It has been suggested that there is some benefit when awake thoracic epidural anesthesia (ATEA) is used for minimally invasive thoracoscopic procedures in critical patients. We tested the hypothesis that ATEA is superior to general anesthesia (GA) when comparing early pulmonary complications in this setting.
MethodsA prospective cohort study was carried out over a 36-month period, comparing patients with malignant pleural effusion scheduled for thoracoscopic talc pleurodesis (TTP) using GA vs. ATEA. Postoperative pulmonary, cardiac, and renal complications, death rate, quality of analgesia and events were analyzed. Univariate and bivariate analyses were performed and time survival probability curves were developed in order to find a possible relation between a particular anesthetic technique and postoperative complications. A p value of <0.05 was considered significant.
ResultsForty-seven patients were included in the analysis. Preoperative characteristics were comparable between groups (GA n=24; ATEA n=23). Incidence of pulmonary complications [GA=19 (86%) vs. ATEA=3 (14%). RR 6.0 (95% CI 2.07–17.7); p<0.001] and severity of postoperative pain (VAS>8) at 24h [GA=7 vs. ATEA=1 RR 6.7 (95% CI 1.13–18.2); p=0.023] were significantly higher when patients received GA. Time required to absence of any postoperative ventilatory support and mobilization with no major restrictions [GA=4 (3.5–5) vs. ATEA=3 (2–3.5) days; p=0.029] and global LOS [GA=10 (3.5–29.5) vs. ATEA=4 (3–15.2) days; p=0.003] were significantly reduced in the ATEA group.
ConclusionsOur study suggests that ATEA is not only a safe anesthetic approach for cancer patients undergoing TTP but is also associated with a significant reduction in pulmonary postoperative events, hospital stay, and a better postoperative pain control. Randomized studies are required to corroborate these findings.
Se ha sugerido que puede haber un beneficio al utilizar la anestesia epidural torácica con el paciente despierto (AETD) para procedimientos toracoscópicos de invasión mínima en pacientes críticos. Sometimos a prueba la hipótesis de que la AETD es superior a la anestesia general (AG) al comparar las complicaciones tempranas en este escenario.
MétodosSe realizó un estudio prospectivo de cohorte durante un período de 36 meses para comparar la AG con la AETD en pacientes con derrame pleural maligno programados para pleurodesis con talco por toracoscopia (PTT). Se analizaron las complicaciones pulmonares, cardíacas y renales, la mortalidad, la calidad de la analgesia y los eventos postoperatorios. Se realizaron análisis univariados y bivariados y se desarrollaron curvas de probabilidad de tiempo de sobrevida a fin de determinar una posible relación entre una técnica anestésica particular y las complicaciones postoperatorias. Se consideró significativo un valor de p < 0,05.
ResultadosSe incluyeron 47 pacientes en el análisis. Las características preoperatorias de los grupos fueron comparables (AG, n = 24; AETD, n = 23). La incidencia de complicaciones pulmonares (AG = 19 [86%] vs. AETD = 3 [14%]; RR: 6,0 [95%CI: 2,07-17,7]; p < 0,001) y la severidad del dolor postoperatorio (EVA > 8) a las 24 h (AG = 7 vs. AETD = 1; RR: 6,7 [95%CI: 1,13-18,2]; p = 0,023) fueron significativamente mayores cuando los pacientes recibieron AG. El tiempo necesario para suspender todo tipo de soporte ventilatorio y para la movilización sin mayores restricciones (AG = 4 [3,5-5] vs. AETD = 3 [2-3,5] días; p = 0,029) y el tiempo de permanencia global (GA = 10 [3,5-29,5] vs. ATEA = 4 [3-15,2] días; p = 0,003) fueron significativamente meno-res en el grupo que recibió AETD.
ConclusionesNuestro estudio sugiere que la AETD no solamente es un método anestésico seguro para los pacientes de cáncer sometidos a PTT, sino que también se asocia con una reducción significativa de los eventos pulmonares postoperatorios y de la permanencia hospitalaria, y con un mejor control del dolor postoperatorio. Se requieren estudios aleatorizados para corroborar estos hallazgos.
Advances in lung ventilation and early developments in anesthetic techniques have led us to reach better outcomes in thoracic surgical procedures.1–3 The challenge to obtain temporal lung collapse for thoracic interventions has stimulated the innovation of practical and safe devices for lung isolation. New concepts about physiology of one-lung ventilation resulted in innovations of ventilatory modes and anesthetic approaches to deal with this purpose.2 Currently, general anesthesia (GA) alone or combined with other techniques is considered the best option for the anesthetic management of patients undergoing thoracic procedures.2,3Recent publications have suggested better clinical outcomes in patients who undergo thoracic procedures under awake thoracic epidural anesthesia (ATEA), including less intraoperative bleeding, a better control of postoperative pain, early mobilization and oral intake tolerance. Other authors have suggested that ATEA for thoracoscopic procedures is associated with a reduction of the surgical stress response, incidence of intraoperative cardiac events, improvement of the myocardial flow determinants and left ventricular function, and a reduction of relevant complications including pulmonary, thrombotic and infectious events.4–11
Epidural Anesthesia has been associated with a low incidence of atelectasis and pneumonia in other surgical settings such as CABG, transternal thymectomies and mastectomies when controlling by age and illness severity.5,9,12–14 Only 3 articles have described the use of ATEA for lung and pleural surgery,5,15,16 but no studies have included cancer patients undergoing thoracoscopic procedures.
We decided to conduct a prospective study analyzing different postoperative outcomes in adult patients scheduled for thoracoscopic pleurodesis when these procedures were done under GA vs. ATEA.
MethodsAfter Institutional and IRB approval, a pilot prospective cohort study was designed to include consecutive adult patients with a diagnosis of refractory pleural effusion secondary to cancer who were scheduled for thoracoscopic talc pleurodesis as palliative treatment during a time interval of 36 months (February 2009–December 2011). The presence of coagulopathy, loculated pleural effusion, pleural infection, or a diagnosis different from cancer, were considered exclusion criteria for this registry.
A standardized CRF was designed to collect relevant perioperative variables. A previously trained nurse not involved in the treatment or decisions about the anesthetic or surgical technique, registered relevant pre and intraoperative data. Blinding was assured by occluding the head of the patient, and the ventilator and capnography screens. For all the cases, a team of cardiothoracic anesthesiologists standardized both of the two anesthetic techniques, but the selection was made by the anesthesiologist responsible for the case. None of the anesthesiologists implicated in the intraoperative management were involved in the postoperative follow-up or in pain management. Another previously instructed technician registered the postoperative outcomes in the ICU and in the ward during the first 30 postoperative days until discharge or death.
When ATEA was selected, an IV bolus of Midazolam 0.02mg/kg was administered. The epidural puncture was performed with a 17G Tuohy Needle at the T3–T4 intervertebral space, and an 18G peridural catheter was inserted and tested for adequate position. A bolus of 8ml of 0.5% bupivacaine with epinephrine was then administered, and the patient was positioned in lateral decubitus. Spontaneous ventilation was supported by supplementary oxygen with a 50% Venturi mask throughout the procedure and monitored by a capnography catheter fixed by a tape to one of the nasal orifices. All the patients were monitored non-invasively. Analgesia was evaluated frequently to maintain levels of sensory blockade between T1 and T7, administering additional boluses of 2–4ml if needed. After surgery, all the patients remained with the peridural catheter for 24h, after which it was removed.
When GA was selected, a double-lumen endotraqueal tube was used in all cases. Use of hypnotics and neuromuscular blocking agents was left to the decision of the anesthesiologist in charge. All patients received 2–3mcg/kg of fentanyl and sevoflurane for anesthesia maintenance during the procedure. Dexamethasone 4mg before induction and ondansetron 4mg before emergence from anesthesia were given for nausea and vomiting prophylaxis, and morphine 6–10mg for transitional analgesia.
All surgical procedures were done by the same surgical team who used a standardized single 20–30mm thoracoscopic port placed at the 5th intercostal space with anterior axillary line through which a camera and a suction tube were simultaneously inserted for aspiration of the pleural effusion and subsequent sprinkling of 5g of talc in the pleural space. After the procedure, all patients went to the ICU and the ward, depending on their clinical course, until discharge. A previously instructed nurse, blinded to the intraoperative management, registered relevant outcomes during the postoperative stay on a second CRF designed for this purpose.
Composite complications during the first 30 postoperative days were classified in 4 categories: Respiratory [respiratory dysfunction (unable to maintain a PaO2/FiO2>200), need for tracheostomy or Bi-PAP, re-intubation, pneumonia or prolonged mechanical ventilation]; cardiac (arrhythmias de novo, cardiac failure, myocardial infarction); pain related [persistent thoracic pain measured by analog visual scale (VAS)≥6/10 at first, second and 30 POP days]; and renal/electrolytic [acute renal failure (creatinine≥2mg/dl), worsening of preexisting renal dysfunction, electrolytic disturbances or oliguria (U.O.<0.3ml/kg/d)]. Overall and composite mortality during the first 30 postoperative days were calculated.
Other secondary outcomes included “time to reach good clinical conditions” (TRGC) defined as time required for achieving an adequate ventilatory function (no dependency of any ventilatory support and/or able to maintain a SpO2>90%), and mobilization with no major restrictions, LOS in UCI and hospitalization, duration of ventilatory support and intensity of pain at 24 and 48h (VAS).
StatisticsResults of previous reports do not specify the rate of early postoperative respiratory complications (EPRC) for patients with malignant effusions after thoracoscopic pleurodesis.15 Size calculation for this study was performed based on a pilot registry that showed a rate of 56% for these types of complications. Considering a significance of 5% and a power of 80% to detect a reduction of 50% in the relative risk for EPCR, it was concluded that 25 subjects were required in each group. Univariate and bivariate analyses were performed to determine the possible relationship between a particular anesthetic technique and outcomes. The Pearson Chi-square test was used to analyze categorical variables and the T-test (if normality criteria were fulfilled) or U Mann–Whitney test were used for continuous variables. Overall and group survival time probability curves for postoperative complications were constructed using the limit product (Kaplan–Meier) method. Differences between groups were analyzed using the Long-Rank test method adjusted by multivariate models of proportional risks (Cox).
The STATA 10 (StataCorp 4905 Lakeway Dr. USA) package was used for statistical analysis. Continuous data are expressed as means±SD when normality criteria were considered, and as medians (if requirements were not met) for scattered data, and categorical variables as absolute and relative frequencies.
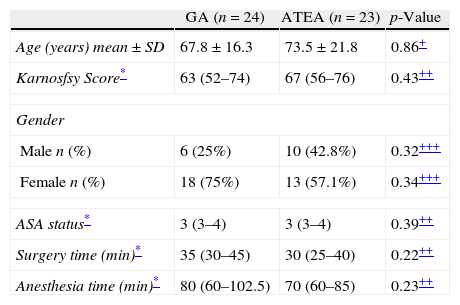
ResultsFifty-one subjects who met the inclusion criteria were invited to participate in this study. Two patients who refused, and 2 with changes in the planned surgical procedure were excluded from the analysis. Ultimately, 47 subjects were included (GA=25 vs. ATEA=22). There were no subjects lost due to follow-up, and there were no omissions of the studied data. Groups were comparable in demographics and perioperative characteristics (Table 1) except for age, which was significantly higher in the ATEA group [55 (34.5–68.5) vs. 70 (57–80) years for GA and ATEA, respectively, p=0.021].
Demographics.
| GA (n=24) | ATEA (n=23) | p-Value | |
| Age (years) mean±SD | 67.8±16.3 | 73.5±21.8 | 0.86+ |
| Karnosfsy Score* | 63 (52–74) | 67 (56–76) | 0.43++ |
| Gender | |||
| Male n (%) | 6 (25%) | 10 (42.8%) | 0.32+++ |
| Female n (%) | 18 (75%) | 13 (57.1%) | 0.34+++ |
| ASA status* | 3 (3–4) | 3 (3–4) | 0.39++ |
| Surgery time (min)* | 35 (30–45) | 30 (25–40) | 0.22++ |
| Anesthesia time (min)* | 80 (60–102.5) | 70 (60–85) | 0.23++ |
GA, general anesthesia; ATEA, awake thoracic epidural anesthesia.
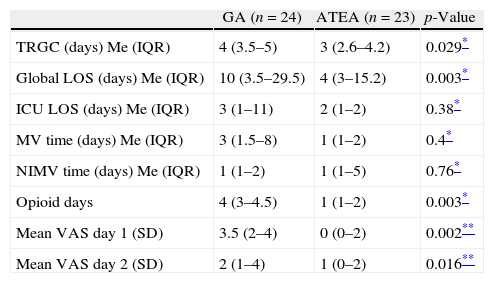
When postoperative outcomes were analyzed, a significant reduction in time to reach good clinical conditions was found in the ATEA group (median 3 (3–4) vs. 4 (3.5–5) days; respectively, p=0.029). Duration of analgesia with opioids as well as pain perception by the patients at 24 and 48 postoperative hours were significantly reduced when ATEA was used [median 4 (3–4.5) vs. 1 (1–5) days; p=0.0185 and VAS 3.5 (2–4) vs. 0 (0–2); p=0.002; respectively]. ICU-LOS and need for mechanical ventilation were similar between groups (Table 2).
Secondary outcomes during the early postoperative period.
| GA (n=24) | ATEA (n=23) | p-Value | |
| TRGC (days) Me (IQR) | 4 (3.5–5) | 3 (2.6–4.2) | 0.029* |
| Global LOS (days) Me (IQR) | 10 (3.5–29.5) | 4 (3–15.2) | 0.003* |
| ICU LOS (days) Me (IQR) | 3 (1–11) | 2 (1–2) | 0.38* |
| MV time (days) Me (IQR) | 3 (1.5–8) | 1 (1–2) | 0.4* |
| NIMV time (days) Me (IQR) | 1 (1–2) | 1 (1–5) | 0.76* |
| Opioid days | 4 (3–4.5) | 1 (1–2) | 0.003* |
| Mean VAS day 1 (SD) | 3.5 (2–4) | 0 (0–2) | 0.002** |
| Mean VAS day 2 (SD) | 2 (1–4) | 1 (0–2) | 0.016** |
Results as medians (Me) for time and means for pain results. IQR, interquartile rank; SD, standard deviation; TRGC, time to reach good clinical conditions; GA, general anesthesia; ATEA, awake thoracic epidural anesthesia; LOS, length of stay; ICU, intensive care unit; MV, mechanical ventilation; NIMV, non-invasive mechanical ventilation; VAS, analog visual scale.
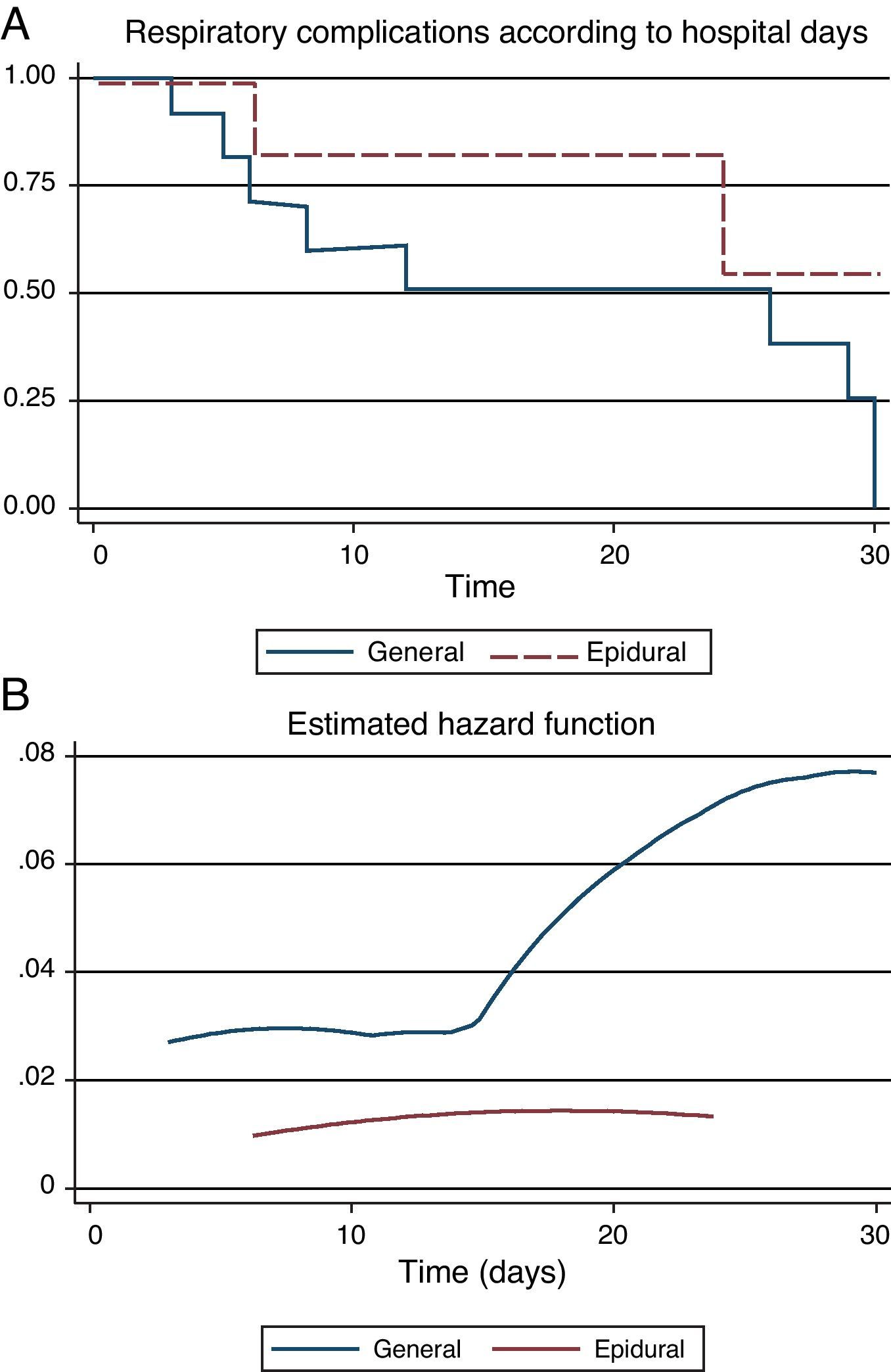
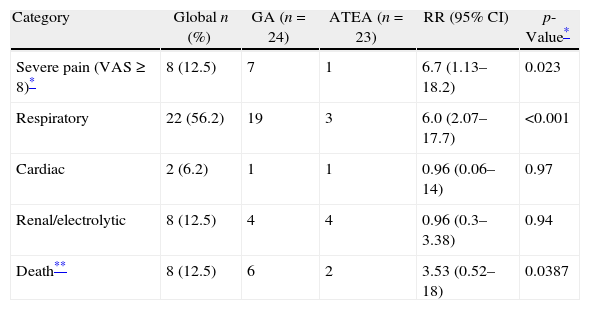
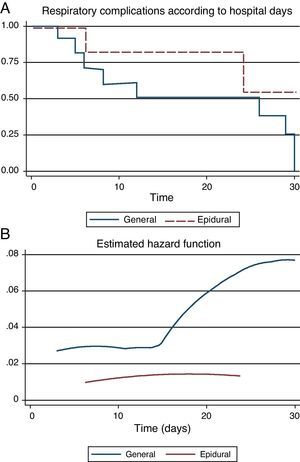
When postoperative morbidity and mortality were reviewed, GA was associated with a significant increase in respiratory complications (re-intubation, pneumonia and need for tracheostomy) during the first 30 postoperative days [GA=23 (79.2%) vs. PA=3 (14.3%); p<0.001]. The incidence of other postoperative complications was not different between groups, although there was a tendency to a high probability of death in the AG group (Table 3). Survival free of respiratory complications (ARDS, reintubation, etc.) was significantly higher in the ATEA group (Log-Rank Test=34.72; p=0.0103), suggesting a high risk of complications in patients under GA, as was confirmed in a hazard analysis [RR=4.37 (95% CI 1.14–16.67); χ2=7.23; p=0.0072] (Fig. 1).
Postoperative complications.
| Category | Global n (%) | GA (n=24) | ATEA (n=23) | RR (95% CI) | p-Value* |
| Severe pain (VAS≥8)* | 8 (12.5) | 7 | 1 | 6.7 (1.13–18.2) | 0.023 |
| Respiratory | 22 (56.2) | 19 | 3 | 6.0 (2.07–17.7) | <0.001 |
| Cardiac | 2 (6.2) | 1 | 1 | 0.96 (0.06–14) | 0.97 |
| Renal/electrolytic | 8 (12.5) | 4 | 4 | 0.96 (0.3–3.38) | 0.94 |
| Death** | 8 (12.5) | 6 | 2 | 3.53 (0.52–18) | 0.0387 |
GA, general anesthesia; ATEA, awake thoracic epidural anesthesia.
At the present time, general anesthesia alone or combined with epidural anesthesia is the preferred technique for patients requiring a thoracic procedure. This work compared two cohorts of patients with pleural effusion due to pleural metastasis from different types of cancers, undergoing thoracoscopic talc pleurodesis under GA or ATEA.
Our findings suggest that ATEA for these procedures in cancer patients is associated with a 25% reduction of the time required to reach an acceptable functional recovery when compared with GA, although we could not prove differences in overall hospital length of stay (LOS). These results may be explained in part because, at our institution, cancer patients have to be under multiple treatments for their condition by the oncology team, thus prolonging their hospitalization. On the other hand, we also found that the VAS scores and opioid consumption were significantly lower in the ATEA group; in the long term, this is related with early initiation of basic activities and a decrease in the incidence of associated complications such as venous/pulmonary embolism, lung atelectasis, pneumonia and urinary tract infections, as mentioned by Waurick and Van Aken.17
Although we did not find differences in ICU LOS, this may be explained by the fact that all patients went to the ICU for monitoring independent of the anesthetic technique used. A significant proportion of ATEA patients were in good clinical condition at the end of the procedure, as indicated by differences in the proportion of patients requiring postoperative mechanical ventilation when compared to the GA group. There is a possibility that ICU LOS in the GA group was longer due to the fact that patients arrived intubated; however, this was precisely what we wanted to avoid by giving them ATEA.
A key observation of our study was the difference in respiratory complications, which were significantly less in the ATEA group. These results are consistent with the results of a previous publication by Tacconi et al.18 who performed unilateral lung volume reduction surgery under ATEA and compared with a control group under GA, showing a low incidence of respiratory complications and a short hospital LOS for the ATEA group. These findings were correlated with a better postoperative pain control and there was no need for mechanical ventilation in many of the cases. Pompeo et al. reported similar findings in procedures for lung wedge resections,19 metastasectomies,20 and spontaneous pneumothorax.21 Patients who were subjected to ATEA had less LOS and reported better satisfaction with the anesthetic technique. Al-Abdullatief et al.15 also found less overall and ICU LOS in 79 patients subjected to ATEA, partly due to the fact that GA was avoided.
Although other authors19–21 have found differences in operatory or anesthesia times, we were not able to prove this. Neither did we find differences in other variables such as duration of mechanical ventilation or severity of postoperative pain, probably due to a multifactorial component or to the small number of patients included. More studies with a large number of patients are required to evaluate special issues like ICU stay, days of mechanical ventilation and other types of surgical procedures.
In conclusion, our work suggests that ATEA for thoracoscopic talc pleurodesis in patients with malignant pleural effusions is a safe anesthetic technique, associated with a reduction in overall hospital LOS, incidence of postoperative respiratory complications and a better control of postoperative pain when compared with GA. Clinical trials with a larger number of patients are required in order to confirm our findings and evaluate other important outcomes.
FundingPersonal funds.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cujiño IF, et al. Anestesia epidural para pleurodesis por toracoscopia: un estudio prospectivo de cohorte. Rev Colomb Anestesiol. 2013;41:10–15.