Retinal vein occlusion (RVO) is mainly related with vascular risk factors (VRF).
ObjectivesTo analyze the lipid profile and serum folate, vitamin B12 and homocysteine levels, in patients with RVO and a population-based control group.
Patients and methodsCase–control study. Patients with RVO were assessed during an 11-year period.
ResultsWe included 368 patients and 325 controls of similar age and sex. HDL cholesterol and folate levels were lower (52 [43–63]mg/dL vs. 55 [46–66]mg/dL; p=0.016 and 7 [5–10]ng/mL vs. 9 [7–13]ng/mL; p<0.0001, respectively) and non-HDL cholesterol and homocysteine levels higher (148.9±37.3mg/dL vs. 142.9±34.5mg/dL; p=0.03 and 13.4 [11.2–18.2]μmol/L vs. 11.1 [9.0–14.4]μmol/L; p<0.001) in patients with RVO than controls. Although total cholesterol, LDL-C, and triglyceride levels were higher and serum vitamin B12 levels were lower in RVO patients, these differences did not reach statistical significance.
ConclusionsRVO-patients have lower serum HDL-C and folate levels and higher non-HDL-C and serum homocysteine levels than population-based controls of similar age and sex. In patients with RVO, apart from the lipid profile, determination of serum homocysteine, folate and vitamin B12 levels might be useful, as well as the treatment of their alterations.
La obstrucción venosa retiniana (OVR) se relaciona fundamentalmente con los factores de riesgo vascular (FRV).
ObjetivosAnalizar el perfil lipídico y las concentraciones séricas de ácido fólico, vitamina B12 y homocisteína, en pacientes con OVR y un grupo control poblacional.
Pacientes y métodosEstudio de casos y controles. Los pacientes con OVR fueron estudiados a lo largo de un periodo de 11 años.
ResultadosSe incluyeron 368 casos y 325 controles de similar edad y sexo. En los pacientes con OVR respecto a los controles, las concentraciones de HDL colesterol y de ácido fólico fueron menores (52 [43-63]mg/dL vs. 55 [46-66]; p=0,016 y 7 [5-10]ng/mL vs. 9 [7-13]; p<0,0001, respectivamente) y las de colesterol no HDL y de homocisteína fueron mayores (148,9±37,3mg/dL vs. 142,9±3; p=0,03 y 13,4 [11,2-18,2]μmol/L vs. 11,1 [9,0-14,4]; p<0,0001). Aunque las cifras de colesterol total, cLDL y triglicéridos fueron mayores y las concentraciones de vitamina B12 fueron menores en los pacientes con OVR, estas diferencias no fueron significativas.
ConclusionesLos pacientes con OVR tienen concentraciones más bajas de colesterol HDL y de ácido fólico y cifras de colesterol no HDL y homocisteína más elevadas que los controles poblacionales de similar edad y sexo. En estos pacientes, además del perfil lipídico, podría ser útil la determinación de la homocisteína, el folato y la vitamina B12, así como el tratamiento de sus alteraciones.
Retinal vein occlusion (RVO) is the second cause of retinal impairment after diabetes mellitus and is therefore a major cause of sight loss.1,2 Depending on the degree of retinal occlusion it is classified as central or peripheral, with the latter being three times more prevalent.3 RVO is essentially related to major vascular risk factors (VRF), especially with high blood pressure (HBP) and as such RVO is considered to be a manifestation of atherosclerosis. Dyslipidaemia is a well defined RVO-associated VRF but the importance of lipid profile characteristics and hypolipemiant treatment in this disease are little known.4,5
However, thrombophilia has been implicated in RVO pathogenesis, although with lower relevance than VRF. Here the importance of acquired thrombophilia stands out, defined by antiphospholipid syndrome and by hyperhomocysteineaemia.6–8 The latter, related both to arthrosclerosis and thrombophilia, is usually the consequence of a deficiency of folate and to a lesser extent of vitamin B6 and B12.9 However, the relationship between serum folate levels and vitamin B12 with the development of RVO10,11 has not been well defined and to our knowledge, there are no existing studies in this regard in Spain.
Based on the above, the aim of this study was to analyse the lipid profile and serum folate levels, vitamin B12 and homocysteine in patients with RVO and in a control group of the general population similar in age and sex.
Patients and methodsAll patients diagnosed with RVO by the Ophthalmology Unit from December 2008 to October 2019, who had been referred to the Internal Medicine Unit (Valdecilla cohort) were studied, based on clinical, fundoscopic and angiographic criteria, together with a control group of people selected consecutively from the annual follow-up check-up of a prospective cohort with a population base (Camargo cohort).12,13 The study took place in the Hospital Universitario Marqués de Valdecilla de Santander, a tertiary benchmark hospital serving a population of 350,000 inhabitants in Cantabria.
All patients were assessed at the Internal Medicine practice within the first 10 days after diagnosis of RVO and in the initial consultation a baseline laboratory study was performed. In the patients with RVO, treatment of the VRF was optimised so that approximately 90% of our patients presented with an LDL cholesterol level <100mg/dL and a BP level <140/80 one year after baseline visit. They were prescribed with vitamin B12, folic acid or both if a deficiency in either was detected. In the case of a finding of hyperhomocysteineaemia (>15μmol/L) with normal folic acid and vitamin B12, levels, a daily tablet of 400μg of folic acid and 2μg of cyanocobalamin for at least three months was prescribed. We did this because we had observed, at the beginning of the study, that in 10 patients with hyperhomocysteineaemia and no evidence of a deficit of folic acid or vitamin B12, the said treatment during three months corrected the hyperhomocysteineaemia en 80% of them.
Data collectionData collection was performed using a standardised questionnaire on a computerised database. The said questionnaire included demographic data (age and sex), clinical data and laboratory test results. The study was approved by the regional ethics committee and all subjects gave their informed consent.
Clinical variablesThe clinical variables recorded were: the differentiation between central or peripheral RVO (temporal or nasal), weight, height, tobacco habit, the presence of high blood pressure (blood pressure higher or equal to 140. 90mmHg or receiving antihypertensive pharmacological treatment), diabetes mellitus, in keeping with the American Diabetes Association14 criteria, dyslipidaemia (total cholesterol or triglycerides above 230mg/dL and 150mg/dL, in at least two determinations after 24h fasting or receiving treatment with hypolipidaemic agents and treatment with statins.
Laboratory parametersBlood samples were taken from the anti-cubital vein between 8:00 and 10:00 a.m., with a period of 12h fasting. The biochemical profile, was carried out in the biochemical laboratory of the same centre with an Advia® 2400 (Siemens) self-monitor and included the determination of: lipid profile (total cholesterol, cholesterol linked to high-density LDL lipoproteins, cholesterol linked to low-density lipoproteins (HDL), non-HDL cholesterol and triglycerides expressed in mg/dL). Several arthrogenic indexes (AI) were estimated: total/HDL cholesterol, LDL/HDL cholesterol, non-HDL/HDL cholesterol, triglycerides/HD.15
Plasma levels of focal acid and vitamin B12 were determined through competitive immunoassay by direct chemiluminiscence (Advia Centaur by Bayer) with reference values of 2.6–20ng/mL for folic acid and 211–911pg/mL for vitamin B12.
Serum homocysteine levels (μmol/L), were initially determined using a nephelometer (Siemens). From June 2012 the technique was changed and levels were determined through chemiluminiscence (Immulite 2000 Xpi; Siemens). Extraction carried out in the hospital was kept on ice, analysed before 3h had passed and was considered high if they were above 15μmol/L. To prevent variations attributable to the change of technique we excluded those made by nephelometer and only analysed the homocysteine made through chemiluminiscence, in patients and in controls. In most patients and controls the folic acid and vitamin B12 controls were determined from June 2012. Prior to this date they were only analysed in patients where there was clinical suspicion or through laboratory findings of said vitamin deficiency.
Statistical analysisQuantitative variables were expressed as a mean±standard deviation or median and interquartile range and the qualitative variables as percentages. Quantitative variables were analysed using the Student's t test or the Mann–Whitney U test, depending on parameter distribution and qualitative variables with the Exact Fisher or χ2 tests. To analyse the possible association of folate levels, vitamin B12 and hyperhomocysteineaemia with the presence of RVO, crude logistic regression models were constructed and adjusted by age, sex, body mass index (BMI), tobacco habit and the presence of classical FRCV (high blood pressure, diabetes and dyslipidaemia). Values of p<.05 were considered significant.
ResultsThree hundred and sixty eight patients with RVO and 325 controls were included in the study. RVO was peripheral in 254 cases (69%); 248 temporal and six nasal and was central in 114 (31%).
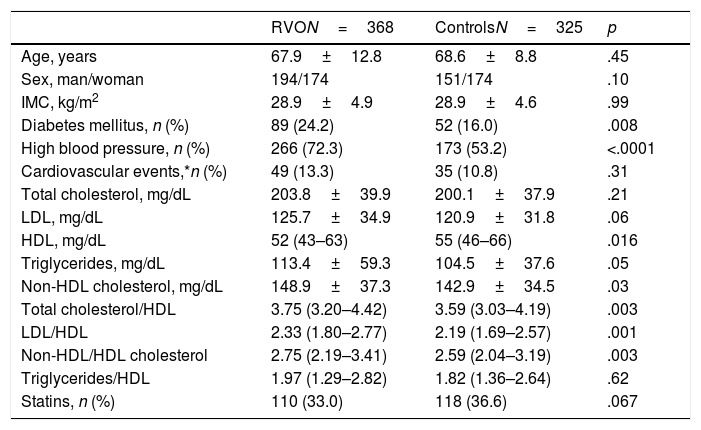
Table 1 contains comparison of demographic variables, serum lipid values (total cholesterol, LDL, HDL, no-HDL and triglycerides), arthrogenic indexes (AI) and the percentage of cases and controls who received treatment with statins.
Demographic data and standard lipid parameter levels and artherogenic indexes in patients with RVO and in controls.
| RVON=368 | ControlsN=325 | p | |
|---|---|---|---|
| Age, years | 67.9±12.8 | 68.6±8.8 | .45 |
| Sex, man/woman | 194/174 | 151/174 | .10 |
| IMC, kg/m2 | 28.9±4.9 | 28.9±4.6 | .99 |
| Diabetes mellitus, n (%) | 89 (24.2) | 52 (16.0) | .008 |
| High blood pressure, n (%) | 266 (72.3) | 173 (53.2) | <.0001 |
| Cardiovascular events,*n (%) | 49 (13.3) | 35 (10.8) | .31 |
| Total cholesterol, mg/dL | 203.8±39.9 | 200.1±37.9 | .21 |
| LDL, mg/dL | 125.7±34.9 | 120.9±31.8 | .06 |
| HDL, mg/dL | 52 (43–63) | 55 (46–66) | .016 |
| Triglycerides, mg/dL | 113.4±59.3 | 104.5±37.6 | .05 |
| Non-HDL cholesterol, mg/dL | 148.9±37.3 | 142.9±34.5 | .03 |
| Total cholesterol/HDL | 3.75 (3.20–4.42) | 3.59 (3.03–4.19) | .003 |
| LDL/HDL | 2.33 (1.80–2.77) | 2.19 (1.69–2.57) | .001 |
| Non-HDL/HDL cholesterol | 2.75 (2.19–3.41) | 2.59 (2.04–3.19) | .003 |
| Triglycerides/HDL | 1.97 (1.29–2.82) | 1.82 (1.36–2.64) | .62 |
| Statins, n (%) | 110 (33.0) | 118 (36.6) | .067 |
BMI: body mass index; HDL: high-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; RVO: retinal venal occlusion.
Values expressed as mean±SD or median (interquartile range) or percentage.
In patients and controls who did not receive treatment with statins, LDL figures were 134.3±32.6 vs. 125.6±26.9mg/dL (p=.04). Distribution of LDL, HDL cholesterol and triglycerides mg/dL in patients with RVO (n, %) was: LDL<116mg/dL, 151 (41%); 89 cases LDL<100 (24.2%) and five patients had a LDL <55mg/dL. HDL: men 149>40 (76.8%), women 115>50 (66.1%), 264 men or women>40 or 50, respectively (71.7%); triglycerides: 63 (17.1%)>150.
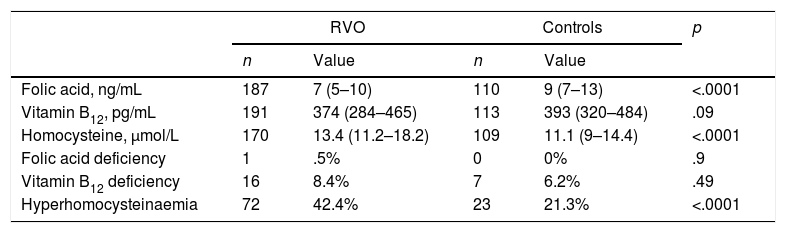
Serum folate levels, vitamin B12, homocysteine and percentage alterations of these parameters in cases and in controls are listed in Table 2.
Serum levels of folic acid, vitamin B12, homocysteine and percentage of their changes in patients with retinal vein occlusion (RVO) and in the control group.
| RVO | Controls | p | |||
|---|---|---|---|---|---|
| n | Value | n | Value | ||
| Folic acid, ng/mL | 187 | 7 (5–10) | 110 | 9 (7–13) | <.0001 |
| Vitamin B12, pg/mL | 191 | 374 (284–465) | 113 | 393 (320–484) | .09 |
| Homocysteine, μmol/L | 170 | 13.4 (11.2–18.2) | 109 | 11.1 (9–14.4) | <.0001 |
| Folic acid deficiency | 1 | .5% | 0 | 0% | .9 |
| Vitamin B12 deficiency | 16 | 8.4% | 7 | 6.2% | .49 |
| Hyperhomocysteinaemia | 72 | 42.4% | 23 | 21.3% | <.0001 |
Folic acid deficiency (<2.6ng/mL); hyperhomocysteinaemia (>15μmol/L); vitamin B12 deficiency (<211pg/mL).
Values expressed as median (interquartile range) or percentage.
The concentrations of folic acid (OR .94; 95% CI .89–.98; p=.011) and hyperhomocysteinaemia (OR 2.73; 95% CI 1.57–4.73; p<.0001) are associated with the presence of RVO. Said values persist significantly after adjustment for age, sex, body mass index (BMI), tobacco habit and FRCV (high blood pressure, dyslipidaemia and diabetes mellitus). No association was found between the RVO and levels of vitamin B12 (OR .99; 95% CI .99–1.00; p=13).
DiscussionThe Valdecilla cohort represents the prospective cohort of patients with RVO with a more prolonged follow-up of those reported in the literature (11 years at present).
High blood pressure and diabetes mellitus were significantly more prevalent in our patients with RVO than in control groups, a fact that is in keeping with that published in the literature.6,7,16,17 We observed no differences in body mass index or in the prevalence of previous cardiovascular events in either group.
In this study we have tried to respond to some often less well-known aspects of the disease such as its relationship with the lipid profile and with folic acid levels, vitamin B12 and homocysteine. We state that, in patients with RVO, HDL levels were significantly lower in the cases than in the controls. We also observed that the serum levels were significantly lower in the cases than in the controls. Serum levels of folic acid were also significantly lower and homocystine levels higher than in the population control group.
Although serum levels of total cholesterol, LDL and triglycerides were higher in patients with RVO than in the controls, the differences were not significant. This lack of significance may be the consequence of the fact that a high percentage of patients and controls, higher in the cases of the latter, had already received treatment with hypolipidaemic agents, essentially with statins, at the time of the RVO. Thus when we analysed the patients who did not receive statins we observed there were significant differences in the LDL levels between cases and controls. However, the HDL, which was modified less with pharmacological treatments, was significantly lower in patients with RVO. HDL is a antiatherogenic lipoprotein particle which acts on the reverse transport of cholesterol and has beneficial vascular and antithrombotic effects.18 Also, in our patients with RVO the non HDL cholesterol and arthrogenic indexes such as that of Castelli (total cholesterol/HDL) or that related to LDL/HDL were significantly higher in patients with RVO. These indexes represent risk markers with a higher predictive value than that of isolated data since they combine two powerful components of vascular risk and subjects with elevation of these ratios have a higher cardiovascular risk based on greater imbalance between the cholesterol transported by the most arthrogenic lipoproteins and that of the lipoproteins with a protector effect. However, as reported by Oriole et al., 19 analysing the anterior lipid profile to a vascular event, the parameters are not overly high so that the majority of our patients with RVO are grouped into the moderate risk lipid intervals (LDL <100–159, HDL >40/50 (men/women), triglycerides <150mg/dL).
Due to all of the above, and bearing in mind that atherosclerosis is the essential pillar in RVO pathogen, the lipid profile of the patients with the disease, with a lower level of HDL and a more unfavourable arthrogenic profile (higher arthrogenic indexes) and higher levels of triglycerides, are congruent with the unfavourable pathogenic role of dyslipidaemia in RVO. The aetiopathogenesis of RVO is multifactorial here. ÓMahoney et al.20 suggest that this is the consequence of the dyslipidaemia in 20%, whilst high blood pressure accounts for it in 48%. Kim et al., 4 in a study of a national cohort, stated there was an association between low HDL levels and the risk of developing an RVO. Newman-Casey et al.21 also suggested that there was a reduction of HDL levels and the raising of serum triglyceride levels were risk factors in the development of peripheral RVO.
Despite presenting with lower serum levels of folic acid and vitamin B12, with the difference not being statistically significant in the latter, the deficiency of these vitamins, defined by the lower limit of normality of our laboratory, is infrequent in patients with RVO. This could be due to the fact that there is no defined cut-off point from which vascular risk increases, but it may increase progressively as the serum folate and vitamin B1222 levels drop.
Studies which have analysed the association between vitamin B12 and folic acid levels and RVO revealed different results. Sofi et al., 10 analysed 262 patients with RVO and the same number of controls and concluded that low vitamin B6 (OR .03; 95% CI 2.58–6.31; p<.0001) and folic acid (OR 6.13; 95% CI 3.85–9.76, p<.0001) levels were associated with RVO and were separate from the standard VRF. However, these authors did not find any significant relationship between the RVO and vitamin B12 levels. Ferrazzi et al., 23 in a series of 64 patients with RVO observed vitamin B12 and folic acid levels dropping, although differences were not significant. Finally, Minniti et al., 11 did find a significant association between low vitamin B12 levels and peripheral RVO, in 94 patients with RVO and 71 controls.
Levels of folic acid, vitamin B12 and homocysteine, which depend on them, are the consequence of both dietary habits and the consumption of some drugs such as meformin or protone pump inhibitors.24 Regarding homocysteine, different studies have stated that their serum levels are higher in patients with RVO, regardless of sex, age and VRF.6,25–30 Hyperhomocysteinaemia is mainly associated with the deficiency of folate and to a lesser degree with deficiencies of vitamin B complex or systemic diseases such as kidney failure.9 Furthermore, this is an independent risk factor of artherosclerosis and has a multiplicative effect with other VRF, such as tobacco or high blood pressure and also a thrombophylic effect.31
Cattaneo et al.31 and Rimm et al., 32 have shown that folic acid and vitamin B12 are also separate risk factors of thrombotic vascular events, both arterial and venous. In this sense, several authors have suggested that the group B vitamins and folate play a role in the appearance of RVO, regardless of their influence on serum homocysteine levels.10,11,23
In population studies, Wright et al.33 observed that oral administration of folic acid reduced homocysteine by 25% and vitamin B12 by 7%. Treatment of hyperhomocysteinaemia and vitamin deficits may therefore revert this VRF, and its use was therefore recommended, in secondary prevention, in the treatment of cardiovascular diseases, including RVO.32,33 However, for the moment, the efficacy of this therapeutic strategy in reducing vascular events has not yet been proven.
Different authors observed a considerable increase in vascular events in patients with RVO, essentially strokes.34–36 In fact, Park et al.34 stated that this increase occurs from the first month of the RVO episode and for this reason, it is recommended that control of the standard VRF be controlled (high blood pressure, dyslipidaemia, diabetes mellitus, a tobacco habit) and they suggested controlling other risk factors (hyperhomocysteinaemia) to prevent new vascular events.30,37
This work presents with the limitation that the folic acid, vitamin B12 and homocysteine levels were only taken in half of the cases and in a third of three controls which suggests results should be interpreted with caution. Also, the presence of asymptomatic RVO cannot be fully ruled out in the control subjects, although this possibility is remote.
To sum up, patients with RVO have serum levels of HDL and folic acid which are significantly lower and non HDL cholesterol levels and homocysteine levels which are higher than the population controls of similar age and sex. In these patients, apart from the lipid profile study, it may be useful to determine the levels of homocysteine, folic acid and vitamin B12, in addition to considering treatment for their alteration. If this treatment is effective in the prevention of RVO recurrences or the development of new vascular events in these patients, this is something which may be worthwhile investigating in prospective studies designed to this effect.
FundingThe study was financed party by funds from a grant from IS Carlos III (PI15/00521).
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Napal Lecumberri JJ, González Bores P, Cuesta Marín A, Caballero Avendaño FA, Olmos Martínez JM, Hernández Hernández JL. Perfil lipídico y concentraciones séricas de ácido fólico, vitamina B12 y homocisteína en pacientes con obstrucción venosa retiniana. Clin Investig Arterioscler. 2021. https://doi.org/10.1016/j.arteri.2020.07.001