A multidisciplinary panel of cardiologists, neurologists, internal medicine and specialists in haemostasis and thrombosis has elaborated this document showing recent scientific evidences supporting a better profile of direct oral anticoagulants (DOACs) vs vitamin K antagonists (VKA), as well as the indications of specific antidotes and haemostatic agents to reverse the anticoagulant effects of DOACs. The analysis reinforces the best profile of DOACs and its special benefit in patients with basal high haemorrhagic risk.
Se presenta un trabajo multidisciplinar realizado por especialistas de Cardiología, Hemostasia y Trombosis, Medicina Interna y Neurología en el que se exponen las evidencias científicas actuales que demuestran el mejor perfil de seguridad de los anticoagulantes orales de acción directa (ACOD) frente a los antivitamina K (AVK) y se discuten indicaciones y el papel de los antídotos específicos y hemostáticos para la reversión del efecto anticoagulante. El análisis sugiere que el mejor perfil de seguridad de los ACOD los hace especialmente útiles en pacientes con alto riesgo hemorrágico.
This paper, drafted by a multidisciplinary team of specialists in Cardiology, Haematology, Internal Medicine and Neurology, concisely sets out the current scientific evidence demonstrating that direct oral anticoagulants (DOACs) have a better safety profile than vitamin K antagonists (VKAs). Additionally, considering that in Spain's health service, financing of DOAC prescribing is subject to government control, this analysis aims to raise awareness about the preference for the use of DOACs in patients with a high bleeding risk profile, as recommended by the European clinical practice guidelines.1
What type of patients do we normally see in the clinic with atrial fibrillation and requiring anticoagulation?They are usually patients with atrial fibrillation (AF) who do not have moderate-to-severe mitral stenosis or mechanical valve prostheses, but very often have structural heart disease and other comorbidities, with a high risk of complications such as stroke, heart failure and dementia and a high mortality rate. They tend to be patients with a high CHA2DS2-VASc score who require oral anticoagulation (OAC) and very often have a high risk of bleeding, either estimated by a score ≥3 on the HAS-BLED score1 or by the presence of modifiable bleeding risk factors.1 Ischaemic stroke is one of the main consequences of AF, with AF responsible for approximately 20% of all ischaemic strokes, even if silently. This type of stroke is generally serious and has a poor functional prognosis, generating a high rate of admissions and a considerable increase in healthcare costs.2,3 To prevent complications of AF, depending on the thrombotic risk estimated by the CHA2DS2-VASc score,1 the patient needs to be started on indefinite OAC. However, the treatment itself is not free of complications, such as major bleeding and the dreaded intracranial haemorrhage (ICH).
DOACs have a better safety profile than VKAs,1 and it would therefore seem wise for patients with a high baseline bleeding risk to be given DOACs when starting anticoagulant therapy; examples are patients with extensive stroke or added acute coronary syndrome. The 2016 modification of the DOAC prescribing document already includes the preferred use of DOACs in one particular case: patients with high thrombotic risk and a HAS-BLED ≥3.
Safety profile of direct oral anticoagulantsThe main studies and the meta-analysis of these studies confirm that stroke prevention in patients with AF is a safer strategy, especially for prevention of brain haemorrhage and major life-threatening bleeding, when the four available DOACs are used as opposed to VKAs (Table 1).
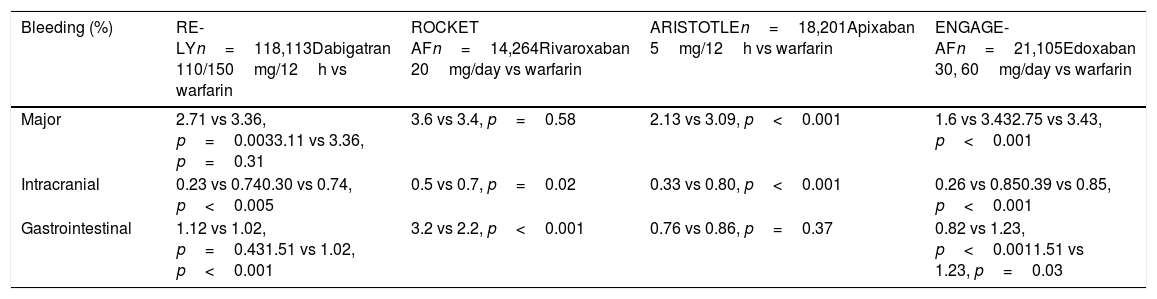
Safety results from pivotal studies of DOACs vs warfarin.
| Bleeding (%) | RE-LYn=118,113Dabigatran 110/150mg/12h vs warfarin | ROCKET AFn=14,264Rivaroxaban 20mg/day vs warfarin | ARISTOTLEn=18,201Apixaban 5mg/12h vs warfarin | ENGAGE-AFn=21,105Edoxaban 30, 60mg/day vs warfarin |
|---|---|---|---|---|
| Major | 2.71 vs 3.36, p=0.0033.11 vs 3.36, p=0.31 | 3.6 vs 3.4, p=0.58 | 2.13 vs 3.09, p<0.001 | 1.6 vs 3.432.75 vs 3.43, p<0.001 |
| Intracranial | 0.23 vs 0.740.30 vs 0.74, p<0.005 | 0.5 vs 0.7, p=0.02 | 0.33 vs 0.80, p<0.001 | 0.26 vs 0.850.39 vs 0.85, p<0.001 |
| Gastrointestinal | 1.12 vs 1.02, p=0.431.51 vs 1.02, p<0.001 | 3.2 vs 2.2, p<0.001 | 0.76 vs 0.86, p=0.37 | 0.82 vs 1.23, p<0.0011.51 vs 1.23, p=0.03 |
The study Randomised evaluation of long-term anticoagulation therapy (RE-LY),4 comparing dabigatran to warfarin, included 18,113 patients and studied two doses of the drug: 110 and 150mg/12h. The safety results showed the incidence of major bleeding to be similar to warfarin at the dose of 150mg and a 20% reduction at 110mg/12h. The incidence of intracranial haemorrhage was significantly lower with both doses of dabigatran. Major gastrointestinal bleeding increased by 48% with the 150mg/12h dose.
The study Rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF)5 included 14,264 patients in whom rivaroxaban 20mg/day was compared to warfarin, and a similar incidence of major bleeding was found in the rivaroxaban and warfarin groups. Fatal bleeding, intracranial bleeding and bleeding in critical organs were less common in the rivaroxaban group. The rate of major gastrointestinal bleeding was 46% higher with rivaroxaban than with warfarin.
In the study Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE),6 which included 18,201 patients with AF and at least one stroke risk factor, apixaban 5mg/12h was compared to warfarin, and a significant 31% greater reduction in major bleeding was found in the apixaban group. The incidence of ICH was significantly lower with apixaban. The relative risk reduction of ICH was even greater in patients with a HAS-BLED ≥3 vs HAS-BLED 0–1 (HR: 0.22 [95% CI: 0.10–0.48] vs 0.66 [0.39–1.12], p=0.064). In addition, although no significant difference was found between apixaban and warfarin in the incidence of gastrointestinal bleeding, it was 11% lower with apixaban.
Edoxaban, at doses of 60 and 30mg/day, was compared to warfarin in the study Effective anticoagulation with factor Xa next generation in atrial fibrillation—thrombolysis in myocardial infarction (ENGAGE-AF-TIMI 48),7 which included 21,105 patients. The annual incidence of major bleeding and intracranial haemorrhage was significantly lower with both doses of edoxaban. The annual rate of major gastrointestinal bleeding was higher than with warfarin at the higher dose of edoxaban, but lower at the lower dose.
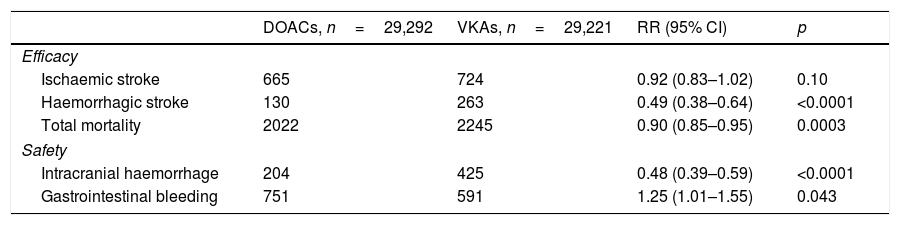
Lastly, a meta-analysis was found that included 71,683 patients from the four main studies, which has made it easier to understand the role of DOACs in stroke prevention in patients with AF. Ruff et al.8 showed that DOACs significantly reduce stroke and systemic embolism by 19% compared to warfarin (RR: 0.81; 95% CI: 0.73–0.91; p<0.0001), mainly due to the decrease in haemorrhagic stroke. DOACs also significantly decrease all-cause mortality and intracranial haemorrhage, but lead to an increase in gastrointestinal bleeding (Table 2).
Meta-analysis comparing events between DOACs and VKAs.
| DOACs, n=29,292 | VKAs, n=29,221 | RR (95% CI) | p | |
|---|---|---|---|---|
| Efficacy | ||||
| Ischaemic stroke | 665 | 724 | 0.92 (0.83–1.02) | 0.10 |
| Haemorrhagic stroke | 130 | 263 | 0.49 (0.38–0.64) | <0.0001 |
| Total mortality | 2022 | 2245 | 0.90 (0.85–0.95) | 0.0003 |
| Safety | ||||
| Intracranial haemorrhage | 204 | 425 | 0.48 (0.39–0.59) | <0.0001 |
| Gastrointestinal bleeding | 751 | 591 | 1.25 (1.01–1.55) | 0.043 |
We also found two studies conducted in the high-bleeding-risk population of patients with revascularisation and stent implantation due to coronary heart disease, PIONEER9 with rivaroxaban and REDUAL10 with dabigatran, which demonstrate a better safety profile with dual antithrombotic therapy compared to the standard triple therapy.
Real world data confirm the better DOAC safety profileThere are now multiple real-life studies available, plus a recent meta-analysis with a substantial number of patients on dabigatran, rivaroxaban and apixaban,11 as there are no data yet on edoxaban. The meta-analysis included 28 rigorous studies comparing VKAs and DOACs in patients with AF: 24 with dabigatran, 14 with rivaroxaban and 7 with apixaban.
The results on the safety of the anticoagulants are as follows:
DabigatranDabigatran was significantly associated with a lower incidence of intracranial haemorrhage in 12 studies with a total of 600,855 patients (HR: 0.42; 95% CI: 0.37–0.49). There were no significant differences in major bleeding between dabigatran and VKAs in 13 studies with a total of 348,896 patients (HR: 0.83; 95% CI: 0.65–1.05).11 Major gastrointestinal bleeding was investigated in 10 studies with 537,770 patients and the incidence of gastrointestinal bleeding was higher with dabigatran vs VKAs (HR: 1.20; 95% CI: 1.06–1.36).
RivaroxabanRivaroxaban was associated with a significantly lower incidence of intracranial haemorrhage compared to warfarin in seven studies with a total of 136,221 patients (HR: 0.64; 95% CI: 0.47–0.86).11 No significant differences were found between rivaroxaban and VKAs in the incidence of major bleeding in eight studies with 167,532 patients (HR: 1.00; 95% CI: 0.92–1.08).11 Four studies with 71,368 patients were identified whose results confirmed a significantly higher incidence of gastrointestinal bleeding with rivaroxaban vs VKAs (HR: 1.24; 95% CI: 1.08–1.41).11
ApixabanWhen compared to VKAs, apixaban was associated with a significantly lower incidence of intracranial haemorrhage in four studies with 66,482 patients (HR: 0.45; 95% CI: 0.31–0.63),11 a lower incidence of major gastrointestinal bleeding in two studies with 33,323 patients (HR: 0.63; 95% CI: 0.42–0.95)9 and a lower rate of major bleeding in four studies with 89,036 patients (HR: 0.55; 95% CI: 0.48–0.63).11
In summary, this real-life meta-analysis shows that, compared to VKAs, DOACs are uniformly associated with a lower incidence of intracranial haemorrhage, with a relative reduction ranging from 36% for rivaroxaban to 58% for dabigatran. In terms of major bleeding, apixaban was associated with a lower incidence than VKAs, and rates were similar for rivaroxaban and for dabigatran. Rates of gastrointestinal bleeding are higher with dabigatran and rivaroxaban and lower with apixaban than with VKAs. These data reinforce the validity of the evidence from the main studies.
What do the guidelines say?The current European Society of Cardiology guidelines1 recommend the preferential use of DOACs over VKAs in patients with AF starting OAC therapy (class I recommendation and level of evidence A). This recommendation is based on several advantages that DOACs have over VKAs, such as better adherence to treatment, limited drug–drug and drug–food interaction and no need for dose adjustment and monitoring, but mainly and fundamentally due to the significant decrease in intracranial haemorrhage caused by DOACs compared to VKAs.
The Sociedad Española de Cardiología [Spanish Society of Cardiology] adopts the recommendations of these guidelines for the management of antithrombotic drugs in AF here in Spain. However, the financing of DOACs prescribing in Spain is subject to a national therapeutic positioning report and separate approval by the different autonomous regions, which means it is important to very clearly define which population benefits most from DOACs.
As we stated above, the scientific evidence on the four DOACs is consistent in demonstrating a better safety profile than VKAs, in particular for the reduction in brain haemorrhages. Therefore, although there are no studies for the specific population at high bleeding risk, it seems reasonable to suggest that patients with AF, and especially those with high bleeding risk, are candidates for preferential prescribing of DOACs when first starting therapy.12
Cerebral haemorrhage and safety profile of direct oral anticoagulantsCerebral haemorrhage (CH) is the most serious complication of anticoagulant therapy, and 10–25% of all CHs occur in patients treated with VKAs. This becomes even more serious because inhibition of coagulation carries a greater risk of haematoma growth and higher mortality rates.13
The increasingly widespread use of anticoagulation in older patients with AF has led to an increase in secondary CH. Therefore, the use of anticoagulants with a lower associated bleeding risk should be prioritised in these patients, especially in those with a history of previous cerebral bleeding.
DOACs are likely to change this trend, as they have shown lower risk of CH than VKAs. The meta-analysis of the clinical trials has confirmed the net benefit of DOACs in all subgroups of patients, with a relative risk reduction of CH of approximately 50% compared to VKAs, although with a higher risk of gastrointestinal bleeding.8 In real life, the use of DOACs in patients with AF confirms the better profile compared to VKAs, with even more favourable data than in clinical trials.11
When using DOACs it is important to prescribe appropriate doses taking into account the factors that increase CH: advanced age, body weight less than 50kg, impaired renal function, thrombocytopenia and concomitant use of anti-platelet agents, inhibitors of P-glycoprotein (amiodarone, quinidine, verapamil) or serotonin reuptake inhibitors (with dabigatran). As far as the concomitant use of antiplatelet agents is concerned, a meta-analysis of the four main DOAC trials comparing patients on dual therapy with antiplatelet agents to patients treated with DOAC monotherapy concluded that the combination does not reduce thromboembolic events and increases the risk of bleeding complications.14
In the absence of studies that directly compare the safety profile of the different DOACs, a meta-analysis of six studies concluded that dabigatran, rivaroxaban and apixaban reduce the risk of intracranial bleeding in AF, with no significant differences between them, and that any of them can be used in patients with a high risk of bleeding.15
Specific factors and management of cerebral haemorrhage associated with DOACsAlthough DOACs are associated with a lower risk of CH, the number of CH related to these drugs is increasing as the number of treated patients increases.16,17 The factors classically related to poor prognosis of CH are the same for DOACs; essentially, clinical severity at admission, the size of the haematoma, its early growth and the presence of intraventricular blood.18
Several studies show that DOAC-related CH is equally serious, with clinical course, prognosis and mortality rates similar to those caused by VKAs or antiplatelet agents19; haematoma growth in the first few days in 38% of cases and secondary increase in intraventricular blood in 18%, with an associated mortality or dependence rate of 65%. However, in another prospective multicentre registry it was found that patients with DOAC-related CH had milder initial neurological involvement and smaller initial haematoma size compared to those with VKA-related CH. In that study, a meta-analysis of observational studies was also conducted, concluding that the prognosis at three months is better in the case of DOAC haemorrhages, with a lower mortality rate.20 In short, it seems that CH caused by DOACs are not more serious and do not have a worse prognosis than those caused by VKAs.
In the specific case of CH caused by oral anticoagulants, there is always the possibility of active treatment by reversing the anticoagulation. Treatment guidelines recommend discontinuing the anticoagulant drug and reversing its effect as soon as possible. However, there is no evidence of an associated improved outcome.18 The treatment of anticoagulant-related bleeding is described in detail below.
Reintroduction of oral anticoagulation in patients with a history of cerebral haemorrhage. Recommendations on the use of direct oral anticoagulantsDeciding in which patients the anticoagulant therapy can be reintroduced and when to do so is no simple task. The clinical guidelines currently recommend making decisions on an individual basis according to thrombotic and bleeding risk and patient characteristics, and reintroducing the anticoagulation in cases of high risk of thromboembolism21 (Table 3). To assess the risk of bleeding recurrence, the recommendation is to consider the risk factors in the HAS-BLED score,1 with particular focus on the control of blood pressure. Certain characteristics of haemorrhages may be associated with an increased risk of recurrence, such as lobar location, microbleeds suggestive of amyloid angiopathy (the guidelines do not recommend reintroducing anticoagulation in these cases) and the radiological signs of vascular leukoencephalopathy or leukoaraiosis. Previous history of heart failure and diabetes is also associated with increased risk.21
Factors that influence the decision to reintroduce anticoagulant treatment after cerebral haemorrhage.
| In favour of reintroducing anticoagulant | Against reintroducing anticoagulant |
|---|---|
| Deep location of the haematomaHigh CHA2DS2-VAScLow HAS-BLEDIndication for secondary prevention (previous ischaemic stroke)Mechanical valveHypercoagulable stateWell controlled blood pressure | Lobar location of the haematomaMicrobleeds on MRILow CHA2DS2-VAScHigh HAS-BLEDPoorly controlled HTN |
HTN: hypertension; MRI: magnetic resonance imaging.
Recent studies show that the reintroduction of anticoagulation in patients with AF after a CH is associated with a significant reduction in the risk of ischaemic stroke and death by any cause, compared to sustained discontinuation or replacement by antiplatelet agents, although there is no significant difference in the risk of bleeding recurrence between the different treatment groups. A meta-analysis of three observational studies also concludes that the reintroduction of anticoagulation is associated with better functional outcome for patients, even with amyloid angiopathy, if the risk of thromboembolism is high.22 In view of these data, it seems that the reintroduction of anticoagulation is safe and, contrary to the current recommendations in the guidelines, anti-platelet agents would not be a useful alternative. In patients with high bleeding risk and contraindication for DOACs, percutaneous left atrial appendage closure could be an alternative to OAC, and although the available studies are inconclusive, a subsequent period of six months of dual anti-platelet therapy is required; the recommendation in the guidelines is class IIB.
As patients with a history of CH were excluded from the main DOAC trials, and given that there are few cases of treatment with DOACs included in observational studies, there is little information on the utility of these drugs in such situations and we will have to wait for the results of ongoing randomised studies. However, DOACs are an alternative in patients with AF who survive VKA-induced CH given their lower bleeding risk, similar to that of aspirin alone.11
The timing of the reintroduction of the anticoagulant is also not clear, with discrepancies in the few available studies. The guidelines recommend from four to eight weeks after cessation of bleeding and consider the possibility of early reintroduction (two weeks) in cases of high risk of thromboembolism (Table 3).
Gastrointestinal bleeding with direct oral anticoagulantsGastrointestinal bleeding (GIB) is the most common haemorrhagic complication of anticoagulant therapy.23 Post hoc studies, different meta-analyses and numerous real-life studies have confirmed the findings of the main DOAC trials. In comparison with warfarin, major GIB is more common with dabigatran at 150mg every 12h and with both rivaroxaban and edoxaban at 60mg per day, but not with apixaban. In the pathogenesis of DOAC-related GIB, in addition to their systemic anticoagulant effect, which predisposes to bleeding from any pre-existing lesion in the gastrointestinal tract mucosa, also involved are a topical anticoagulant effect due to incomplete absorption of the drug, a direct caustic effect in the case of tartaric acid with dabigatran, and an inhibitory effect on mucosal healing. Compared to major GIB related to warfarin, anti-platelet agents or non-steroidal anti-inflammatory drugs (NSAIDs), bleeding related to dabigatran (and possibly the other DOACs) most often occurs in the lower gastrointestinal tract, usually the small intestine. The association of major GIB with advanced age in patients taking DOACs may be suggestive of topical anticoagulant activity exerted on frequent, vulnerable and asymptomatic lesions, such as erosions in mucous membranes or angioectasia.24
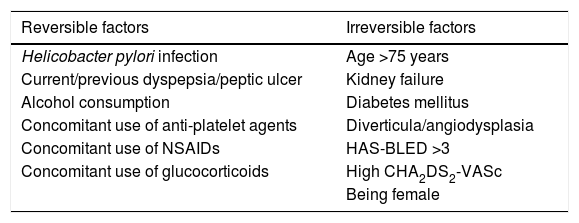
The risk of GIB with DOACs increases with reversible and treatable and non-reversible factors (Table 4). A new predictive scale model has been developed for the risk of acute GIB in patients taking OACs based on five factors (non-use of proton pump inhibitors [PPIs], chronic kidney disease, COPD, history of peptic ulcer and liver cirrhosis), and it has proved to be superior to the HAS-BLED score.25 In contrast, the use of PPIs reduces the risk of GIB in patients receiving DOACs, particularly dabigatran. This association is stronger for upper GIB than lower GIB and in patients with a history of peptic ulcer or previous GIB.
Factors that increase the risk of gastrointestinal bleeding with DOACs.
| Reversible factors | Irreversible factors |
|---|---|
| Helicobacter pylori infection | Age >75 years |
| Current/previous dyspepsia/peptic ulcer | Kidney failure |
| Alcohol consumption | Diabetes mellitus |
| Concomitant use of anti-platelet agents | Diverticula/angiodysplasia |
| Concomitant use of NSAIDs | HAS-BLED >3 |
| Concomitant use of glucocorticoids | High CHA2DS2-VASc |
| Being female |
Gastrointestinal contraindications for the use of anticoagulants are: active bleeding, active ulcers, haemorrhagic angiodysplasia, recurrent bleeding requiring repeated transfusions, the presence of potentially haemorrhagic gastrointestinal lesions inaccessible to endoscopic or surgical treatments and liver cirrhosis in Child–Pugh grade C. A previous history of GIB entails a risk of recurrence but does not definitively contraindicate anticoagulation. However, most gastrointestinal contraindications for the use of DOACs are temporary.26
If an anticoagulated patient has an episode of GIB, the timing of the endoscopy will depend on the severity of the GIB and the patient's haemodynamic status. In stable patients, endoscopy can be delayed 12–24h, which will increase visualisation and the safety and effectiveness of the intervention, and ensure better cleansing of the colon in lower endoscopy. In contrast, in patients with severe and haemodynamically unstable GIB, endoscopy should be performed at the earliest opportunity after stabilisation. Radiological or surgical procedures, or both, are the last resort when endoscopic treatment fails.26
The decision to resume DOAC therapy and the optimal time for doing so after GIB should be made on an individual and often multidisciplinary basis, and depends on the thromboembolic risk, the risk of bleeding recurrence and the DOAC indication. In the case of venous thromboembolism (VTE), if ≥3 months have elapsed since the acute episode and there has been no recurrence, it was not severe thrombophilia or a massive or sub-massive pulmonary embolism, definitive discontinuation of anticoagulant therapy should be considered. In most cases of non-valvular atrial fibrillation (NVAF), if the cause of GIB has been resolved, DOAC therapy can and should be resumed as soon as possible.26 In addition, if the patient was taking rivaroxaban, dabigatran at a dose of 150mg every 12h or edoxaban, the treatment should be changed to apixaban, with a lower risk of GIB in any age group. In the over-75s, the dose should be carefully adjusted to renal function, which is often fluctuating.
One in every 12 major cases of GIB in patients with AF who take VKA or dabigatran is caused by an underlying cancer. Performing the faecal occult blood test for all patients on DOACs has been recommended, particularly during the first period of treatment. Frank or occult GIB in a patient receiving anticoagulant therapy always requires investigation.27
Although GIB can be serious, the clinical repercussions are never as severe as with cardioembolic stroke or ICH, and in general the net benefit is considered to be in favour of DOACs.28 The mortality rate for GIB related to anticoagulants is generally low, and may be less serious in patients taking DOACs.29
What to do in the event of bleeding?Patients receiving DOACs may require rapid neutralisation of anticoagulant activity where bleeding is life-threatening or in critical organs (intraocular, intraspinal, retroperitoneal, etc.), or persists despite haemostasis measures, and in cases of emergency surgery in patients with high bleeding risk. The initial assessment of these patients requires the severity of the bleeding and the time of the last DOAC dose to be determined, along with monitoring of renal function and of coagulation, using specific tests to determine the intensity of anticoagulation, such as diluted thrombin time and ecarin clotting time for dabigatran and anti-Xa activity in the case of factor Xa inhibitors.30,31 For the treatment of bleeding, the half-life (approximately 12h in adults) and the degree of renal elimination of the drug (higher with dabigatran) should also be considered. The treatment will depend on the severity of the condition: in mild bleeding, discontinuing the drug accompanied by local haemostasis will be sufficient; in moderate bleeding, in addition to surgical haemostasis and support measures (fluid therapy, haemodynamic support and blood products), gastric lavage will be considered if ingestion was in the previous 2h, and haemodialysis if dabigatran was administered; in severe or life-threatening haemorrhages, in addition to discontinuing the DOAC and supportive measures, surgery or embolisation, dialysis (if dabigatran) and the use of antidotes should be considered31,32 (Table 5).
Treatment of the bleeding in patients on DOACs.
| Review |
| Stop anticoagulation |
| Time of last dose |
| Previous medications |
| Coagulation parameters |
| Renal function |
| Fluid therapy |
| Blood transfusion |
| Remove |
| Gastric lavage |
| Activated charcoal |
| Dialysis (dabigatran) |
| Repair |
| Surgical haemostasis |
| Reverse |
| Dabigatran: idarucizumab |
| Factor Xa inhibitors: prothrombin complex concentrate |
The therapeutic options for the reversal of DOACs tend to be non-specific agents, such as prothrombin complex concentrates, whose efficacy and safety are limited, with only very few series showing that they are partially effective and quite safe in the control of bleeding due to factor Xa inhibitors.
Among the specific antidotes, currently only idarucizumab (Praxbind®) has obtained approval here in Spain for clinical use in the reversal of dabigatran. Idarucizumab is a humanised monoclonal antibody which specifically inhibits the anticoagulant effect of dabigatran. In the REVERSE-AD phase III study, a rapid reversal of the anticoagulant effect induced by dabigatran was found when idarucizumab was administered at a dose of 5g intravenously, with cessation of bleeding at 3–5h. At 90 days, 6.3% of the patients had thrombotic complications. The results of this study indicate that idarucizumab is very effective for the control of dabigatran-related bleeding if administered early.33 Consequently, idarucizumab is the recommended agent for dabigatran reversal in cases of severe bleeding or emergency surgery, while the efficacy of agents used for VKA reversal, such as prothrombin complex concentrates, is still open to debate. Clinical trials are currently being carried out with specific antidotes for factor X inhibitors. One such is andexanet alfa, which is very advanced and was recently approved by the FDA. Also being tested are small molecules, such as ciraparantag, which is also active against heparins, and fondaparinux.
In summary, DOAC antidotes would be indicated in patients with major or life-threatening bleeding, in haemodynamically unstable patients, patients with impaired renal function or patients who do not respond to supportive measures.34
ConclusionsThis review confirms that DOACs have a better safety profile than VKAs in patients with AF who require anticoagulant therapy. In patients at high risk of bleeding, initial anticoagulant therapy with DOACs should be considered.
Conflicts of interestNone.
Cardiovascular Thrombosis Group of the Sociedad Española de Cardiología [Spanish Society of Cardiology]: Francisco Marín, José Luis Ferreiro, Vanessa Roldán, David Vivas.
Neurology: Ana Morales.
Internal Medicine: Luciano López Jiménez, José María Cepeda Rodrigo
Haematology: Pere Domenech.
The members of the Cardiovascular Thrombosis Forum are listed in Appendix A.
Please cite this article as: Roldán Rabadán I, Alonso de Leciñana M, Barba Martín R, Páramo Fernández JA, por el Foro de Trombosis Cardiovascular. Perfil de seguridad de los anticoagulantes directos. Uso preferente en fibrilación auricular. Clín Investig Arterioscler. 2019;31:263–270.