Parry–Romberg Syndrome is a rare degenerative disease characterized by unilateral atrophy affecting the skin, connective tissue, muscle and bone. The end result is facial asymmetry associated with other skin, dental, visual, cardiovascular, and neurological disorders.
Clinical findings, diagnostic evaluation and interventionsThe case of a patient with Parry–Romberg Syndrome programmed for frontonasal flap remodeling is discussed. The patient's history includes trigeminal neuralgia, epilepsy, and two previous surgical interventions. Uneventful endotracheal intubation with the Glideoscope® video laryngoscope was performed, upon adequate pre-oxygenation followed by anesthetic induction.
ConclusionThe phenotypical characteristics of Parry Romberg Syndrome are severe facial hemiatrophy and craniofacial anomalies that require careful preoperative evaluation and management of a potentially difficult airway. Consequently, the use of video laryngoscopes is a first-line approach. Due to the syndrome's associated disorders, it is essential to maintain hemodynamic stability and prevent any potential seizures.
El Síndrome de Parry-Romberg es una enfermedad degenerativa poco común, caracterizada por una atrofia unilateral que afecta la piel, el tejido conjuntivo, el músculo y el hueso. El resultado final es una asimetría facial cursando con otras alteraciones cutáneas, dentales, oculares, cardiovasculares y neurológicas.
Hallazgos clínicos, evaluación diagnóstica e intervencionesPresentamos un caso de un paciente con Síndrome de Parry-Romberg programado para remodelación de colgajo frontonasal. Entre sus antecedentes destacan neuralgia del trigémino, epilepsia y dos intervenciones quirúrgicas previas. Tras una adecuada preoxigenación y posterior inducción anestésica, se realiza una intubación endotraqueal sin incidencias mediante el videolaringoscopio Glideoscope®.
ConclusiónEl Síndrome de Parry Romberg presenta como características fenotípicas hemiatrofia facial grave y anomalías craneofaciales, que requieren una cuidadosa evaluación preoperatoria y el manejo de una vía aérea potencialmente difícil. Es por esto que los videolaringoscopios resultan una alternativa de primera línea. Debido a sus trastornos asociados, es esencial mantener la estabilidad hemodinámica y la prevención de posibles crisis convulsivas.
Parry–Romberg Syndrome (PRS), also known as progressive facial hemiatrophy, is a rare degenerative condition characterized by unilateral atrophy with variable involvement of the skin, the subcutaneous tissue, the muscles, the connective tissue and the bones.1,2 It may occasionally extend to the neck and even the body as a whole.3 Typically, the onset of the atrophy is during the early decades of life,1,3 with a slow progression until the disease stabilizes.3 The end result is facial asymmetry, which may present associated with other skin, dental, visual, and neurological disorders. It is usually more frequent in females and generally involves the left side of the face.2 The incidence and precise etiology is jet unknown,2,3 although the most recent and reliable theory is a genetic alteration during the embryogenesis of the central nervous system,2 together with cerebral sympathetic nervous system hyperactivity, probably of autoimmune origin.4
The treatment goal in PRS is remodeling of the facial contour, minimizing any atrophy-related complications,2,3 including a difficult airway. We would like to discuss the case of a PRS patient with difficult airway predictors and a successful orotracheal intubation using the Glideoscope® video laryngoscope (VL).
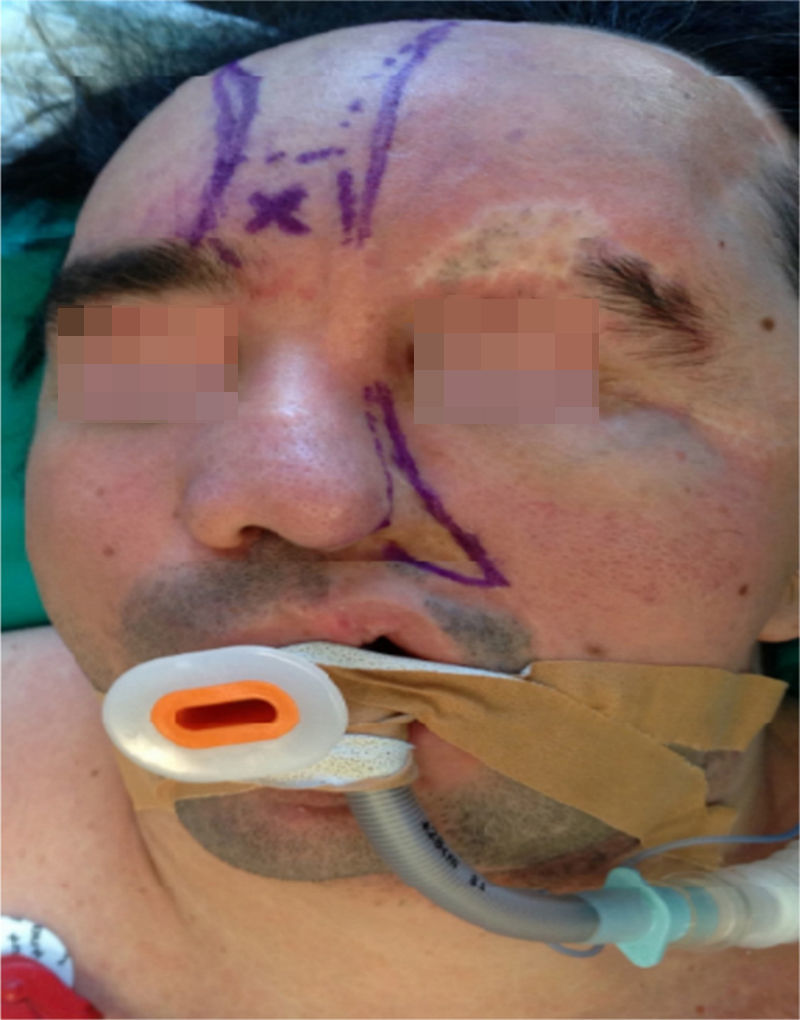
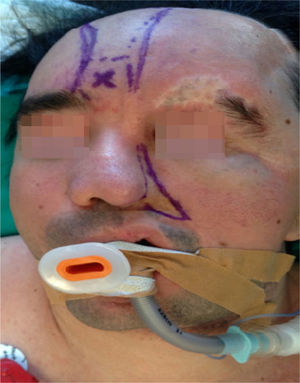
Clinical case33-year old patient diagnosed with PRS since childhood, programmed for surgical remodeling of a frontonasal flap (Fig. 1). The patient's history includes trigeminal neuralgia and epilepsy treated with carbamazepine and valproic acid. No relevant cardiac has been identified and the patient underwent two facial reconstruction procedures ten years ago.
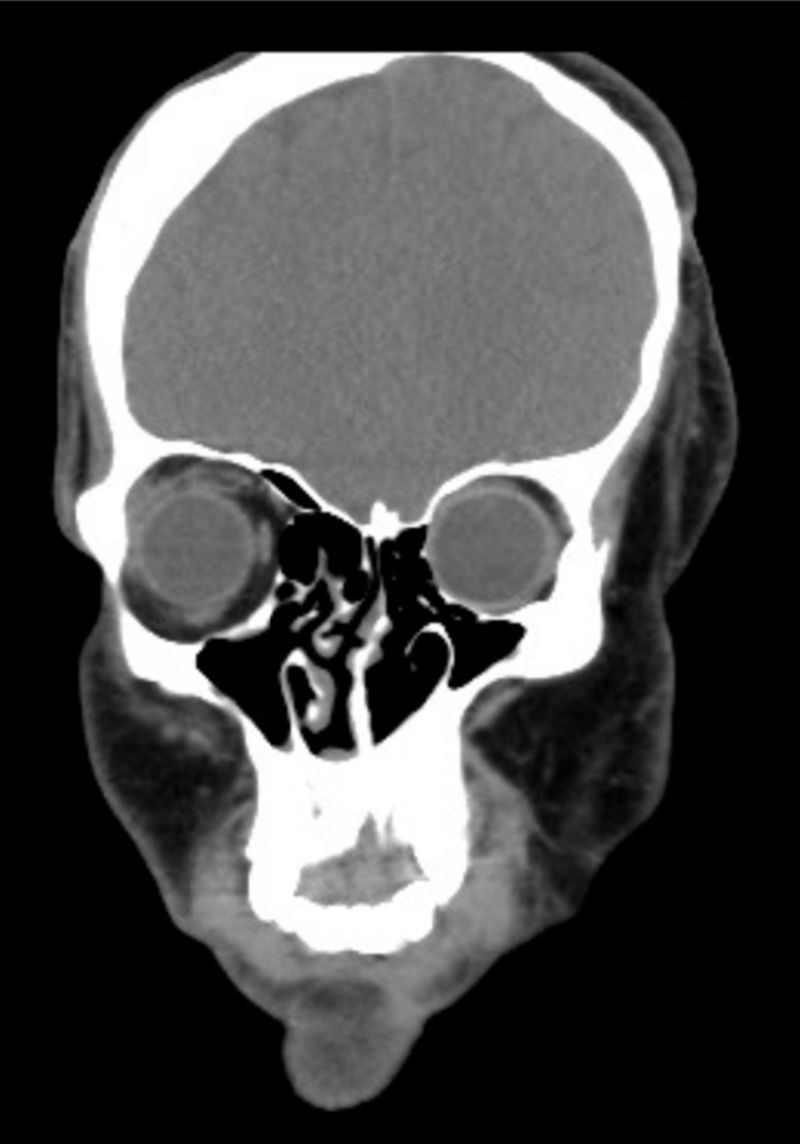
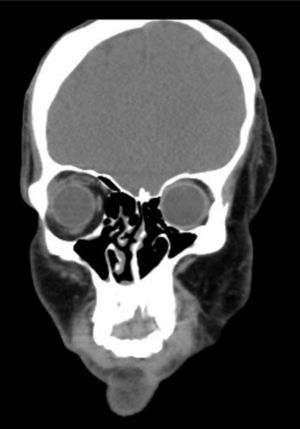
Clinical findings, diagnostic evaluation and interventionsA comprehensive physical examination was performed during the pre-anesthesia visit and complementary tests were reviewed. The findings include evidence of a marked facial asymmetry, atrophy of the left side of the face, left alar retraction, nasal septum deviation, hypoplasia of the frontal, malar, and maxillary bones and of the left hemimandible. There is also evidence of a temporal bone and cheek overgrowth resulting from the hyper development of the temporal muscle, the masseter and the left parotid gland (Fig. 2). The patient has an eye prosthesis (following the loss of his left eye due to endophthalmus) and his bodyweight is 80kg.
The airway evaluation resulted in Mallampati grade III, a 5cm thyromental distance, and a 4cm interdental distance, deviation of the tongue and uvula and discrete limitation of the cervical extension. No mobile teeth were identified. Reviewing past anesthesia records, a Cormack–Lehane IIb classification was established that required intubation with the Eschmann guide.
When admitted to the OR, the patient was under standard non-invasive blood pressure monitoring, pulse oximeter, and electrocardiography. A peripheral venous catheter was placed and the patient was properly pre-oxygenated. The anesthetic technique used was IV induction with 150μg of fentanyl and 180mg of Propofol. There was no airway obstruction during manual face mask ventilation. Using the Glideoscope® VL proper visualization of the glottis was obtained, prior to administering a neuromuscular block (Rocuronium 50mg) for uneventful orotracheal intubation at the first attempt. Upon verification of the correct positioning of the tracheal tube with capnography and auscultation of the right and left hemithorax, the tube is secured in place and mechanical ventilation established. Sevoflurane 1 CAM was used for maintenance of anesthesia and fentanyl according to the analgesia requirements. The surgery lasted 120min and was uneventful.
TimelineNo specific timeline is specified based on the clinical case set-up.
Follow-up and results2g of metamizol and 4mg of ondansetron were administered at the end of the procedure; 160mg of sugammadex were administered for reversal of the residual neuromuscular blockade with for a successful extubation. The patient was then transferred to the post-anesthesia recovery suite for postoperative monitoring.
DiscussionMedical literature reviewOnly one case report of PRS anesthesia management was found in the medical literature search.1 However, PRS is a multisystem craniofacial disease with associated disorders for which the anesthetic implications have not been clearly established. A comprehensive knowledge of PRS and of the many systems involved is important from the perioperative management perspective, with particular emphasis on the difficult airway.
The phenotypical characteristics of PRS patients are severe facial hemiatrophy, facial asymmetry, dental and craniofacial anomalies. There is often a deviation of the mouth and nose toward the affected side, as well as deviation of the tongue and uvula.1 Similar to other craniofacial syndromes,5 all of these characteristics may result in difficult airway.
In case of an expected difficult airway, orotracheal intubation with a flexible fibrobronchoscope is an effective and safe technique considered as the gold standard. However, based on the last updated algorithm of the American Society of Anesthesiologists (ASA) in 2013,6 VLs are also considered first-line devices for the approach of the difficult airway. In contrast to direct laryngoscopy the Glidescope® video laryngoscope offers improved visualization of the glottis,7 through the alignment of the oropharynx-laryngeal axis that is frequently difficult in patients with craniofacial syndromes5 such as PRS and may then be helpful in difficult airway cases.7 Its unique 60° angle, the real time visualization, improved Cormack–Lehane classification, and the availability of the stylette, all contribute to a sound orotracheal intubation.8 A recent simulated trial comparing several devices used for airway management9 concluded that the Glideoscope® video laryngoscope results in a lower rate of failed intubations and shorter times for proper exposure of the glottis and successful orotracheal intubation.9 Furthermore, the Glideoscope® was rated as the most user friendly and the preferred approach for a difficult airway patient.9
PRS has been associated with hypertrophic cardiomyopathy.10 Based on the cardiac history, both an electrocardiogram and an echocardiography should be made prior to surgery, considering the administration of a perioperative beta blocker to optimize ventricular filling.10 Two cases of patients with PRS and congenital heart disease11 and one case of supraventricular tachycardia1 have been reported. This fact, in addition to the hyperactivity of the sympathetic nervous system4 absolutely demands adequate pain control and proper maintenance of the intravascular volume.12
Some of the neurological manifestations include: trigeminal neuralgia, facial paresthesia, migraine, and focal epilepsy.2,3 The latter has a higher frequency and has been described in 10% of the PRS patients as a result of the frontoparietal lesions usually evidenced in imaging studies. Good treatment compliance and the administration of a preoperative benzodiazepine for anxiolysis are both recommended.
PRS is associated with various autoimmune disorders, including thyroid disease vitiligo, rheumatoid arthritis, anchylosing spondylitis, systemic lupus erythematous, and torticollis.3 Special mention should be made of linear scleroderma en coup de saber. Some authors believe that thee two conditions co-exist, while others consider PRS a variant of linear scleroderma en coup de saber.3
Identifying any moving teeth is relevant in these patients (the dental roots may be underdeveloped) in order to prevent any lesions during the orotracheal intubation, as well as any nasal or oral telangiectasias (in cases associated with sclerodermia) that may bleed during the procedure. There is also a need to evaluate the cervical mobility due to the typical PRS fibrosis or due to any prior surgeries, and based on the association with rheumatoid arthritis and torticollis.
Therefore, our key objectives are: a perioperative evaluation to identify any new manifestations of the disease, careful planning and approach of a difficult airway, ensure a constant hemodynamic stability, and preventing any neurological disorders, particularly seizures.1
Patient managementBased on the two previous surgeries, we were aware of the Cormack (IIb) classification, although these procedures had been done over a decade ago. Considering the progressive nature of the disease, this fact was just additional information and the decision was made to do the airway management plan based on the current situation. The asymmetric airway due to the left facial atrophy could lead to an airway obstruction during face mask ventilation. Based on the type of surgery suggested, the decision was made to secure the airway with orotracheal intubation using the Glideoscope® video laryngoscope (based on our considerable experience with the device) and to take advantage of what in our opinion is one of its most salient characteristics: the possibility to assess the patient's airway in situ on a screen – under adequate hypnosis following the administration of a neuromuscular block. This approach provides for a dual advantage which is the evaluation of the level of glottis exposure and the feasibility to intubate avoiding the risk of a difficult airway patient under neuromuscular blockade. The administration of the blocking agent facilitates the intubation and prevents any iatrogenic injuries of the vocal folds under safe conditions. Whichever the case, there has to be an alternate plan such as having available a fully equipped difficult airway cart. In response to these recommendations, a Supreme® laryngeal mask is always available in case of difficult ventilation and/or intubation and sugammadex for a no ventilation/intubation scenario that requires awakening the patient.13
The patient has no associated cardiac disease. However, based on the patient's history of epileptic seizures, anticonvulsive therapy is maintained, in addition to a perioperative benzodiazepine.
Lessons learnedWe highlight the importance of a continuous and careful preoperative evaluation due to the progressive facial changes, proper difficult airway management, and the prevention of any potential cardiovascular and neurological complications that may arise, in order to provide a safe anesthetic technique to PRS patients. The role of the Glideoscope® video laryngoscope for excellent visualization of the glottis in patients with craniofacial syndromes must be emphasized as a first-line approach by expert practitioners.
Patient outlookThe patient's perception was that he received the best anesthetic management for the surgery involved and the anesthesia-associated risks.
Informed consentA written informed consent was obtained for the publication of the case and its pictures to protect the patient's identity.
Ethical responsibilitiesProtection of persons and animalsThe authors hereby claim that this research involved no experiments in human beings or in animals.
Confidentiality of informationNo patient data are disclosed in this article.
Right to privacy and informed consentNo patient data are disclosed in this article.
FinancingNo financing was received for this paper.
Conflicts of interestThere are no conflicts of interests to disclose.
Please cite this article as: Fernández-Castellano G, Guerrero-Domínguez R, López-Herrera-Rodríguez D, Jiménez I. Implicaciones anestésicas del Síndrome de Parry-Romberg; reporte de un caso. Rev Colomb Anestesiol. 2017;45:26–30.