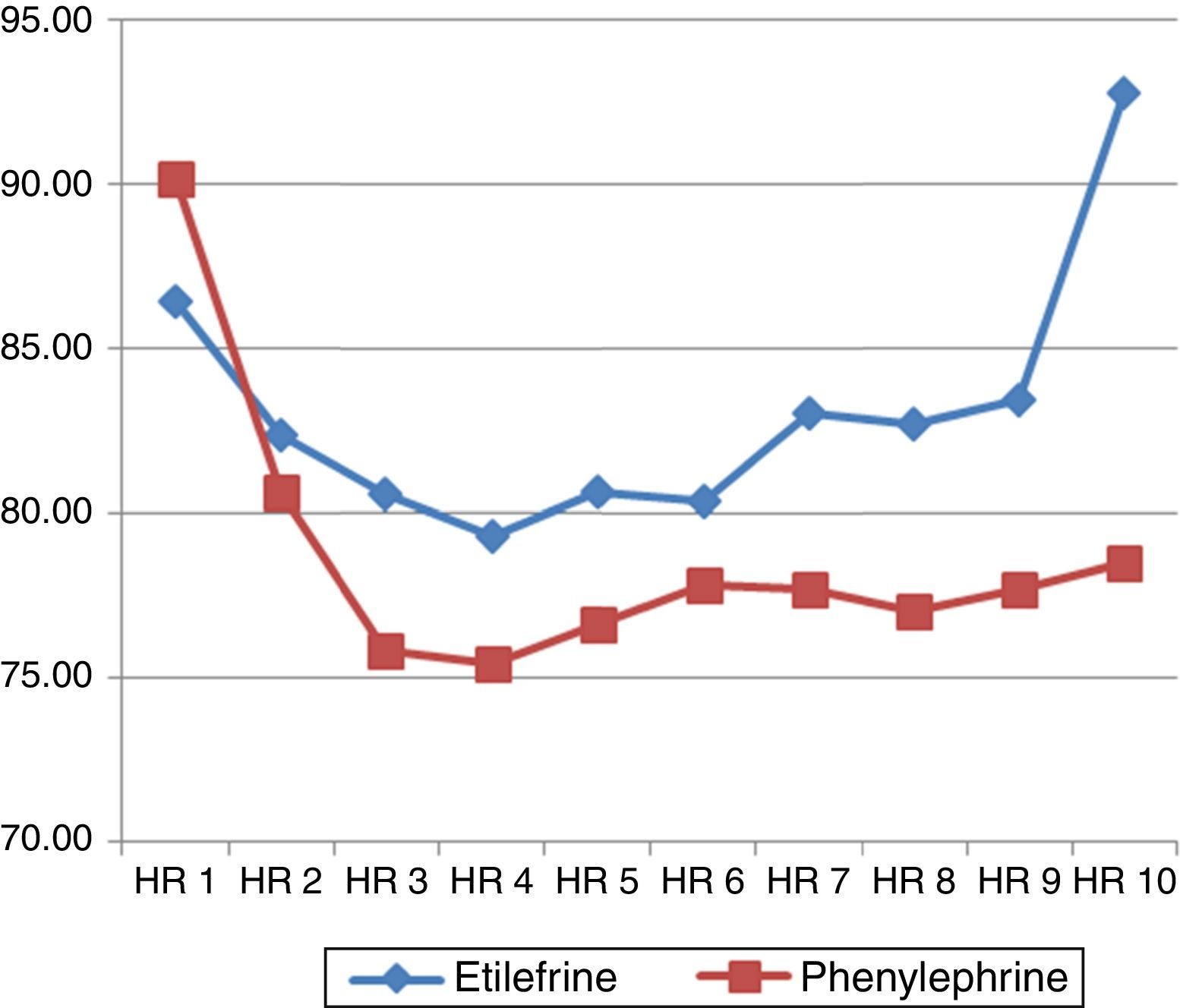
The secondary outcomes for the newborn babies were: fetal acidosis defined as an umbilical vein pH<7.20; low Apgar score at minutes 1 and 5, established at <7; need to intubate and NICU admission. The secondary outcomes for mothers were: time of hypotension, total dose of vasopressor, need to use atropine, total intravenous fluids volume during the intraoperative period, and the incidence of nausea and vomiting. Additionally, a graphical representation and a comparison of the systolic blood pressure, the mean blood pressure, and the heart rate during the first 10min in both groups were developed.
The quantitative variables were evaluated with the Kolmogorov–Smirnov test to verify the hypothesis of normality. The continuous variables with normal distribution are presented as means±standard deviation and the non-normal distribution variables as medians with interquartile range. The categorical variables are presented as percentages. The comparison of the continuous variables between both study groups was made with the student-t test when the distribution was normal, and with Mann–Whitney's when the distribution was not normal. The comparison of the categorical variables was done using chi square, or Fisher's exact test when required based on the magnitude of the result. The intention-to-treat analysis was performed. A two-tailed P value of less than 0.05 was considered statistically significant. The statistical analysis was done under the SPSS software, version 15.0.
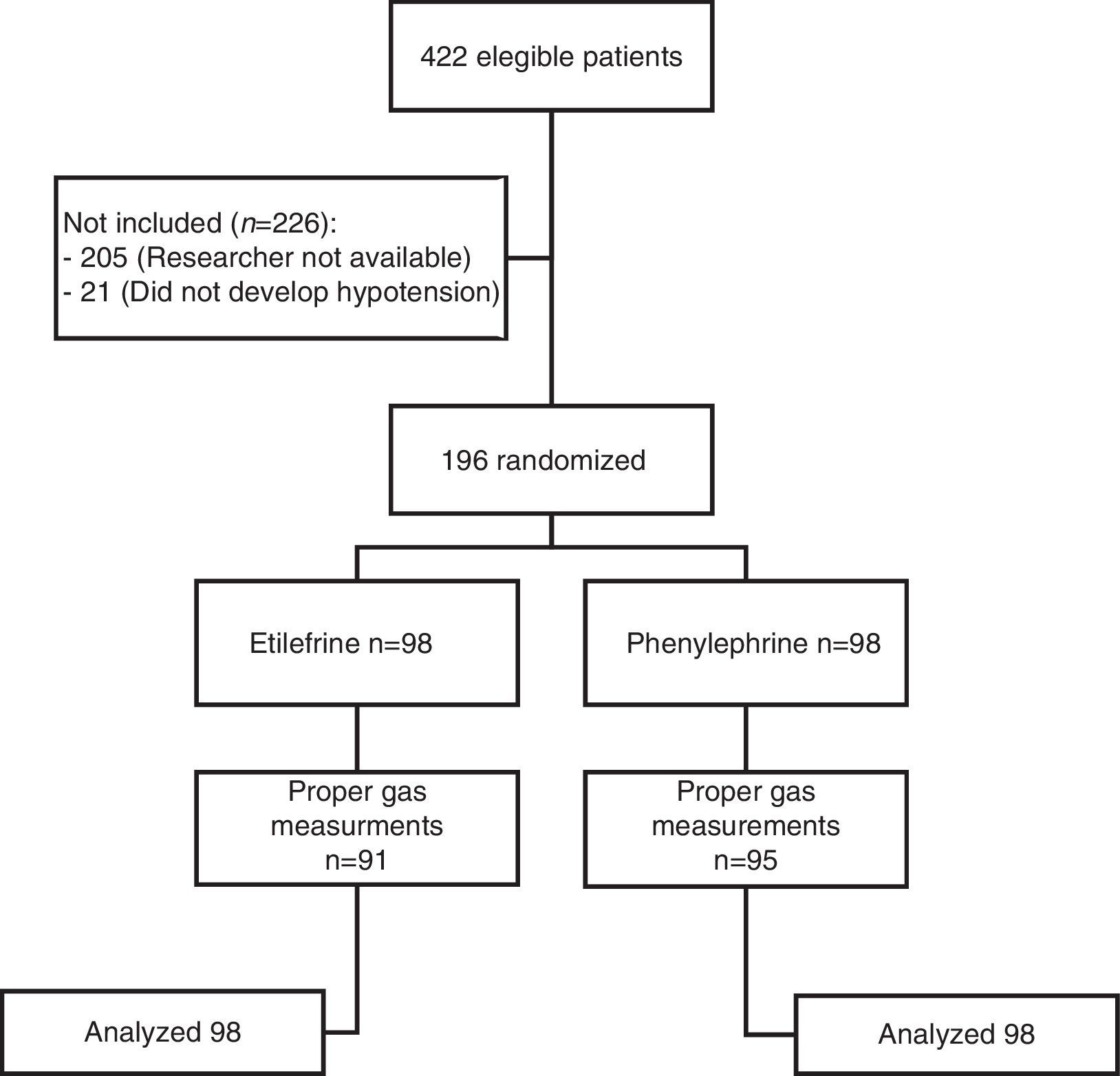
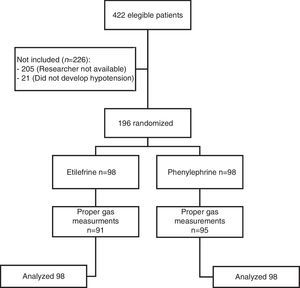
ResultsA total of 422 patients were evaluated to participate in the trial from July 2009 through November 2010. 196 were included, of which 98 received etilefrine and 98 received phenylephrine. Fig. 1 illustrates the flow of participants.
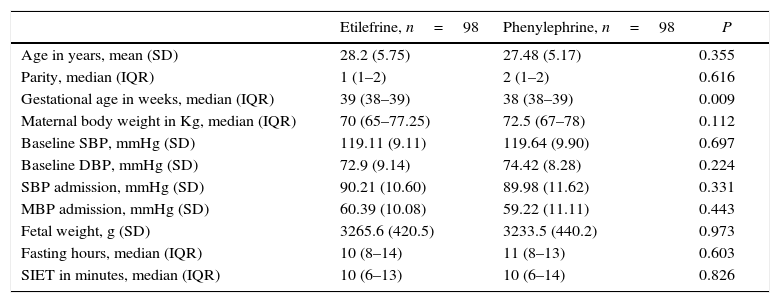
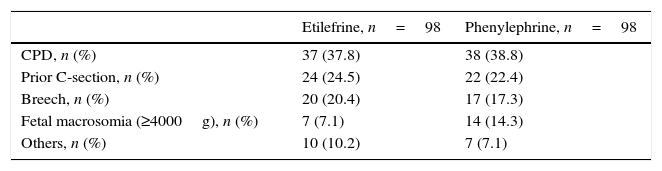
Although there was a statistically significant difference in the gestational age between the two study groups, such difference was not clinically relevant. All other baseline characteristics were similar (Table 1). The most frequent indication for cesarean section was cephalopelvic disproportion, which occurred in 37.8 and 38.8% of the patients in each group (Table 2).
Baseline characteristics of the study population.
| Etilefrine, n=98 | Phenylephrine, n=98 | P | |
|---|---|---|---|
| Age in years, mean (SD) | 28.2 (5.75) | 27.48 (5.17) | 0.355 |
| Parity, median (IQR) | 1 (1–2) | 2 (1–2) | 0.616 |
| Gestational age in weeks, median (IQR) | 39 (38–39) | 38 (38–39) | 0.009 |
| Maternal body weight in Kg, median (IQR) | 70 (65–77.25) | 72.5 (67–78) | 0.112 |
| Baseline SBP, mmHg (SD) | 119.11 (9.11) | 119.64 (9.90) | 0.697 |
| Baseline DBP, mmHg (SD) | 72.9 (9.14) | 74.42 (8.28) | 0.224 |
| SBP admission, mmHg (SD) | 90.21 (10.60) | 89.98 (11.62) | 0.331 |
| MBP admission, mmHg (SD) | 60.39 (10.08) | 59.22 (11.11) | 0.443 |
| Fetal weight, g (SD) | 3265.6 (420.5) | 3233.5 (440.2) | 0.973 |
| Fasting hours, median (IQR) | 10 (8–14) | 11 (8–13) | 0.603 |
| SIET in minutes, median (IQR) | 10 (6–13) | 10 (6–14) | 0.826 |
IQR, interquartile range; SD, standard deviation; kg, kilograms; SBP, systolic blood pressure; mmHg, millimeters of Mercury; DBP, diastolic blood pressure; MAP, mean arterial pressure; g, grams; SIET, skin incision extraction time.
Indications for cesarean delivery in the study population.
| Etilefrine, n=98 | Phenylephrine, n=98 | |
|---|---|---|
| CPD, n (%) | 37 (37.8) | 38 (38.8) |
| Prior C-section, n (%) | 24 (24.5) | 22 (22.4) |
| Breech, n (%) | 20 (20.4) | 17 (17.3) |
| Fetal macrosomia (≥4000g), n (%) | 7 (7.1) | 14 (14.3) |
| Others, n (%) | 10 (10.2) | 7 (7.1) |
CPD, cephalo-pelvic disproportion; g, grams; Others, non-favorable cervix (4); human immunodeficiency virus (1); congenital hip dislocation (1); hip dysplasia (1); giant myomas (2); hip fracture (2); thin uterine segment (1).
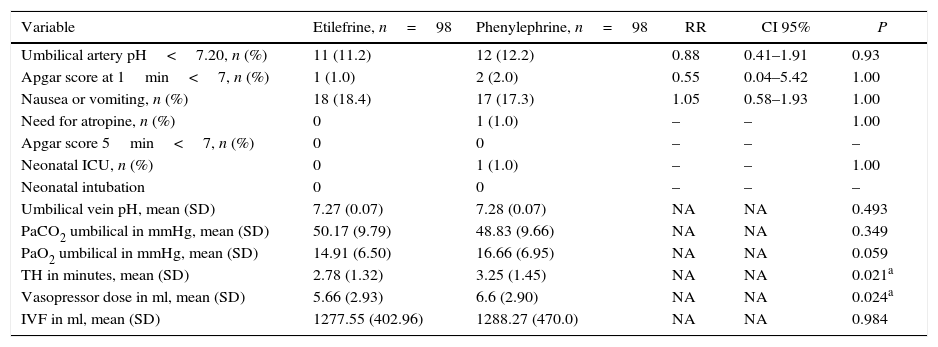
No statistically significant difference was found in the primary outcome – umbilical arterial pH – between the etilefrine and phenylephrine groups (7.27±0.07 vs. 7.28±0.07 respectively. P=0.493). Neither were there any differences in most of the secondary outcomes. There were no statistically significant differences in the PaCO2 and PaO2 values of the umbilical cord samples (Table 3). The incidence of fetal acidosis in the etilefrine group was 11.2% vs. 12.2% in the phenylephrine group, with no evidence of statistical significance (RR: 0.88; CI 95%: 0.41–1.91; P=0.93). Only one of all the neonates examined had to be admitted to the NICU and he was from the phenylephrine group (Table 3).
Final results.
| Variable | Etilefrine, n=98 | Phenylephrine, n=98 | RR | CI 95% | P |
|---|---|---|---|---|---|
| Umbilical artery pH<7.20, n (%) | 11 (11.2) | 12 (12.2) | 0.88 | 0.41–1.91 | 0.93 |
| Apgar score at 1min<7, n (%) | 1 (1.0) | 2 (2.0) | 0.55 | 0.04–5.42 | 1.00 |
| Nausea or vomiting, n (%) | 18 (18.4) | 17 (17.3) | 1.05 | 0.58–1.93 | 1.00 |
| Need for atropine, n (%) | 0 | 1 (1.0) | – | – | 1.00 |
| Apgar score 5min<7, n (%) | 0 | 0 | – | – | – |
| Neonatal ICU, n (%) | 0 | 1 (1.0) | – | – | 1.00 |
| Neonatal intubation | 0 | 0 | – | – | – |
| Umbilical vein pH, mean (SD) | 7.27 (0.07) | 7.28 (0.07) | NA | NA | 0.493 |
| PaCO2 umbilical in mmHg, mean (SD) | 50.17 (9.79) | 48.83 (9.66) | NA | NA | 0.349 |
| PaO2 umbilical in mmHg, mean (SD) | 14.91 (6.50) | 16.66 (6.95) | NA | NA | 0.059 |
| TH in minutes, mean (SD) | 2.78 (1.32) | 3.25 (1.45) | NA | NA | 0.021a |
| Vasopressor dose in ml, mean (SD) | 5.66 (2.93) | 6.6 (2.90) | NA | NA | 0.024a |
| IVF in ml, mean (SD) | 1277.55 (402.96) | 1288.27 (470.0) | NA | NA | 0.984 |
SD, standard deviation; mmHg, millimeters of mercury; TH, total hypotension time; ml, milliliters; IVF, total intravenous fluids in the intraoperative period; RR, relative risk; CI, confidence interval; min, minute; ICU, intensive care unit; (–), O value variables where RR, CI or P cannot be calculated. NA, not applicable.
There were significant differences in terms of time of hypotension between the two groups, with shorter times in the etilefrine group (2.78±1.32min vs. 3.25±1.45min, P=0.021) and in the total dose of vasopressor which was also lower in the etilefrine group (5.66±2.93ml vs. 6.6±2.90ml; P=0.024) (Table 3). Two patients from the phenylephrine group and one from the etilefrine group had to be unblinded, but treatment remained unchanged in all three; just additional doses were given and none developed fetal acidosis.
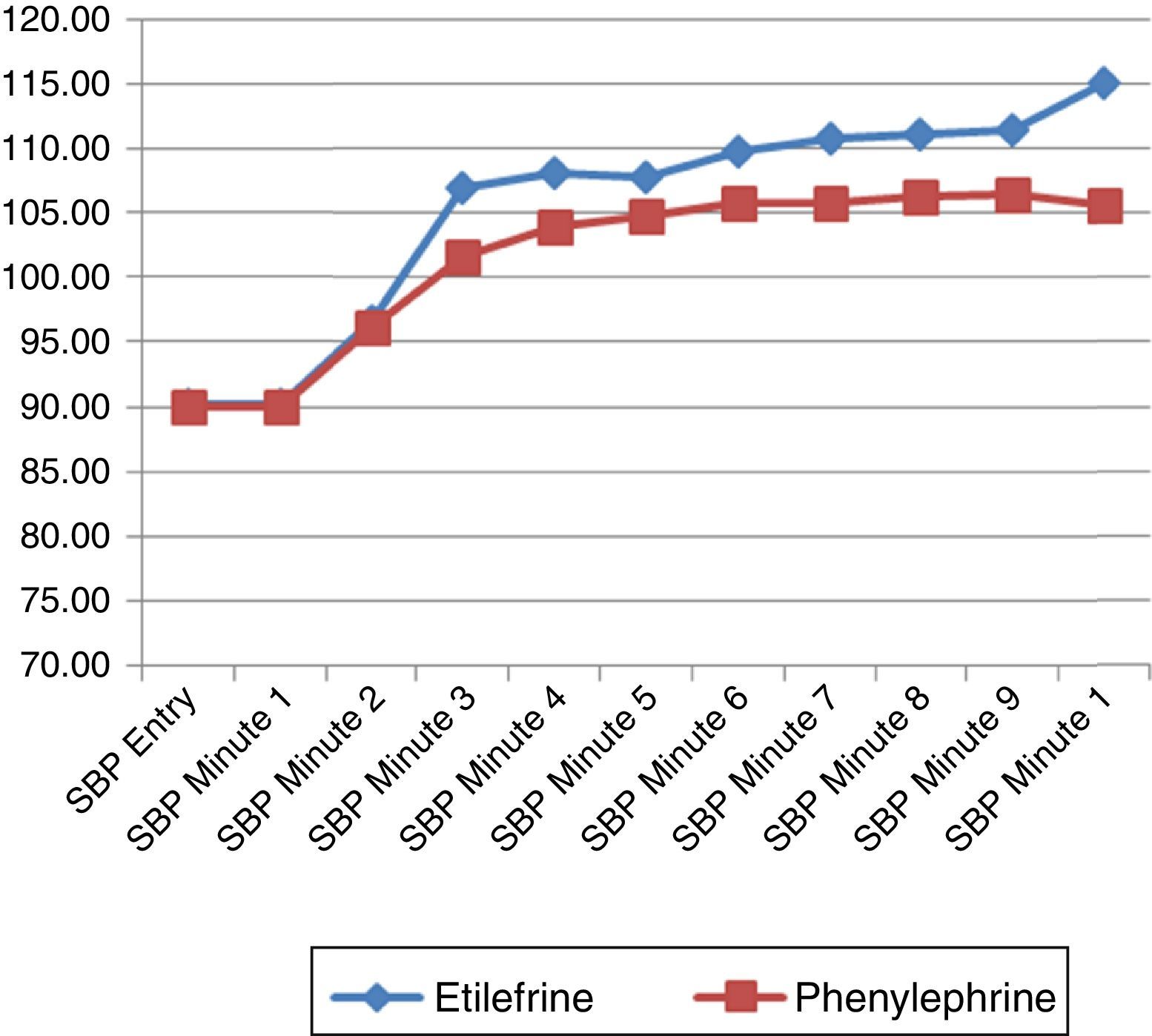
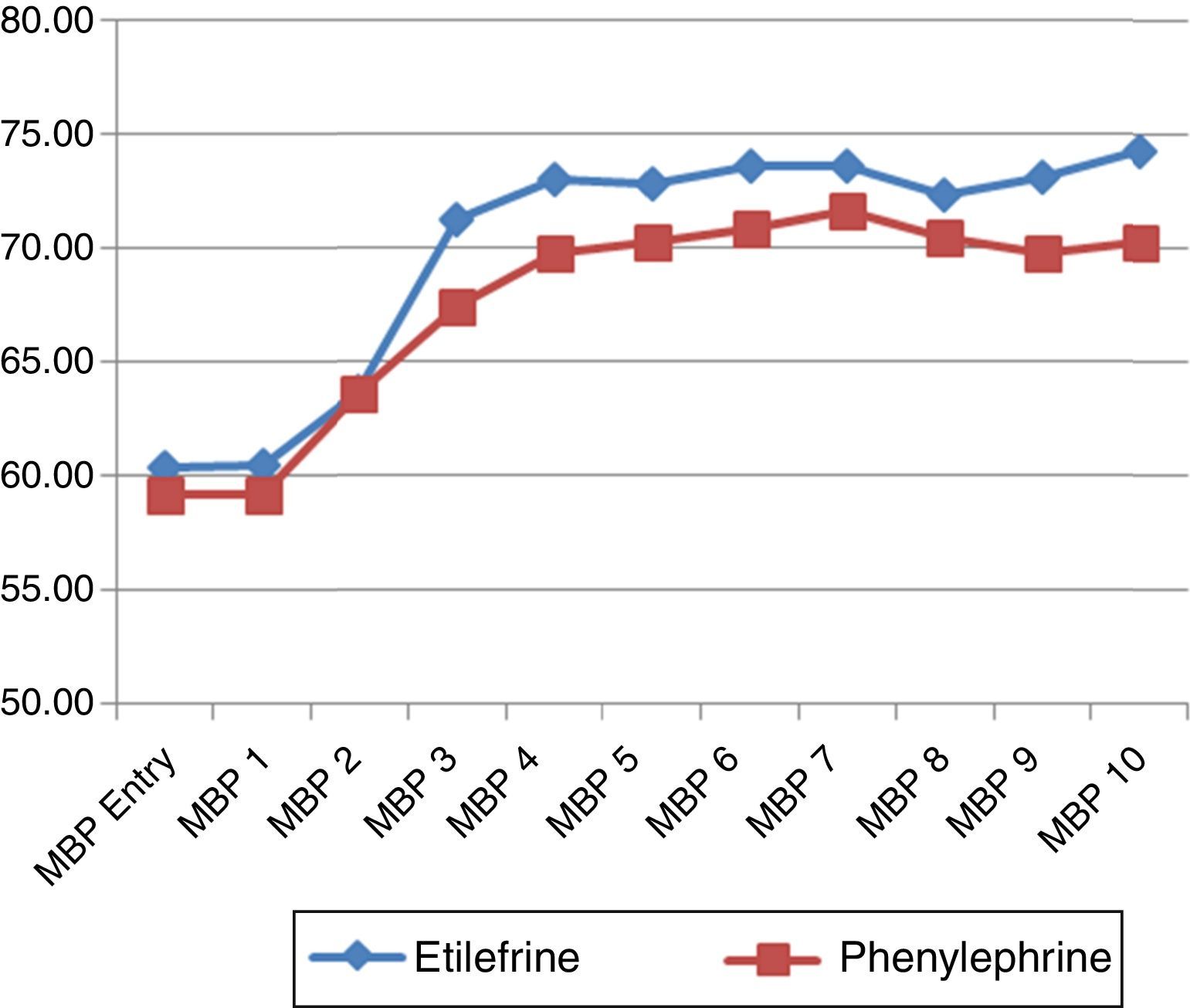
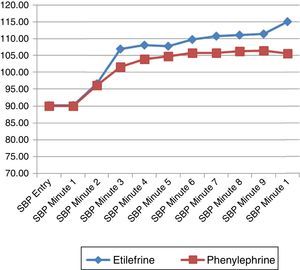
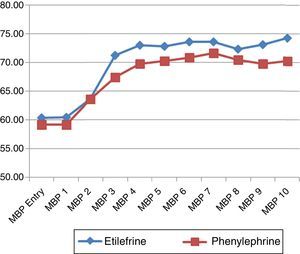
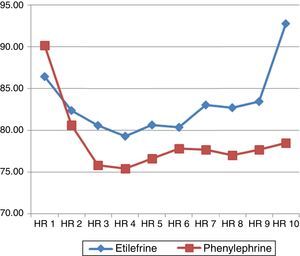
The hemodynamic behavior of both SBP and MBP in both groups during the first 10min following the first episode of hypotension showed no significant differences, though the heart rate was higher in the patients receiving etilefrine (Figs. 2–4).
The remaining secondary outcomes including Apgar score, incidence of nausea and vomiting, atropine requirement and need for neonatal intubation were low and there were no differences between the two groups (Table 3).
DiscussionThis study shows that etilefrine and phenylephrine are equally effective and safe vasopressors for the treatment of spinal anesthesia hypotension during cesarean delivery. There were no differences in the fetal or maternal outcomes in pregnancies between 36 and 42 weeks, in women undergoing elective or programmed cesarean section.
The recommendation to achieve adequate anesthesia for cesarean section is to deliver a sensory block up to dermatome T5 as a minimum. This trial used an anesthetic technique with hyperbaric bupivacaine, fentanyl, and morphine aimed at obtaining a block up to level T4. This results in a sympathetic block that leads to a reduction in the systemic vascular resistance causing hypotension in 55–90% of the patients.5,6
Toward the end of pregnancy, the uterine-placental vessels exhibit maximum dilatation and low resistance, resulting in loss of self-regulation. Maternal hypotension may lead to utero-placental hypoperfusion and fetal distress.4 Different approaches have been considered to prevent and to quickly treat such event.5,20,21 Our group used the concomitant administration of a co-load of 500ml of 0.9% saline solution following spinal anesthesia, since it has been established that the result is the same using crystalloids or colloids and that it is better to do co-load rather than pre-load.4,21,22 We also did left uterine displacement placing a wedge below the pelvis, although this approach has not been useful23,24 but is part of the routine management in our setting.
To offset the drop in vascular resistance caused by spinal anesthesia, which leads to hypotension, the use of vasopressors to preserve the α and β adrenergic activity is a logical approach. The two vasopressors most commonly used and researched are ephedrine (an α and β adrenergic agonist) and phenylephrine, a α-1 receptor agonist and with β-agonist action only at high doses.6 Phenylephrine is currently considered the vasopressor of choice since it has been associated with improved fetal acid-base status as compared to ephedrine.15 Notwithstanding the fact that a meta-analysis including 142 trials failed to acknowledge the superiority of phenylephrine over ephedrine when comparing the results of the Apgar scores in neonates,25 it has been proven that ephedrine can cross the placenta and result in lower fetal pH due to its metabolic effects from stimulation of the fetal β-adrenergic receptors14 and that fetal acidosis – defined as a pH in the umbilical cord artery <7.20 – is associated with a two and four-fold increase in morbidity and mortality, respectively.26
The most popular vasopressor in Colombia is etilefrine, which being an isomer of phenylephrine could have similar effects. We failed to find a trial comparing the maternal-fetal effects of these two vasopressors and only found two trials comparing etilefrine with efedrine. Belzarena reported that there were no differences in the Apgar scores of newborn babies when comparing these two drugs for the treatment of hypotension.27 Räsänen et al., showed that etilefrine caused no detectable changes in the fetal hemodynamic behavior or myocardial function, whilst ephedrine reduced the renal and cerebral artery flow rate and led to increased ventricular contractility and a reduction in the left ventricular dimension at the end of diastole.17
Although Valli et al. reported an increase in the uterine vascular resistance during the prophylactic administration of etilefrine infusion to maintain the blood pressure in patients under spinal anesthesia, no adverse medical neonatal effects were shown as measured by the Apgar score and the acid-base status of the arterial and venous blood of the umbilical cord.28 No significant difference was found in this study between the arterial pH values of the umbilical cord among the neonates born from mothers receiving etilefrine or phenylephrine; hence, as shown in previous studies, the medications did not cross the placenta. Neither did this study find statistically significant differences between the two groups in terms of the incidence of fetal acidosis; however, the incidence in the phenylephrine group was higher than the incidence reported in the literature.12,29,30 This could be explained by the use of a smaller volume of intravenous fluids as compared to other studies, or because our group included a neonate with non-previously diagnosed heart disease and another one with macrosomia (4110g). The latter neonate experienced a difficult and prolonged uterine extraction (>3min).
As far as the neonatal results are concerned, Casey et al.,31 claim that the Apgar score is a better predictor of the neonatal outcome than the umbilical cord pH measurement. There were no statistically significant differences in the Apgar score measurement between the two groups in this trial. There were only three neonates with a score <7 at the first minute, two from the phenylephrine group and one from the etilefrine group. The Apgar score at 5min of every neonate in both groups was >7, none of them required tracheal intubation and only one from the phenylephrine group – the same baby who developed acidosis – had to be admitted to the NICU and was diagnosed with congenital heart disease that was not identified in the prenatal period.
The intravenous dose of phenylephrine has an immediate onset of action and lasts for 5–10min. There is yet no agreement among the anesthetists about the proper administration regime for phenylephrine31; our group used bolus doses because although some advocate infusion, it has been proven that the total bolus dose used is less, pressure control is adequate, simpler and no pumps or perfusion syringes are needed.32,33
Several studies suggest that the effective dose of phenylephrine is 122–147μg34,35; however, a 50μg dose (2ml) was chosen because the 40μg and 100μg bolus doses continue to be the usual practice36 and because we use the same dose used in other studies.32–39
The 2mg dose (2ml) of etilefrine was chosen on the basis of the management protocols used by the local work groups and by the Belzarena trial27 since no bioequivalence data comparing both drugs are available.
LaPorta and Thomas documented that the development of bradycardia was more likely in women receiving phenylephrine than in those treated with ephedrine. This is a side effect and a reflex mechanism due to increased vascular resistance without stimulation of the β receptors.38,40 The etilefrine group in this trial developed a higher heart rate, though not clinically significant, probably because of a stronger stimulating effect over the β-adrenergic receptors.
Although there was a statistically significant difference in the time of hypotension, such difference was not clinically relevant. There was also a difference in the dose of vasopressor used measured in ml, but we cannot make any claims about an actual difference since the bioequivalence of these drugs is yet unknown.
Spinal anesthesia induces nausea and vomiting through various mechanisms such as intestinal ischemia, cerebral ischemia or via a reflex mechanism in response to a decreased venous return.9 There were no differences in the incidence of vomiting between both groups in this trial and it was similar to other reports.27,38
This trial has several limitations: 205 patients that could have been included in the randomization were not evaluated because the researchers were not available at the institutions throughout the trial and, although there could have been an equitable distribution based on the methodological design, the impact on the results cannot be ruled out, and neither is it possible to indicate how that effect would have been since their behavior is unpredictable. Furthermore, the trial was carried out at only two institutions and the results cannot be generalized to other institutions using different doses, different block levels, pre-loads or other co-load volumes. Etilefrine is a very popular vasopressor in our country, but this is not necessarily the case in other regions.
There are still many unanswered questions about vasopressors used for the management of hypotension in patients undergoing cesarean section that require further research. Currently there is no clear evidence about the adequate vasopressor for non-elective surgery; the bioequivalent doses of phenylephrine and etilefrine are unknown; and the specific vasopressor that can be safely used in mothers of nonreassuring fetal status babies has not been identified.
The conclusion from the results of this trial are that etilefrine may be safely used in mothers during the 36 and 42 weeks of pregnancy undergoing elective or programmed cesarean section, following the protocol recommendations, and develop spinal anesthesia-associated hypotension.
FundingCentro de Investigación para el Desarrollo y la Innovación – CIDI de la Universidad Pontificia Bolivariana. Número de radicado: 379A-12/08-40.
Conflicts of interestNone of the authors has conflicts of interest.
The authors express their gratitude to:
- •
Clínica Universitaria Bolivariana.
- •
Clínica del Prado.
- •
Anesthesiologists and obstetricians that collaborated with the collection of information.
- •
All students graduate in anesthesiology who collaborated with the collection of information.
- •
Diarlen Milene Arboleda B. from Laboratorios Arrow Medical de Occidente S.A.
- •
Hernando Muñoz P. from Laboratorios SUN Pharmaceutical Service.
Please cite this article as: Bolaños-Arboleda D, Fonseca-Ruiz NJ, Socha-García NI, García-Peñuela E, Monsalve-Mejía G. Etilefrina vs. fenilefrina en hipotensión por anestesia espinal para cesárea: ensayo clínico multicéntrico, controlado, aleatorizado y doble ciego. Rev Colomb Anestesiol. 2016;44:89–96.