Craniosynostosis is a congenital disorder requiring extensive reconstructive surgery that entails a high probability of severe bleeding, massive transfusion and difficult airway management. Considering that the anaesthetic management for this procedure has special requirements and priority targets, presenting the experience of the anaesthesiology department working under the programme for surgery of craniofacial abnormalities is of the greatest importance.
ObjectiveDescribe the behaviour of anaesthetic variables during the perioperative period in patients taken to craniosynostosis correction at Hospital Infantil Universitario de San José.
MethodsRetrospective observational cohort study in patients taken to surgery between January 1st 2008 and January 31st 2012. Data were collected from electronic clinical records and anaesthesia records.
ResultsThe most relevant data were haemorrhage and transfusion. Blood loss was 35.6cc/kg (SD=17.4), considered as severe haemorrhage. Patients receiving tranexamic acid did not show lower intra-operative levels of blood loss or packed red blood cell (PRBC) transfusions, shorter mechanical ventilation or ICU (intensive care unit) length of stay. We observed a smaller number of PRBC transfusions in patients in the ICU who received desmopressin.
ConclusionsWe suggest that neither tranexamic acid (14mg/kg) nor desmopressin (0.36mg/kg) in this cohort correlated with reduced haemorrhage or smaller volumes of intra-operative PRBCs. We only describe a smaller volume of transfused PRBCs in the ICU associated with the use of desmopressin.
La craneosinostosis es un trastorno congénito que requiere intensas cirugías reconstructivas que implican alta probabilidad de hemorragia severa, transfusión masiva y difícil abordaje de la vía aérea. Debido a que el manejo anestésico previsto para este procedimiento presenta particularidades y metas de prioritario alcance, la presentación de la experiencia del departamento de anestesiología en el programa de cirugía de anomalías craneofaciales toma gran importancia.
ObjetivoDescribir el comportamiento de variables anestésicas en el perioperatorio de pacientes llevados a corrección de craneosinostosis en el Hospital Infantil Universitario de San José.
MétodosEstudio observacional de cohorte retrospectiva en pacientes intervenidos entre el1 de enero de 2008 y el 31 de enero del 2012. Se realizó extracción de datos de historias clínicas electrónicas y registros anestésicos.
ResultadosLos datos de mayor relevancia fueron la hemorragia y la transfusión. La hemorragia quirúrgica fue de 35,6 cc/kg (DE=17,4), lo cual consideramos como hemorragia severa; allí los casos que utilizaron ácido tranexámico no presentaron inferiores volúmenes hemorrágicos o transfusionales de glóbulos rojos empaquetados (GRE) intraoperatorios, menor tiempo de ventilación mecánica o estancia en la unidad de cuidados intensivos (UCI). Observamos menor cantidad de GRE transfundidos en la UCI en los casos que recibieron desmopresina.
ConclusionesSugerimos que probablemente el ácido tranexámico (14mg/kg) y la desmopresina (0,36 mg/kg) en esta cohorte no se relacionaron con disminución de la hemorragia, ni menor cantidad de GRE transfundidos durante el intraoperatorio; solo describimos menor cantidad de GRE transfundidos en la UCI asociados al empleo de desmopresina.
Craniosynostosis is known in the medical setting for its difficult treatment, usually requiring invasive procedures with a high impact on the patient's functional reserve and the economics of our healthcare system. In response to this situation, the surgical programme for craniofacial abnormalities at Hospital Infantil Universitario de San José was designed to give back to the child the expectation of functional performance and the ability to live in its social environment. The outcomes of the past years lead us to believe that this exercise might make the difference in terms of impact on quality of life when compared with treatments in other referral centres treating similar cases. This study describes the results for perioperative variables in patients intervened for craniosynostoses between January 1st 2008 and January 31st 2012 by the Anaesthesia Department at Hospital Infantil Universitario de San José, Bogota, Colombia.
Materials and methodsThis study was conducted with the approval of the Ethics and Research Committee of the Fundación Universitaria de Ciencias de la Salud (FUCS) Medical School, and required no informed consent because of its nature. Demographic, anaesthetic and critical data were described by gender. Means and standard deviations were estimated for continuous variables, and frequencies and percentages were estimated for nominal variables. Mean values for outcomes such as haemorrhage, transfusion, days on mechanical ventilation and length of stay in the ICU were compared, diverse variables were stratified and, finally, hypotheses for future work were postulated.
A p value of <0.05 was used to designate statistically significant differences. A non-systematic review of the medical literature was conducted, and our data were discussed in relation with those found in the international literature. The STATA 10 statistical software package was used.
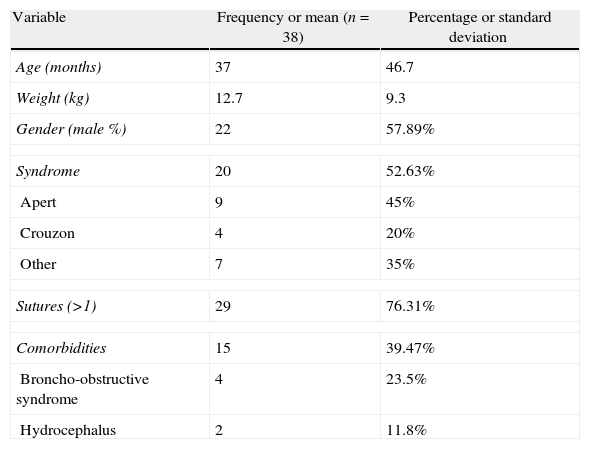
ResultsA total of 41 patients were operated between January 1st 2008 and January 31st 2012. Three were excluded because of failure to meet the inclusion criteria, and no intra-operative anaesthesia data were obtained in three cases. The remaining 35 cases contributed 100% of the data (Table 1).
Demographic data.
| Variable | Frequency or mean (n=38) | Percentage or standard deviation |
| Age (months) | 37 | 46.7 |
| Weight (kg) | 12.7 | 9.3 |
| Gender (male %) | 22 | 57.89% |
| Syndrome | 20 | 52.63% |
| Apert | 9 | 45% |
| Crouzon | 4 | 20% |
| Other | 7 | 35% |
| Sutures (>1) | 29 | 76.31% |
| Comorbidities | 15 | 39.47% |
| Broncho-obstructive syndrome | 4 | 23.5% |
| Hydrocephalus | 2 | 11.8% |
Male %: % of men; comorbidities: conditions present in the patients before surgery.
The pre-anaesthesia airway assessment did not reveal a significant prevalence of difficult airway predictors. Only 17.6% of the patients in this cohort were classified as Cormack 3, 2.9% had difficult mouth opening, and only 5.2% were considered to have true difficult airways on intubation, despite the fact that 50% had a Mallampati score greater than, or equal to III (IV=8.3%).
Basic and invasive blood pressure monitoring was used in all cases, in 60% of the cases the lowest mean temperature was 35.09°C (SD=0.78), and central venous catheter monitoring and therapy were used in 94.7%.
The anaesthetics most commonly used for induction were sevoflurane (64.7%) and propofol (32.4%). Only in 71.4% was a specific analgesic used during the induction phase (remifentanil 88% of the time). Neuromuscular blockade was given in 91.2% of cases, with pancuronium and rocuronium being the most widely used (37.5% and 25%, respectively). For maintenance, the remifentanil/sevoflurane combination was chosen in 88.6% of cases, while sevoflurane was the inhaled drug most commonly used (94.3%).
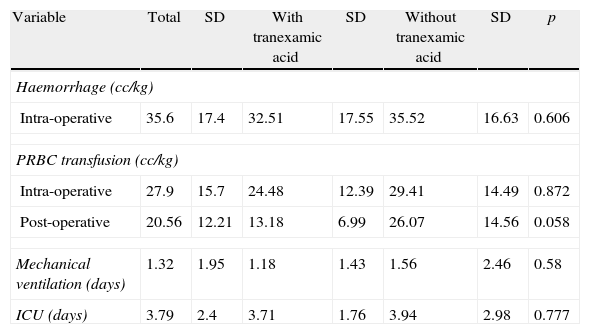
Average blood loss during surgery was 35.6cc/kg (SD=17.4), requiring transfusion of 27.9cc/kg (SD=15.7) of PRBC in the operating room, and of 20.55cc/kg (SD=12.21) of PRBC in the ICU. Moreover, an important difference was found in the volumes of other blood products transfused in the operating room and in the ICU (surgery 2.7% vs. ICU 36.8%, or 1 vs. 14 cases) (see Table 2). On arrival to the operating room, haemoglobin (Hb) was 13.27g/dL (SD=1.14), while on discharge Hb was 11.46g/dL (SD=2.04), with excess base (EB) of −8.55 (SD=2.98) consistent with metabolic acidemia, despite the fact that the mean difference was only 1.69g/dL between Hb on admission to the operating room and admission to the ICU.
Blood losses, PRBC transfusion, days on mechanical ventilation and length of stay in the ICU.
| Variable | Total | SD | With tranexamic acid | SD | Without tranexamic acid | SD | p |
| Haemorrhage (cc/kg) | |||||||
| Intra-operative | 35.6 | 17.4 | 32.51 | 17.55 | 35.52 | 16.63 | 0.606 |
| PRBC transfusion (cc/kg) | |||||||
| Intra-operative | 27.9 | 15.7 | 24.48 | 12.39 | 29.41 | 14.49 | 0.872 |
| Post-operative | 20.56 | 12.21 | 13.18 | 6.99 | 26.07 | 14.56 | 0.058 |
| Mechanical ventilation (days) | 1.32 | 1.95 | 1.18 | 1.43 | 1.56 | 2.46 | 0.58 |
| ICU (days) | 3.79 | 2.4 | 3.71 | 1.76 | 3.94 | 2.98 | 0.777 |
PRBC: packed red blood cells; ICU: intensive care unit; SD: standard deviation.
Tranexamic acid was used in 44.7% of the cases, at a mean bolus dose of 14mg/kg and a mean infusion dose of 1.1mg/kg/h; only eight (8) patients received an additional bolus dose of desmopressin 0.36 mcg/kg. Of the 17 patients (44.7%) who received tranexamic acid, 41.2% received a single bolus, while 47.1% received a combined bolus and infusion dose.
Volemia was estimated in all patients according to age and weight as an indirect measurement of the effect of tranexamic acid, and blood loss goals of 25%, 33%, 40% and 50% were established. When describing bleeding volumes and comparing between those who received the drug and those who were not exposed, no significant differences were found between the patients who lost less than 40% and 50% of the estimated volemia ([9 vs. 4; p=0.105] for blood loss less than 40% and [9 vs. 9; p=0.746] for blood loss less than 50%). Also, no significant differences were found for blood losses less than 25% and 33% of the estimated blood volume.
No reduction in blood loss was observed in the group receiving desmopressin. In contrast, the higher proportion of patients with bleeding below 50% of the estimated volemia were among those who were not exposed to the drug (p=0.001). We do not describe differences for estimated losses less than 25%, 33% and 40% of the blood volume.
When transfusion mean values were stratified at operating room with the use of tranexamic acid, averages varied from 24.48cc/kg (SD=12.39) in patients exposed to 29.41cc/kg (SD=14.49) in those who did not receive the drug, albeit with no significant differences (p=0.872). In the ICU, transfusion mean values showed marginal differences, not considered significant, for the use of PRBCs ([13.18cc/kg, SD=6.99 vs. 26.07cc/kg, SD=14.56] for p=0.058), but in no way does this mean that they should not be used and, on the contrary, it supports the decision of setting up experiments to evaluate this hypothesis.
When the effect of desmopressin was stratified, we did not find differences in PRBC transfusion averages in the operating room (29.17cc/kg, SD=12.92 vs. 26.26, SD=13.85; p=0.949); but when PRBC transfusion volumes were stratified for the ICU, significant differences were found (15.82cc/kg, SD=3.01 vs. 22.81cc/kg, SD=16.32; for a p=0.027), suggesting that desmopressin might reduce microvascular bleeding in the ICU and, consequently, the PRBC transfusion requirements, which we found not to be related to the transfusion of other blood products.
Of the patients, 13.5% required haemodynamic support in the operating room, with frequent use of norepinephrine (66.7%), while 42.1% received dopamine and/or norepinephrine (87.5%) during their stay in the ICU.
Operative time was 303.1min (SD=92) and was not associated with increased bleeding in the operating room or the ICU. Post-operative comorbidities occurred in 42.1% of patients, with disseminated intravascular coagulation (DIC) being the most frequent complication (35%), followed by post-extubation CROUP and acute renal failure (10.5%, each). The mean time on mechanical ventilation was 1.32 days (SD=1.94). The mean length of stay in the ICU was 3.79 days (SD=2.39) and none of the patients died during hospitalization.
For this cohort, we did not observe a relationship between syndromic craniosynostosis and increased rates of bleeding, morbidity or mortality. In contrast, we describe a slight increase in the length of stay in the ICU (2.78, SD=1.22 vs. 4.7, SD=2.83; p=0.011) and the time on mechanical ventilation (0.39, SD=1.03 vs. 2.15, SD=2.21; with p=0.004). When analysing the independent variables – number of sutures and complexity (advancement surgery vs. correction of several sutures and advancements) – no differences were found in terms of bleeding and/or transfusion outcomes, or in changes in surgical time.
DiscussionThe surgical procedures developed for correcting craniosynostosis are well known for the high volumes of blood loss and maximum risk of massive transfusions, as described by Koh and Soriano.1,2 Patients are usually infants or school children, and have comorbidities associated with the use of drugs required for their adequate control and which impact the outcome of the surgical procedures.3
In the world literature, difficult airways and haemorrhage control are cited as the main issues requiring anaesthesia intervention. In this study, although 17.6% of the patients were classified as Cormack 3 and 5.9% were considered to have a difficult airway, there were no complications at the time of intubation. We do not suggest the absence of a difficult airway in these patients; on the contrary, we would like to expand our protocols, which we hold with great respect at the Anaesthesiology Department, to include the potentially difficult airway in paediatric craniofacial dimorphism.
Intra-operative bleeding rates were consistent with the reports from other authors, but analysis of bleeding associated with different pro-coagulation strategies revealed some differences. For Goobie et al.4 (in an experiment using tranexamic acid in patients undergoing craniosynostosis correction), there was less haemorrhage in the tranexamic acid group (62cc/kg, DE=22) compared to placebo (101cc/kg, SD=63). In a similar study, Dadure et al.5 found the same bleeding rates for tranexamic acid (51.4cc/kg, SD=28.3) compared with placebo (61.1cc/kg, SD=16.8); p=0.25. However, they estimated an 85% reduction in intra-operative transfusion in the tranexamic acid group (p=0.02), related to a rigorous protocol with erythropoietin and iron supplementation.
The intra-operative transfused volume in this study was 27.9cc/kg (SD=15.7), which correlates with transfusion requirements published in the world literature. For Goobie et al.4 the mean transfusion value during surgery was 33cc/kg (SD=13), and the PRBCs used postoperatively were 3cc/kg (95% CI between 0 and 25), whereas Dadure et al.5 report an intra-operative transfused volume of 1.6cc/kg in the tranexamic acid group vs. 11cc/kg in the placebo group (p=0.01).
In the world literature, tranexamic acid is used in bolus (10–100mg/kg) and infusion (1–10mg/kg/h). In the case of our patients, a mean bolus dose of 14mg/kg was used, occasionally associated with a 1mg/kg/h infusion; however, this was not related with a lower bleeding volume or smaller amounts of PRBCs transfused intra-operatively or in the ICU. This analysis begs a question about the true efficacy of standard doses of tranexamic acid in preventing major bleeding and reducing the use of blood products, consistent with the report by Neilipovitz.6
Dadure et al.5 used similar doses of tranexamic acid as those used in our group of patients (15mg/kg followed by infusion at a rate of 1mg/kg/h), but combined with preoperative subcutaneous erythropoietin and iron supplementation, and observed no reduction in the rate of bleeding but a reduction in the transfusion volume, similar to that reported by Meneghini7 and Fearon.8
In an interesting reflection about the methods and results published by the authors mentioned above, Holcomb9 discusses the accuracy and depth of their conclusion and advices physicians to base their judgement on solid evidence when it comes to starting these patients on this anti-fibrinolytic agent. Finding specific doses (bolus or infusion), developing protocols and forecasting transfusion goals based on the diversity of effects and with the guidance of modern simultaneous coagulation monitoring (in real time) might result in more accurate indications, follow-up and goals for tranexamic acid.9
The use of desmopressin as an adjuvant or as a single therapy in this cohort of patients did not reduce bleeding volumes or the amount of PRBCs used in surgery. It is worth noting that transfused volumes of PRBCs observed in the ICU were significantly smaller (15.82 vs. 22.81; p=0.027), which suggests a potential benefit associated with their application in the operating room and leads us to recommend the experimental verification of these observations. It is clear that bleeding in the immediate and early post-operative period has different aetiologies, it varies depending on the age group, and it is more the result of an intrinsic coagulation defect than of a persistent vascular disruption in the paediatric patients, which might justify its potential efficacy in these patients.10
Although the restrictive use of blood products has resulted in lower rates of complications when compared to their liberal use, it does not necessarily prevent associated morbidity, unlike what happens with the incidence of transfusion-related complications in adults (37:100,000 units of PRBCs).11 Of significant importance is the study of alternatives such as normovolemic/hypervolemic haemodilution, deliberate hypotension, anti-fibrinolytics, blood cell savers, fibrinogen concentrate and autotransfusion, among other blood saving methods, which have not been studied or applied in this age group.11–15
This study outlines the anaesthesia model used at Hospital Infantil Universitario de San José for craniosynostosis, which includes intravenous and inhaled agents endorsed by the world literature for induction and maintenance, given their advantage of prompt and easy neurological assessment after surgery.16 Its conclusions lead us to create a hypothesis regarding the preventive management of severe haemorrhage in the high-risk paediatric population.
The premise that tranexamic acid in a mean dose of 14mg/kg may provide the same benefits as higher doses might not be true; for this retrospective cohort, bleeding and transfusion volumes at different points in time in relation to the administration of tranexamic acid were similar.
Although massive transfusions contributed to the improvement of perfusion indices and the optimization of haemoglobin levels, they did not prevent DIC or diminish metabolic acidosis, just as was reported by Choi.17 Only one of our patients received fresh frozen plasma (FFP) during surgery, similar to what was reported by Kerner,18 who published that the use of FFP during surgery did not reduce blood loss volumes, transfusion requirements, or the length of stay in the ICU. In our research, the high rate of DIC associated with aggressive PRBC transfusion, fluid resuscitation with crystalloids free from significant metabolic acidosis, and the presence of mild hypothermia point to the need of reconsidering the early administration of FPP and PLA guided by modern coagulation monitoring (thromboelastography), as well as timely replacement of serum ionic calcium.
In accordance with worldwide publications, the strategy to approach the airway in patients with facial abnormalities must be based on meticulous pre-operative planning. For Beer and Bingham,19 the key to success is to identify the site of the abnormality, the degree of mouth opening and neck mobility, and control of all those factors that may exacerbate intracranial pressure and/or decompensate pathological cardiac conditions; these authors also consider that the invasive technique to approach the airway must be tailored individually.19 In our study, cases were rarely associated with a difficult intubation or perioperative respiratory complications. These findings were similar to those of Barnett, Moloney and Bingham20 who found a low rate of complications in Apert's syndrome (4.5%). Unlike reported difficulty in approaching the airway of patients with mid-facial hypoplasia due to irregular inter-maxillary proportions and reduced temporomandibular mobility,19 in our cohort only a minority of patients (17.6%) were Cormack grade III and only 5.26% had a difficult airway. This may be related to the use of modern advanced management techniques (video laryngoscopy and fibreoptic intubation).
Air embolism is quite frequent during craniectomy in patients with craniosynostosis, with an incidence ranging between 36% and 82.6%.21–23 In this cohort, there was no study or diagnosis of this condition, which is a weakness when it comes to analyze the reason for haemodynamic support in the ICU.
Just as reported in the world literature, the syndromic aetiology of craniosynostosis, the comorbidities, the drugs used up to the moment of surgery, the number of sutures, and the complexity were not correlated with the increased volume of bleeding, blood product transfusion or morbidity. Only a slight increase in mechanical ventilation and length of stay in the ICU was found to correlate with the syndromic aetiology.
In this cohort, the anaesthetic medium apparently did not modify bleeding, transfusion, time on mechanical ventilation, fast-tracking strategy or the length of stay in the UCI.
ConclusionsOur observations regarding haemorrhage and needs for transfusion lead us to suspect that tranexamic acid (at a mean dose of 14mg/kg) in children taken to carniosynostosis correction might not prevent massive bleeding or reduce the volume of PRBC transfusion, the days on mechanical ventilation or the length of stay in the ICU; we suggest that its use must be subject to the availability of the best evidence on paediatric doses, administration regimen and coagulation monitoring.
We suggest that invasive monitoring, adequate airway management planning and early restrictive transfusion based on cell perfusion and coagulation goals are the pillars for the anaesthetic management. We believe that these may have contributed to reducing the frequency of adverse events and mortality in this cohort, compared with what has been reported in the world literature.
We strongly recommend conducting randomized clinical trials to determine the effectiveness of different doses of anti-fibrinolytics in preventing severe bleeding and high transfusion volumes, as well as research on alternatives to transfusion and blood saving in paediatric patients taken to surgery with a high risk of bleeding. Likewise, we recognize the need for clinical practice guidelines for this anaesthetic challenge.
FundingResearch Division, Fundación Universitaria de Ciencias de la Salud – Bogotá, Colombia.
Conflicts of interestNone declared.
To Fundación Universitaria de Ciencias de la Salud, Hospital Infantil Universitario de San José and its Departments of Anaesthesiology, Plastic Surgery, Neurosurgery, and the Reconstructive Surgery Program for Craniofacial Abnormalities (CSAP).
Please cite this article as: González Cárdenas VH, Vanegas Martínez MV, Rojas Rueda ME, Guevara Nelly S, Prada JR, Baquero P. Anestesia para Craneosinostosis. Rev Colomb Anestesiol. 2014;42:199–204.