Epidural analgesia is the safest and most effective method for the treatment of pain during childbirth. Epidural bupivacaine provides excellent analgesia for labor and remains the most widely used local anesthetic in obstetric anesthesia.
ObjectiveTo evaluate the analgesic efficacy of two concentrations of bupivacaine in women in labor.
Methods114 patients in labor with term pregnancy were included in the study. They were grouped randomly into two groups: patients who received bupivacaine at 0.125% (group A) and those who received 0.25% bupivacaine (group B). Patients in group A received a bolus injection of 10ml of 0.125% bupivacaine. The patients in group B received a bolus of 10ml bupivacaine 0.25%. Pain intensity according to VAS, blood pressure, heart rate, respiratory rate, and the degree of motor block was assessed using the Bromage scale at different periods of time.
ResultsDemographic characteristics and parity were compared with no statistically significant differences found. By comparing the values of the VAS measure at 0, 15, 30, 60 and 90min, statistically significant differences in favor of the group with 0.25% bupivacaine were found with decreased pain perception after 30min, p-value=0.02. No differences in arterial pressure, heart rate and respiratory rate were found between the two groups.
ConclusionThe concentration of 0.25% bupivacaine has greater analgesic efficacy compared to 0.125% bupivacaine.
La analgesia epidural es el método más seguro y eficaz para el tratamiento del dolor del parto. La Bupivacaína epidural proporciona una analgesia excelente para el parto y sigue siendo el anestésico local más utilizado en anestesia obstétrica.
ObjetivoEvaluar la eficacia analgésica entre dos concentraciones de Bupivacaína en mujeres en trabajo de parto.
MétodosSe incluyeron 114 pacientes en trabajo de parto con embarazo de término. Se agruparon de forma aleatoria en dos grupos; pacientes que recibieron Bupivacaína al 0,125% (grupo A) y Bupivacaína al 0,25% (grupo B). Las pacientes del grupo A recibieron 10ml de Bupivacaína al 0,125% en bolo. Las pacientes del grupo B recibieron 10ml. de Bupivacaína al 0,25% en bolo. Se valoró la intensidad del dolor según la EVA, la presión arterial, frecuencia cardiaca, frecuencia respiratoria, el grado de bloqueo motor según la escala de Bromage en diferentes periodos de tiempo.
ResultadosLas características demográficas y de paridad se compararon, sin encontrar diferencias estadísticamente significativas. Al comparar los valores de la EVA medida en el minuto 0, 15, 30, 60 y 90 se encontraron diferencias estadísticamente significativas a favor del grupo con Bupivacaína al 0,25% con disminución de la percepción del dolor a partir del minuto 30, valor de p de 0,02. No se encontraron diferencias en la Presión Arterial, frecuencia cardiaca y frecuencia respiratoria entre ambos grupos.
ConclusiónLa concentración de Bupivacaína al 0,25% mejora la eficacia analgésica en comparación con Bupivacaína al 0,125% en mujeres con trabajo de parto activo en 6 puntos a los a partir de los 60 minutos.
Pregnancy and childbirth are among the main reasons for care in hospitals around the world1. Pain during childbirth is conditioned by uterine contractility, which, in turn, is modulated by the secretion of endogenous catecholamines that activate beta-2 andrenergenic receptors2, causing the sensation of pain3.
Apart from being an unpleasant feeling, pain causes anguish and stress and limits cooperation during labor4, which may end in a reduction of fetoplacental flow leading to fetal acidosis.
The Visual Analog Scale (EVA) is a validated, subjective, and widely used tool to stratify the intensity of patient pain perception5.
In 1847, James Young Simpson was the first to use ether for analgesia during childbirth6. Currently, neuraxial analgesia is the most used procedure and its benefits are widely known7. The most used pharmaceuticals are ropivacaine and bupivacaine in different concentrations8,9. Bupivacaine is preferred because of its greater affinity for plasmatic proteins in pregnant women10, although cardiotoxic properties have been attributed to it since it affects calcium channels. However, in low concentrations, it is far from causing this cardiotoxic effect11.
There is scientific evidence regarding the use of low dosages of epidural analgesia compared to high doses or combined analgesia (p<0.05), such as the Comparative Obstetric Mobile Epidural Trial (COMET)12 from the Study Group in the United Kingdom. In this study, the results of different concentrations of anesthetics, like bupivacaine and ropivacaine, in epidural anesthesia during labor are compared, along with their relationship with the incidence of assisted vaginal childbirth, and their effect at a variety of doses. Nevertheless, there is no evidence of the efficacy for pain management of the use of different concentrations of a single local anesthetic, in this case bupivacaine, in patients undergoing childbirth13,14.
ObjectiveTo assess the analgesic efficacy (VAS) between two concentrations of bupivacaine in women in labor.
Materials and methodsA randomized, triple blind clinical trial was conducted on pregnant patients in labor with registration in ClinicalTrials.gov (NCT02244086) and authorization from the Mexican Federal Commission for Health Risk Protection (Comisión Federal para la Protección Contra Riesgos Sanitarios – No. 2013-2301-21). The study took place in 2013 in Regional Hospital #17 of the Mexican Social Security Institute (IMSS) in the state of Quintana Roo, Mexico.
Patients were invited to participate with previously signed informed consent in physicians’ offices once active labor was corroborated and before entering the Labor and Delivery room. Here, they were fully informed of the characteristics and risks of the study. The selection criteria were as follows: women with normal, to term pregnancies that requested obstetric analgesia with any number of gestations, a singleton pregnancy, in active labor (cervical dilation ≥4cm), with gestational age greater than 34 weeks, and an ASA (American Society of Anesthesiology) physical state of I or II. Patients who had problems like language barriers or allergies to local anesthetics were excluded. Also excluded were those women that presented any of the following situations in their last trimester: pregnancy induced hypertension, placenta previa, anomalies in the variety of fetal presentation, cephalopelvic disproportion, or hypertonic uterus. This was also the case for obstetric emergencies such as: severe preeclampsia, abruption of normally inserted placenta, fetal distress, or any alteration to the anatomy of the spinal column, or previous surgery that impede or limit the administration of epidural analgesia. Also excluded were those women who, despite having received epidural analgesia, finished the pregnancy with a cesarean section or prolonged labor, or those for whom there were failures in the epidural technique or related complications that merited three or more extra doses.
The groups were formed randomly and the triple blind trial was guaranteed for the patient (who was unaware of the concentration administered due to the similar appearance of the syringes and their content) and for the anesthesiologist (the bupivacaine was prepared by the researcher in a special area and delivered with a label that did not reveal the concentration but only the preparation with the letters A and B). An extra dose was prepared to be used in case extra medication was required, and for the data analyst the database was handed in and the lack of specificity guaranteed anonymity.
The result variables were as follows: pain perception as measured with VAS, heart rate, respiratory rate, systolic/diastolic blood pressure, and unfavorable events at two different concentrations of bupivacaine (0.125 and 0.25%). Centimeters of cervical dilation pre- and post-anesthetics were obtained directly from the partogram.
During the progress of labor, if the patient showed a desire for obstetric analgesics, the gynecologist requested their administration. The appointed anesthesiologist would assess the general characteristics of the patient and decide whether or not she was a candidate for the procedure. The study was executed by residents and anesthesiologists with experience in more than 100 procedures of this nature and who were previously offered retraining on anesthetic technique in order to achieve standardization.
The main researcher created a coding sheet for the main information that was obtained from the Delivery Room admittance sheet. This information included: general information about the patient, age, number of gestations, history of previous analgesia, and complications during pregnancy. The VAS was measured with a standardized table at a time when the patient did not present contractions. The variables of heart rate, diastolic and systolic blood pressure, and respiratory rate were obtained by the anesthesiologist who applied the analgesia at different times. After the administration of analgesia, the variables of interest were measured initially, and at 15, 30, 60, and 90min.
The sample size was determined with the mean difference formula15, considering a difference between the concentrations of bupivacaine of 3 points on the VAS at 30min, and expecting a minimal decrease of 2 points at 30min in both applications. A risk of 0.05 and a statistical power of 90% were accepted, calculating a sample size of 38 patients per group and forecasting 10% in losses, with a total estimated sample size of 84 women.
A partial analysis was conducted after obtaining ¼ of the expected sample size to corroborate that the proposed treatment did not compromise the health of the child or the mother.
Statistical analysisWith the program SPSS version 20.00 for windows, and previous analysis of normality with the Kolmogrov–Smirnov test, descriptive statistics were conducted with frequencies and percentages for qualitative variables, measures of central tendency (mean, median) and of dispersion (interquartile ranges, standard deviation), and with comparison of the two therapeutic groups with Student's T-test or the Mann–Whitney U-test for quantitative variables and the Chi-squared test or Fisher's exact test for qualitative variables. The dependent variables (VAS, heart rate, respiratory rate, systolic and diastolic blood pressure) were compared in the two groups with different measures including the ANOVA repeated measures test or Friedman's test, depending on the distribution. A value of p<0.05 was considered significant.
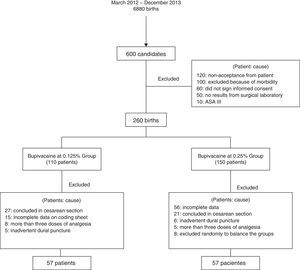
ResultsFrom March 2012 to December 2013, 6880 births were attended of which 600 were eligible for this study. Losses and exclusions of patients are shown in Fig. 1.
The total population was 114 patients with 1 or more gestations, undergoing induction/conduction of labor, divided into two balanced groups of 57 women (bupivacaine at 0.125% and bupivacaine at 0.25%). The average age was 23.8±4.6 years. The average weight was 64.5±8kg. The mean number of gestations was 1 (1–3). 70 (61%) were primiparous and 30 (26%) were induced births.
During the management of labor, 20 (16%) received oxytocics, and the median of centimeters of dilation was 4 both after (3–7) and before (3–9) analgesia. The latency time of the analgesic effect was 8±1.5min and there were no complications reported (hypotension, inadvertent dural puncture, intravascular injection, motor blockade) during the procedure in any of the patients.
Upon comparing the bupivacaine at 0.125% group to the bupivacaine at 0.25% group, there were differences found only in the history of previous births: 20% of the first group against 35% of the second group (p-value=0.04) (Table 1).
Differences in demographic variables between the two groups of women with obstetric analgesia at 0.125 and 0.25%.
| Variable | Bupivacaine 0.125%n=57 | Bupivacaine 0.25%n=57 | p-Value |
|---|---|---|---|
| Previous birthsc | 11 (20%) | 20 (35%) | 0.04 |
| Previous C-sectionsc | 7 (12%) | 6 (10%) | 0.5 |
| Previous analgesiad | 0 (0%) | 2 (4%) | 0.2 |
| Oxytocin (yes/no)c | 45 (78%) | 49 (86%) | 0.6 |
| Weight (kg)b | 64±8 | 65±7 | 0.3 |
| Age (years)b | 23±4 | 24±5 | 0.1 |
| Pre-cervical dilation pre (cm)a | 4 (3–7) | 4 (3–7) | 0.4 |
| Post-cervical dilation post (cm)a | 5 (3–9) | 5 (3–8) | 0.9 |
| Fetal heart rate (min)b | 140±7 | 139±6 | 0.8 |
| Latency time (min)b | 8±1.1 | 8±1.6 | 0.4 |
| Required second bolus | 2 (4%) | 0 (0%) | 0.1 |
| Later difficulty walking | 3 (5%) | 4 (7%) | 0.8 |
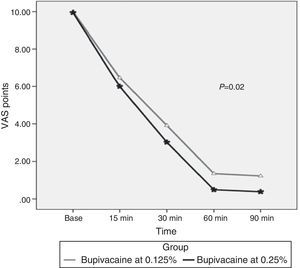
Upon comparing the values of the visual analog scale (VAS) measured at 0, 15, 30, 60 and 90min (Table 2), statistically significant differences were found in favor of the group with bupivacaine at 0.25% with a reduction in pain perception starting at minute 30 (p-value=0.02) (Fig. 2). No differences were found in systolic blood pressure, diastolic blood pressure, heart rate, or respiratory rate between the two groups.
Difference in measures between two groups at 5 measurement times.
| Variable | Bupivacaine at 0.125% | Bupivacaine at 0.25% | P-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | 15′ | 30′ | 60′ | 90′ | Base | 15′ | 30′ | 60′ | 90′ | ||
| VASa (points) | 10 (10) | 6 (3–9) | 4 (0–7) | 1 (0–6) | 1 (0–5) | 10 (0–9) | 6 (2–10) | 3 (0–7) | 0(0–4) | 0 (0–4) | 0.02 |
| SBPb (mm/hg) | 122±8 | 116±5 | 114±6 | 115±5 | 115±5 | 120±7 | 117±5 | 115±5 | 114±6 | 114±6 | 0.2 |
| DBPb (mm/hg) | 74±9 | 71±8 | 70±8 | 70±7 | 70±7 | 72±9 | 70±6 | 70±7 | 67±6 | 68±7 | 0.4 |
| HRb (min) | 91±8 | 83±6 | 80±4 | 78±4 | 78±3 | 90±9 | 83±6 | 79±4 | 77±4 | 77±4 | 0.7 |
| RRb (min) | 19±2 | 18±1 | 18±1 | 18±1 | 18±1 | 19±2 | 18±1 | 18±1 | 18±1 | 18±1 | 0.8 |
VAS, visual analog scale; SBP, systolic blood pressure; TAD, diastolic blood presure; HR, heart rate; RR, respiratory rate.
The current tendency in obstetric epidural analgesia is to use local anesthetics at the minimum effective concentration16 in order to reduce possible secondary effects to the mother and to the progress of labor17–19.
The safety and efficacy of bupivacaine compared to other anesthetics is known20. Nevertheless, the dose used varies depending on the study, with variations between 0.0125 and 0.37%21. The well-known risk is that labor may end in an instrumental delivery or with complications that affect the mother and the child22.
The results of this study demonstrate that, at two different concentrations of bupivacaine, no complications occurred and the only significant difference was in pain perception.
The general characteristics of the population are similar to those found in any second-level of care hospital that offers obstetric services. Thus, the external validity of the study is good.
There were no statistically significant differences between the two groups in terms of age, previous cesarean sections, the use of oxytocin, and the number of previous gestations and analgesia. Therefore, initially, the populations are homogenous. Kolmogorv–Smirnov p>0.05.
Usually, bupivacaine is used in combination with other pharmaceuticals like fentanyl or meperidine, and it has been found that the higher the concentration, the higher the risk or instrumental delivery and prolonged labor23. Bupivacaine, compared to other pharmaceuticals used during labor, has safer results if it is used exclusively24,25, though user satisfaction may vary depending on dosage or combination26.
Previous studies, with less “blind” procedures and dependent variables other than pain, report that there are no differences in the concentration of bupivacaine27–29, which differs with our results.
Since the procedure was controlled under methodological rigor, this is a study of efficacy. The main evidence is that the perception of pain reported by the patient appears to be statistically less in those women that received a concentration of bupivacaine at 0.25%. Most remarkable is that there are no differences in the rest of the vital signs and in instrumental delivery.
Our study is innovative in that there are no published protocols with the same characteristics comparing bupivacaine at different concentration levels. The results that we obtained suggest that, by using the 0.25% concentration, analgesic effectiveness can be increased without causing adverse effects. While the difference was not statistically significant, patients who received bupivacaine at 0.25% did not merit a second dose. We decided to not include those patients that merited three or more doses due to the probability of loosing the purity of the procedure (due to the lack of blindness from the anesthesiologist with respect to the concentration). Nevertheless, the application of three or more doses was greater in the group with 0.125% bupivacaine compared to the 0.25% group (8 vs 5).
The most pronounced weakness lies in that the dependent variable used, in this case VAS, is merely subjective and that this information is supplied entirely by the patient. For this reason, there may be a bias of poor classification and poor memory. This suggests that a study should be conducted comparing the most efficacious medication for treating pain in this kind of patient compared to the 0.25% concentration of bupivacaine. The methods and the limiting factor of not comparing it to other analgesics may limit its application in real clinical practice.
One of the adverse effects that may appear during epidural analgesia in labor is motor block produced by local anesthetics. This block may be the cause, in some cases, of a greater duration of the second stage of labor, greater incidence of instrumental deliveries and cesarean sections, and more discomfort for the mother30. With both concentrations, patient comfort is obtained without prolonging the second stage31–33. In our study, there were no significant differences found in terms of the presence of motor block.
Epidural bupivacaine provides excellent analgesia for labor and continues to be the most used local anesthetic in obstetric anesthesia34. The minimal local anesthetic concentrations (MLAC) have been used to determine the power of epidural analgesics like bupivacaine and its homologue levobupivacaine, establishing that concentrations of bupivacaine at 0.125 and 0.25% have analgesic effect and are safe35.
ConclusionAnalgesic efficacy of bupivacaine at 0.25% concentration was better compared to bupivacaine at 0.125% concentration, with no associated complications such as difficulty walking, requirements of extra doses, or complications for the mother–child unit. The measurement of other variables of interest merits study to enrich the results.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNone.
Conflict of interestThe author has no conflicts of interest to declare.
Please cite this article as: Rodríguez-Ramón R, Márquez-González H, Jiménez-Báez MV, Iparrea-Ramos IC. Eficacia analgésica entre dos concentraciones de Bupivacaína en mujeres en trabajo de parto. Ensayo clínico contralado aleatorizado triple ciego. Rev Colomb Anestesiol. 2015;43:179–185.