This article by Gillies et al.1 attempts to find a response to the use of hydroxyethyl starch (HES) 6% in surgical patients and its possible connection with an increase in mortality at 30 days (primary outcome), the appearance of acute kidney injury (AKI) and the need for dialytic support. These aspects are associated with patients in intensive care, especially with sepsis. To do this, they designed a systematic review of literature since 1946, choosing those essays that compared HES 6% with other resuscitation solutions. Finally, the selected 19 randomized clinical studies, obtaining 1567 patients with whom they carried out a meta-analysis.
No differences were found in surgical patients or in mortality between the use of HES 6% and of other types of fluids in terms of mortality — RD 0.00, CI 95%: 0.02–0.02 — or the need for dialysis support — RD 0.01, CI 95%: 0.04–0.02 — or AKI — RD 0.02, CI 95%: 0.02–0.06.
Finding no additional benefit from the use of HES 6% and taking into account the cost, they do not recommend its use in surgical patients.
Critical assessmentMethodologyAfter applying the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)2 criteria, we observed that the authors do not describe the risk of bias of the individual studies. They do not present additional analysis of the results but conclude that 6% HES showed no additional benefit even though this was not the objective of the study. They did not report means of financing, however, in the conflict of interest declaration, they stated that the authors have received honorariums or donations from enterprises related to the debate on the use of HES. The funnel plot for the main outcome shows a low probability of publication bias. The method is clear and is limited to HES at 6% (molar substitution ratio 0.4 and 0.42) and the adult population, thereby eliminating the possibility of other kinds of HES. The definition of Acute Renal Injury was defined differently in the selected studies. Some did not describe any criteria, others used the AKIN (Acute Kidney Injury Network) criteria and others still used the RIFLE (Risk, Injury, Failure, Loss y End Stage Kidney Disease) criteria. In the online version of the article, the clarify that a clinical trial with approximately 19,000 patients would be required to demonstrate any benefits of HES.3 This study excluded neurosurgical, burn, transplant, and obstetric patients.
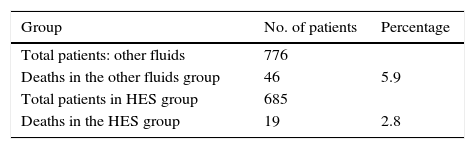
ResultsMortalityThe authors describe more than double mortality when HES is not used (5.9% vs. 2.8%) and it is not easy to understand why there is no statistical difference between the two. Applying a contingency table, as if all of the individuals were from the same study (there is minimal heterogeneity according to their descriptions based on the I2 test), the absolute reduction of risk shows a significant decrease in mortality in the HES group: RD: 0.031, RR 0.47; CI 95%: 0.27–0.79, with a reduction of 53% when HES 6% is used. Thus, there is some doubt about the true value of the results for mortality (Table 1).
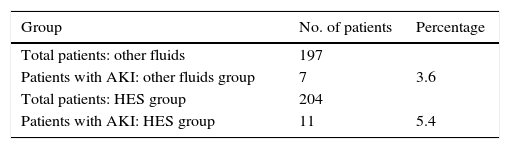
Acute Kidney Injury (AKI)In the HES group, the incidence of kidney damage was 5.4% versus 3.6% in the group of other fluids. In the HES group, the incidence of kidney damage was 5.4% versus 3.6% in the group of other liquids. When we apply these results in a contingency table, we find that there is a greater incidence of acute kidney injury in the HES group, without statistically significant differences: RD −0.018, RR: 1.51, CI 95%: 0.6–3.8 (Table 2).
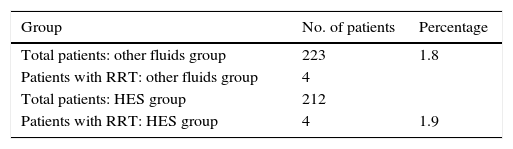
Requirement of Renal Replacement Therapy (RRT)The results between the groups are almost equal and evidently there are no clinical or statistical differences: RD −0.0009, RR 1.0, CI 95%: 0.2–4.15 (Table 3).
DiscussionThe debate on the use of colloids vs. crystalloids is not over.4 Studies that defended starches, presented by Boldt et al.,5 ended with methodological errors, duplicity, and fraud. Later, multi-center studies (clinical study 6S – The Crystalloid versus Hydroxyethyl Starch Trial (CHEST)) reignited the debate and lead to the November 2013 alert from the European Medicines Agency (PRAC) recommending the suspension of HES.6
The study 6S7 (Scandinavian Starch in Severe Sepsis and Septic Shock) compared HES 130/0.42 at 6% to Ringer's acetate, reporting greater mortality after 90 days in patients to whom HES was administered along with greater use of RRT, blood transfusions, and greater incidence of severe bleeding. The CHEST8 study, in critical patients, compared HES 130/0.4 to saline solution at 0.9% and found no differences in mortality at 90 days. However, it did find greater frequency of RRT in the HES group. The studies above present methodological questioning: they included patients after the initial phase of resuscitation and did not include protocols and objectives related to the administration of fluids or a unification of the procedures for initiating RRT.
The CRISTAL (The Colloids Versus Crystalloids for the Resuscitation of the Critically Ill)9 study, compared colloids (gelatins, dextrans, HES, and albumin) to crystalloids (isotonic or hypertonic saline solution, Ringer's lactate) in septic patients and patients in hypovolemic shock, showing a mortality at 28 days of 25.4% vs. 27% in favor of colloids with no statistically significant difference. The heterogeneity of the evaluated groups is in question.
In surgical patients, we have the analysis by Van Der Linden et al.10 with 2139 patients comparing HES and other kinds of solutions, finding no adverse effects at the renal level, nor in terms of the transfusion of red blood cells. The heterogeneity of the groups and the variety of HES types is questionable. The meta-analysis of Claude Martin et al.,11 with 17 randomized studies including 1230 patients, found no evidence of renal dysfunction caused by the second generation HES (derived from corn).
The effects of fluid therapy depend on the type of fluid, the quantity administered, and the characteristics of the patients that receive it. Today we know that the basement membrane of the endothelium is covered in proteoglycans and glycoproteins, the so-called “glycocalyx layer”, that act as a second barrier to limit the extravasation of fluids to the interstitial space in addition to filling other roles such as preventing the adhesion of leukocytes and platelets.12 In critical patients this layer tends to be altered by different inflammatory mediators, favoring the extravasation of proteins to the interstitial space. This leads to edema in the tissues and cell aggregation.13 The surgical patient, however, tends to have an initially intact glycocalyx.
The effects of hydroxyethyl starches differ depending on whether they derive from potatoes or corn and on their molecular weight, their molar substitution ratio, and their substitution pattern.14 Thus the results of the studies on “HES” without specifying what kind can not be extrapolated since clearly the worst results come from HES with high molecular weights and molar substitution ratio.
ConclusionThe characteristics of the patient and the type of HES used may be responsible for the controversial results of the studies.
Shortfalls persist in the methodological designs and especially in the lack of a sufficient sample size to demonstrate statistical differences as clinically significant.
Currently there is no consensus regarding the safety of HES use in surgical patients. However, HES use does represent a high monetary cost when compared to crystalloids. Therefore, the use of HES cannot be recommended until further studies explaining these problems appear.
FinancingThe authors did not receive any sponsorship to produce this article.
Conflict of interestsThe authors declare having no conflicts of interest.
Please cite this article as: Rivera-Tocancipá D, Tovar-Cardozo JH, Garcés LD, Salcedo JA, Galeano DA. Comentario sobre “Incidence of Postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta-analysis”. Rev Colomb Anestesiol. 2016;44:78–80.