The inclusion of statins and stents in coronary disease management during the 1980s has marked a dramatic change in the natural history of the disease. Separately, each of these therapies have progressed rapidly and have achieved a prime position in the current armamentarium. The simultaneous use of statins in patients undergoing percutaneous coronary revascularization procedures with stent implantation has shown a significant beneficial synergistic effect by reducing ischemia and necrosis, and improving coronary blood flow in patients with stable coronary disease, as well as in acute coronary syndromes. The use of high dose statins in conjunction with coronary angioplasty with stent implantation has shown great efficacy and safety in patients with severe coronary disease.
La inclusión de las estatinas y stents en el manejo de la enfermedad coronaria durante la década de 1980 marcó un cambio dramático en la historia natural de la enfermedad. Cada una de estas terapias por separado han mostrado una rápida evolución y ha alcanzado una posición privilegiada en el arsenal terapéutico actual. El uso simultáneo de las estatinas en los pacientes sometidos a procedimientos de revascularización coronaria percutánea con implantación de stent ha demostrado un efecto sinérgico significativo para reducir la isquemia y necrosis, mejorando el flujo sanguíneo coronario en pacientes con enfermedad coronaria estable, así como en los síndromes coronarios agudos. El uso de estatinas dosis altas en relación con la angioplastia coronaria con implantación de stent ha demostrado una gran eficacia y seguridad en pacientes con enfermedad coronaria severa.
The relationship between elevated serum cholesterol and coronary artery disease (CAD) was established at the end of the 1950s with the Framingham study.1 This observation led to a wide development of new pharmacological treatments to reduce CAD mortality by reducing cholesterol. Around 1984, the NIH concluded that reducing elevated LDL-C levels with diet and medications should reduce the risk of CAD.2
The first 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, was discovered in 1970 by Akira Endo through fermentation of Penicillium citrinum during antimicrobial agent research.3,4 In 1978, even though another HMG-CoA reductase inhibitor (lovastatin) was discovered through the fermentation of Aspergillus terreus,5 it was not until 1982 that lovastatin was used in patients with familial hypercholesterolemia, showing a important reduction of LDL-C with a favorable safety profile.6,7
Around the 80s decade, large-scale, randomized, double-blind clinical studies showed the beneficial effect of lovastatin, achieving FDA approval in 1987, and becoming the first commercially available statin.5,8,9 A 40% reduction in average LDL-C levels was obtained with maximum doses of 80mg of lovastatin.8–10 Other statins have been developed and are available today: simvastatin in 1988, pravastatin in 1991, fluvastatin in 1991, atorvastatin in 1994, rosuvastatin in 2003, and pitavastatin in 2009. In the mid 1990s the beneficial effect of statins in primary and secondary prevention of CAD was clearly established.11–13 As an understanding of statins grew, percutaneous coronary intervention techniques and stents were developed in parallel.
Percutaneous coronary interventionsDuring the 60s decade, Dotter and Judkins proposed the concept of the percutaneous use of devices to maintain the luminal integrity of stenotic blood vessels.14 When A. Grüentzig introduced percutaneous transluminal coronary angioplasty (PTCA) in 1977, the field of percutaneous coronary intervention was born.15 In spite of the new technology available for the treatment of CAD, PTCA was limited by the relatively high incidence of acute vessel occlusion and the need for repeated revascularization procedures due to the phenomenon of restenosis. The introduction of bare metal stents (BMS), demonstrated an important advance in interventional cardiology.
In 1987, Sigwart et al. were the first to describe the use of BMS for emergency management of an acute occlusion during a PTCA.16 The first commercially available BMS was the Palmaz–Schatz (Johnson and Johnson Interventional Systems, Warren, NJ) stent, showing superior results to PTCA alone according to the first two large studies,17–19 the Belgium Netherlands Stent (BENESTENT) study and the North American Stent Restenosis Study (STRESS). These results led to an era of “elective coronary stent” use resulting in an 84% penetration of BMS use in all PCI by 1999.20 The most recent advance in interventionist cardiology was the introduction of drug-eluting stents (DES), which have significantly been proven to be the best currently available therapy to reduce instent restenosis. The sirolimus-eluting stent (Cipher®, Cordis Johnson & Johnson) was the first DES to provide positive clinical results. The Sirolimus-Eluting Stent in De novo Coronary Lesions (SIRIUS) trial was a large, randomized, double-blind study which showed a low incidence of instent restenosis compared with BMS (3.2 vs. 35.4%, respectively; p<0.001).21
Recently, the percutaneous treatment of CAD has had different local options using mechanical techniques with stents and in some cases rotational atherectomy, angioplasty balloons with special characteristics (cutting balloon, drug eluting balloons, etc.) which allow focal treatment of the lesion, and in addition drug options are available with systemic action such as the statins. In spite of the fact that these two therapeutic methods act in different ways, there is growing evidence that suggests that they have a significant positive synergistic effect.
Arterial effects after coronary stents implantationThe microembolization of bioactive particles of the atherosclerotic plaque is a frequent phenomenon following PCI,22–24 producing microinfarcts which favor an inflammatory process characterized by local leukocyte infiltration which negatively affects contractile function.23–26 However, this contractile dysfunction induced by local inflammatory phenomena seems to spontaneously recover within one week following coronary microembolization.27
Stent implantation in coronary circulation inherently causes mechanical trauma in the vessel wall and induces an inflammatory response of varying degrees of severity.28,29 In addition to distal embolization of micro-particles, endothelial stripping and dysfunction, media dissection, and exposure of the plaque components to inflammatory mediators, platelets and coagulation factors are induced.30 Within a few minutes after stent implantation a local invasion of inflammatory cells (macrophages and T cells) is generated, and a small wall thrombus is formed31,32 which is generally self limited by physiological mechanisms and routine antithrombotic medications. The local production of inflammatory mediators stimulates the liver's production of PCR, VSMC and local macrophages from the vessel wall,33,34 reduces the synthesis of nitric oxide (NO) even more, incites thrombotic processes, increases the expression of adhesion molecules, modulates the chemotaxis of monocytes and modifies the local capture of LDL-C by macrophages.35–37 Systemically, a proinflammatory response is seen with the higher levels of PCR.38–40
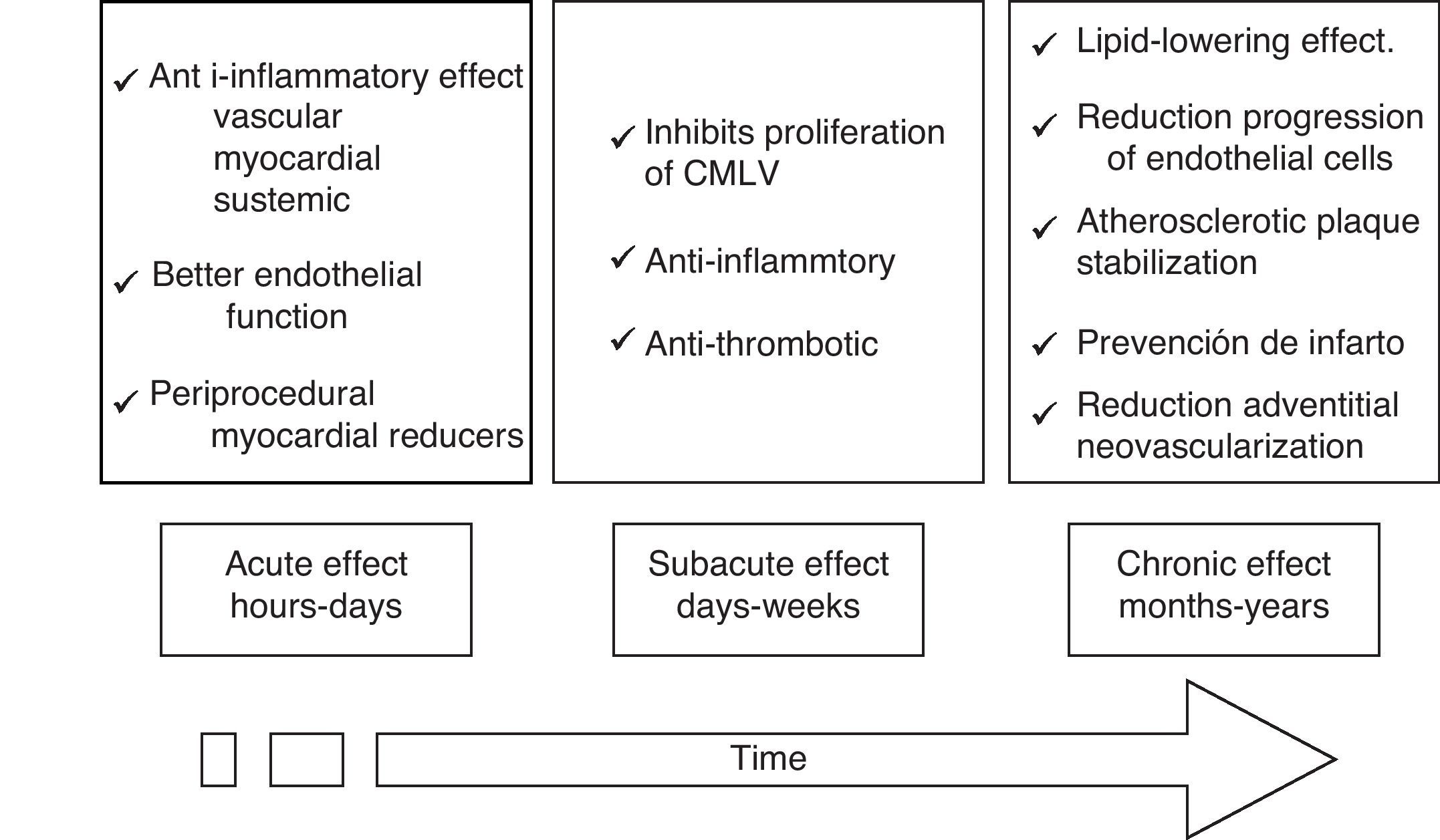
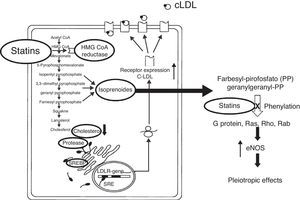
Potential vascular protection mechanisms of statinsWhile the beneficial effects of statins in improving clinical results in patients undergoing PTCA is well established, the mechanisms that seek to explain these findings are less well understood. These mechanisms suggest that there are acute (hours to days), subacute (days to weeks) and chronic (months to years) effects (Fig. 1).
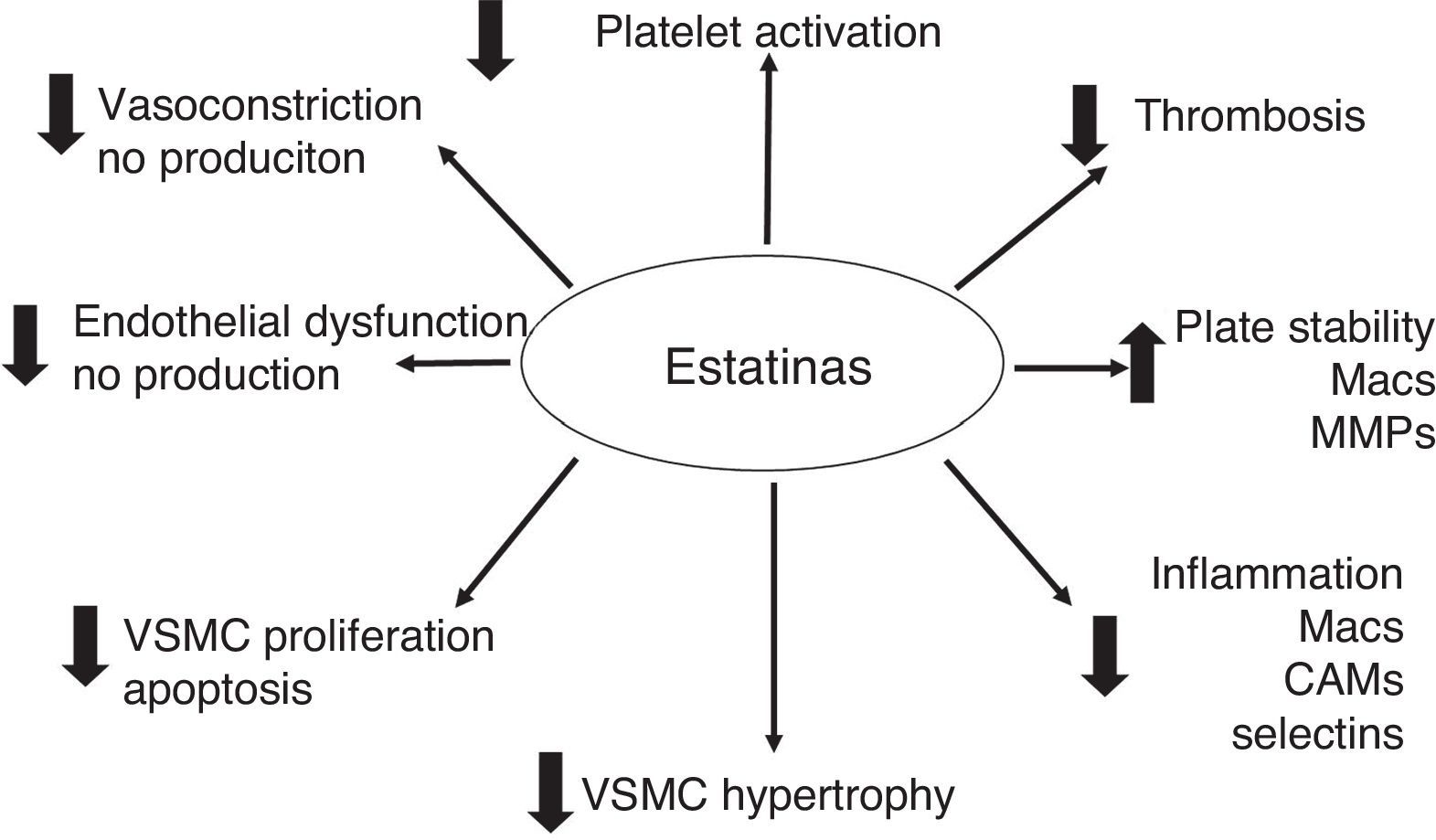
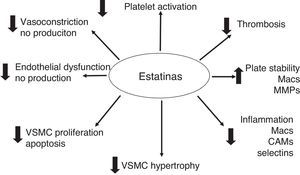
The hypolipemic effect of statins has been widely accepted through all the clinical studies carried out. However, beneficial vascular effects that are independent of the hypolipemic effect, and which have generated a lot of attention, have also been described. These are known as the “pleiotropic” effects, and they have significant antioxidant, anti-inflammatory and anti-thrombotic properties. Experimental and clinical data have shown that statins involve a large number of cellular pathways, reducing the inflammatory response, adhesion of leukocytes, activation of monocytes and macrophages, platelet aggregation and proliferation of VSMC.41–47
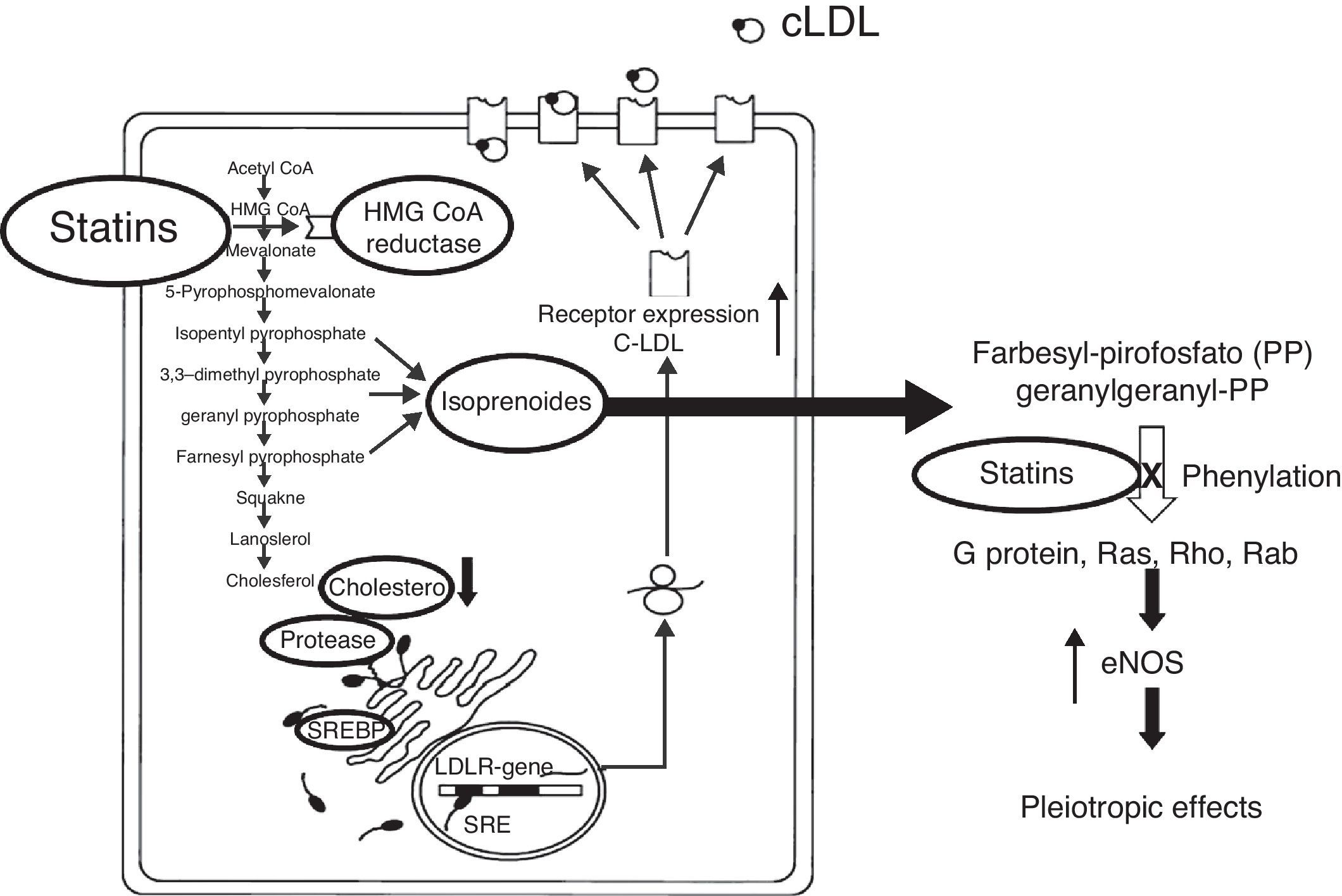
The pleiotropic effects can improve endothelial function by increasing the expression and activity of NO constitutive synthase (eNOS)48 and the bioavailability of NO, stabilizing atherosclerotic plaque, reducing oxidative stress and minimizing the thrombogenic response (Figs. 2 and 3).49–57 At times as early as 3h following the administration of statins, a rapid increase in the bioavailability of NO and a better endothelial response has been demonstrated.54 And inversely, the sudden suspension of statins reduces the bioavailability of NO through a negative feedback mechanism.58 There are a series of cellular mechanisms involved, such as a reduction of caveoline-1, an increase in Hsp90,59 stabilization of the eNOS mRNA,54 a decrease in free radicals,60 phenylation of RhoGTPase by GGPP,61 and activation of serine/treonine kinase Akt.62
Many other pathways of action have been proposed. Among them is the anti-oxidant effect of statins at the vascular level. It has been described that statins reduce the effect of LDL-ox on eNOS, reduce the oxidation of LDL-C, VLDL-C and HDL-C (OH-Atorvastatin metabolites),63 inhibit the capacity of macrophages to oxidize lipoproteins,64 reduce the activity of the macrophage CD36 receptor,65 decrease electronegative LDL-C,62 suppress up-regulation of CD36, SRA-I, SRA-II, and increase the activity of serum paraoxanase (35%).
Atherosclerosis has been considered to be a systemic and inflammatory disease. Statins have proven to have a potent anti-inflammatory effect. They reduce inflammation in hypercholesterolemic and normocholesterolemic animal models,66 they show a reduction in post-transplant vasculopathy, a reduction in hs-CRP levels and a reduction in IL-6 and TNFα levels.67 The systemic effects of statins transcend the arterial vessel wall and manifest themselves in attenuation of serum PCR levels.43,55,68–70
The pleiotropic effects of statins also show results in patients undergoing PCI. The Atorvastatin for Reduction of Myocardial Damage during Angioplasty-Cell Adhesion Moleculaes (ARMYDA-CAMs) substudy43 revealed that pretreatment with statins 7 days prior to PCI attenuates the increase of cellular adhesion molecules (ECAM, VCAM) and E-selectin that are generally elevated following PTCA-stents. This anti-inflammatory effect has been considered a protector for myocardial necrosis. Its exact mechanism has not been made clear, but it seems to be a sum of the anti-inflammatory effects, improvement in endothelial function, reduction in microembolization and stabilization of atherosclerotic plaque.
While elevated PCR levels are known to be predictors for MACE following PCI-stent, it has been reported that statin therapy with these patients reduces PCR levels and attenuates the risk.71,72 In animal models, it has also been demonstrated that the reduction in cholesterol favorably modifies the biology of atherosclerotic plaque, and increases its stability.53 This is due to a reduction in oxidative stress, macrophage and foam cell infiltration, and immature VSMC deposits around the plaque's lipid nucleus. The extracellular matrix metaloproteinases secreted by active macrophages and immature VSMC contribute to the destruction of the fibrotic capsule which protects the lipid nucleus and all the internal prothrombotic components.53,73 The anti-thrombotic effects of statins are evident in reducing the expression of tissue factor (TF),74 thrombin generation reduction,75 attentuation of pro-coagulant factors: fibrinogen, factor V and factor XIII, and reduction in the production and activation of factor VII.76
At the platelet level, statins have a direct cellular effect. They mediate eNOS up-regulation, favor a reduction in the production of A2 thromboxane, modifications in the membrane cholesterol content, inhibit platelet deposits, reduce the formation of platelet thrombi, and inhibit the expression of TF in macrophages (in vitro).77,78 Statins have been shown to have a very interesting effect on the recruitment of mother–progenitor endothelial cells, allowing a regulation of local neovascularization, favoring the differentiation of EPCs and EPC migration in response to VEGF.79
All these cellular effects have been complemented with an “immunomodulation” action. They modulate the immune response allowing recruitment, differentiation, proliferation and secretion of immune cells: monocytes/macrophages, T cells. They inhibit the expression of MHC-II, selective blocking of β2 integrins, direct beneficial effects on: atherosclerosis, rheumatoid arthritis, multiple sclerosis and post-transplant vasculitis.80,81
There is strong evidence to support that lipid reduction with statins is the major protective factor against coronary events.82 With a significant hypolipemic effect (LDL-C reduction) statins can reduce the risk of progression or favor the reduction of plaque volume in an estimated time of 6 months.83–85 Schartl et al.,84 evaluated the effects of statin therapy on plaque composition (lesions<50%) using intravascular ultrasound measurements. In 12 months, the patients who were assigned to atorvastatin therapy and who maintained levels<100mg/dL had a significantly larger increase in the fibrotic content of the plaque (p=0.021). These findings have been proven, demonstrating plaque stabilization, less disease progression and less expression of inflammation biomarkers such as PCR.86,87
The use of more novel technologies (angioscopy, virtual histology, MsCT, MRI, etc.) have allowed the quantification of different components of atherosclerotic plaque: lipid nucleus, fibrotic capsule and tissue and calcium.88,89 It has been shown through these studies how statins reduce plaque predisposition to rupture, microembolization, progression and to induce acute coronary events including death.88–97
Use of chronic statins therapy following PCIThe available evidence suggests that statins improve survival and reduce myocardial infarction (MI) following PCI (Table 1).98–101 However, many of these studies were carried out when stents were just positioning themselves in clinical practice, and therefore a large proportion of the patients included in these studies received balloon PTCA alone. The first long-term benefit description of a statin following PCI was made during the Lescol Intervention Prevention Study (LIPS) using fluvastatin 80mg/day.98 A four year follow-up of hypercholesterolemic patients treated with fluvastatin following their first PCI (64% received a stent) for stable and unstable angina showed a significant reduction in risk of major adverse cardiovascular events (MACE) vs. placebo (relative risk (RR): 0.78; 98% CI: 0.64–0.95; p=0.01).
A National Heart, Lung and Blood Institute Dynamic Registry observational study carried out from 2004 to 2006 evaluated the effects of statins following hospital discharge on the clinical results of 3227 patients undergoing PCI and treated predominantly with DES.102 At one year follow-up, patients with statins (n=2737) were associated with a significant decrease in death (HR:0.58; 95% CI: 0.36–0.93; p=0.02), CABG (HR: 0.49; 95% CI: 0.24–1.00; p=0.05) and repeat revascularization (HR:0.74; 95% CI: 0.56–1.00; p=0.05), compared to the group of patients without statins (n=490). A post hoc analysis of the Treating to New Targets (TNT) study investigated the long-term results (up to 4.9 years) of patients with a history of PCI randomized to receive high doses (80mg/day) or low doses (10mg/day) of atorvastatin.103 Of the 5407 patients studied, the high dose group showed a sustained reduction in the occurrence of the first MACCE and 27% reduction in repeat revascularization, compared to the low dose control group.
In the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study, PCI plus an optimal medical treatment (OMT) did not produce differences compared with OMT alone in patients with stable CAD in mortality (OR: 0.87; 95%CI: 0.65–1.16) and in MI (OR: 1.13; 95% CI: 0.89–1.43).104 Treatment with statins is a fundamental part of OMT, and in this study, the high use of statins (93% in each group) and the aggressive control of LDL-C (at ∼72mg/dL) for 5 years were fundamental. Based on this available information, the American College of Cardiology/American Heart Association Guidelines included the use of statin therapy for the reduction of LDL-C as part of an integrated management for the reduction of cardiovascular risk following PCI.105 In summary, studies have consistently shown a beneficial effect of statins on patients with stable CAD undergoing PCI.
Use of statins in patients with acute coronary syndromes undergoing PCIThe role of early and agressive statin therapy in patients with acute coronary syndromes (ACS) was suggested in the Myocardial Ischemia Reduction with Aggresive Cholesterol Lowering (MIRACL) study.106 Atorvastatin (80mg/day) begun 24–96h after admission and used for up to 16 weeks in patients with ACS (excluding MI with ST elevation) provides a reduction in end points such as death, non-fatal MI, resuscitated cardiac arrest, and emergency hospitalization for symptomatic ischemia, compared with placebo (RR: 0.84; 95% CI: 0.070–1.00; p=0.048). In the PCI-Pravastatin or Atorvastatin Evaluation and Infection Therapy-thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI-22) substudy, 2868 patients who underwent PCI in ACS (both without and with ST elevation) were randomized to intensive statin therapy (atorvastatin 80mg/day) vs. moderate therapy (pravastatin 40mg/day).107 The intensive statin therapy was associated with a significant reduction in death, MI, unstable angina and cerebrovascular accident (21.5% vs. 26.5%; HR: 0.78; 95% CI: 0.67–0.91; p=0.002) and a low incidence of treated vessel revascularization (TVR) and not −TVR (p=0.017).
Atorvastin for reduction of Myocardial Damage During Angioplasty-Acute Coronary Syndromes Study (ARMYDA-ACS), was a study which examined the role of statin therapy in patients with non-STEMI who underwent early PCI.108 Patients who had not been receiving statin therapy were randomized to receive atorvastatin or placebo prior to PCI. The atorvastatin group received a loading dose of 80mg 12h prior to PCI, followed by an additional 40mg dose 2h before the procedure. They were maintained on 40mg/day during follow-up. The incidence of MACE at 30 days was significantly lower in the statin group (5.0 vs. 17%; p=0.01). This difference was mainly due to a reduction in periprocedural MI (7.0 vs. 27%; p=0.001). These ARMYDA-ACS results were later confirmed,109 studying patients with ACS who received rosuvastatin 40mg/day vs. no statin therapy prior to PCI. They showed a similar reduction in MACE at 30 days (6.7% vs. 15.9%; p=0.002), and it was also related to a reduction in periprocedural MI. In contrast with other studies, in this one DES were highly used (>95%).
While most supported information is for non-STEMI, it is limited for patients with MI with ST elevation (STEMI). A study published by Kim et al.110 compared atorvastatin doses, 80mg/day vs. 10mg in 71 patients with STEMI. With high atorvastatin doses they demonstrated a 46% reduction in MACE at 30 days compared to low doses (5.8% vs. 10.6%). It also showed a significant improvement in TIMI arterial flow (p=0.01) with high doses of atorvastatin compared to low doses. In general, the available information suggests that the early use of statins in patients with ACS undergoing PCI is associated with better short term results.
Statins and periprocedural infarctsThe elevation of cardiac biomarkers is relatively common following PTCA.111 It is generally associated with complications during the procedure such as dissection, lateral branch obstruction, distal embolization, and non-reflux phenomenon. Minimal biomarker elevations are generally benign, while significant elevations are associated with adverse results.112–115 Based on this principle, levels more than three times greater than normal cardiac biomarker values have been considered to be a periprocedural MI.116
The efficacy of statins in reducing periprocedural MI's following coronary stent implants has been demonstrated in several clinical studies.116–121 Herrmann et al.119 compared the incidence of periprocedural MI between 229 patients treated with statins (atorvastatin, pravastatin and simvastatin) for at least 1 week prior to PCI with a group of 67 patients without treatment. The statins were correlated with a significant reduction of periprocedural MI (0.4% vs. 6.0%; p=0.01). These findings were confirmed117 when compared with 275 patients undergoing elective PCI who received statins for more than one week with a control group of 150 patients. They also showed a reduction in periprocedural MI (6% vs. 18%; p=0.001).
The first randomized, controlled, double blind study aimed at evaluating the incidence of periprocedural MI was the Atorvastatin for Reduction of Myocardial Damage during Angioplasty (ARMYDA) study.120 This study administered 40mg of atorvastatin per day (n=76) or placebo (n=77) 7 days prior to elective PCI (>90% received a stent implant). A marked reduction in biomarkers (CK-MB) was observed (5% vs. 18%; p=0.025). It should be noted that the protective effect for periprocedural MI in this study was superior with statins than that observed with other medications such as beta blockers, ACE inhibitors and GIIb/IIIa inhibitors. Another randomized, open study118 described the incidence of periprocedural MI (>5 times increase in biomarkers) following treatment with statins begun at least 3 days prior to elective PCI. It showed a beneficial effect of 8% vs. 15.6% (p=0.012). A meta-analysis of 9 studies which investigated periprocedural MI following elective PCI, with a total of 5355 patients,121 showed how statins reduce periprocedural MI (9% vs. 17.5%; OR: 0.45; 95% CI: 0.33–0.62; p<0.01). It should be noted that DES were not used routinely in these studies.
Briguori et al.122 showed in the Novel Approaches for Preventing or Limiting Events (NAPLES II) trial that even a one-time pre-procedural high dose of statins is beneficial in reducing the incidence of periprocedural MI. Patients who were not taking statins previously (n=668) were randomized to receive 80mg atorvastatin within the 24h prior to the procedure and had a low incidence of periprocedural MI vs. those patients who did not receive statins (9.5% vs. 15.8%; OR: 0.56; 95% CI: 0.35–0.89; p=0.014). A similar benefit was achieved, using an oral loading dose of 40mg rosuvastatin 24h prior to the PCI.123 The group treated with rosuvastatin had a low incidence of elevated biomarkers such as CK-MB (0.7% vs. 11.0%; p<0.001) vs. the control group.
Recently, the ARMYDA-RECAPTURE trial124 described the concept of administering additional loading doses of statins. It consisted of a randomized, prospective, double blind clinical trial including 352 patients with stable angina (53%) and STEMI (47%). They received placebo or atorvastatin 12h (80mg) and 2h (40mg) prior to PTCA; they continued with 40mg/day post-procedure. The incidence of MACE at 30 days was reduced from 9.4% to 3.7% (p=0.037), mainly due to a 2.4 times reduction in the incidence of periprocedural MI. The results show that in order to prevent one adverse event (with atorvastatin), 17 patients must be treated.
Statins and slow flowThe phenomenon of slow flow has been a relatively frequent complication in PTCA-stent cases. It is generally associated with interventions during acute coronary syndromes, the presence of thrombi, and venous bridge interventions. The phenomenon of slow flow is associated with anginal episodes during the procedure, ischemic electrocardiographic changes, and when it is not resolved, the presence of increased myocardial trauma biomarkers and infarct. Statins (atorvastatin) in patients undergoing PTCA-stent have been shown to increase coronary blood flow at rest and in hyperemic conditions, increase coronary reserve flow velocity,125 and improve fractional reserve flow after 8 weeks of treatment (p<0.001). Statins have also been shown to improve the function of coronary microcirculation.126
Statins and contrast media induced nephropathyContrast media induced nephropathy (CIN) is recognized as a complication following PCI. It is associated with an increase in early and late cardiovascular events. CIN pathogenesis is not completely known.127 It is thought to be due to inflammatory mechanisms and oxidative stress.
Statins have proven that they could have a beneficial effect on the prevention of CIN by mechanisms such as anti-inflammatory effects and improvement of endothelial function;128 they could modulate renal hypoperfusion, downregulation of angiotensin receptors, a reduction in the synthesis of ET-1, and inhibition of the κB nuclear factor.129 Patti et al.130 developed a randomized, multi-center and double blind study to evaluate the effect of pretreatment with atorvastatin of patients undergoing PCI in acute coronary syndromes on the incidence of CIN (from the AMYDA-CIN – Atorvastatin for reduction of myocardial damage Turing angioplasty-contrast-induced nephropathy trial). In this study (carried out with 241 patients of 1318 recruited), they demonstrated that atorvastatin reduces the incidence of CIN from 13.2% in the placebo group to 5% (p=0.46) and has a significant tendency to maintain normal and high creatinine clearance levels (p=0.034). These benefits were mainly evident in the population of patients with high PCR levels.
Statins and coronary restenosisAmong the non-lipidic effects of statins is the inhibition of vascular smooth muscle cell (VSMC) proliferation. This behavior has caused statins to be considered for the reduction of neointimal formation following PTCA. The first reports when only balloon PTCA was used gave conflicting results.131–134 A multi-center, randomized, double blind study40 evaluated restenosis following PTCA in patients treated with pravastatin (40mg/day) vs. placebo for 6 months. Angiographic follow-up showed no difference in minimal luminal diameter (p=0.21), and binary restenosis (39.2% vs. 43.8%; p=0.26). In contrast, The Regression Growth Evaluation Statin Study (REGRESS) showed a reduction in stenosis at one year follow-up in patients treated with pravastatin vs. placebo (p<0.0001); in addition, clinical restenosis, was lower in the group treated with pravastatin (7% vs. 29%; p<0.001). A meta-analysis of six clinical randomized studies evaluated the risk of new revascularization procedures in a total of 2979 patients.102 Compared with the control group, the group treated with statins for six months significantly reduced the risk of repeat revascularizations by 27% (RR: 0.73; 95% CI: 0.55–0.98; p=0.04).
In the stent era, there is also conflicting evidence supporting the benefit of statins in reducing restenosis.135–139 A first report,135 retrospectively analyzed the available information of patients undergoing stent implantation and 6 month follow-up who were treated with statins (n=258) and those who were not (n=267). The incidence of new revascularization of the treated vessel (TVR) was significantly lower in the statins group (27.9% vs. 36.7%; p=0.04). In addition, the minimal lumen diameter was greater (1.98±0.88 vs. 1.78±0.88mm; p=0.01) and reduction in angiographic restenosis (25.4% vs. 38%; p<0.005). In the study by Bunch et al.,136 patients treated with stents vs. untreated patients did not show a reduction in restenosis (12.6% vs. 13.0%; p=0.12). Likewise, other study137 found no difference in restenosis when they evaluated the use of atorvastatin 10mg/day compared to therapy without a statin (26.8% vs. 36.1%; p=0.177).
At present it is clear that the available evidence is not sufficient to come to conclusions regarding the beneficial effects of statins on the reduction of restenosis, and more currently in the era of DES.
Management guidelines and statins in PCIThe available published clinical evidence allowed the 2011 publication of ACCF/AHA/SCAI Guidelines for Percutaneous Coronary Intervention to consider that the administration of high doses of statins is reasonable prior to PTCA-Stent to reduce the risk of periprocedural MI (Class IIa, level of evidence A for patients without previous consumption of statins, and evidence level B for those on chronic statin therapy).140
ConclusionStents and statins, fortunately for our patients, are commonly used for the treatment of coronary disease, with significant synergistic vascular effects. While stents provide a mechanical and local mechanism in the affected arterial segment, statins have a systemic effect on the disease. The simultaneous use of stents and statins offers the best available therapy for the treatment of coronary disease. Statins in patients undergoing PTCA-stents allow a reduction in symptoms, plaque stabilization and regression, periprocedural MI prevention, periprocedural MACE reduction, prevention of restenosis, prevention of CIN, and improvement of slow flow. The statin with most clinical evidence supported by the literature is atorvastatin. The recommended doses are high. The recommended administration time of prior dose to the procedure is at least 24 h before, and to continue the dose “chronically”.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflicts of interest.