The objective of this study is to explore the longitudinal progression of atherosclerosis and the correlation between methods to measure the lesion in apolipoprotein E-deficient mice. Atherosclerosis progression was assessed by measurements of foam cell-rich depositions in their proximal aortas, and/or in surgically excised arteries, to assess the histological luminal narrowing. A longitudinal study was performed by comparing the values for carotid, aorta, and femoral and iliac arteries using common histological techniques. There were no significant differences in progression between different arteries, but correlation with the classical measurement of atherosclerosis in the aortic root was poor. Each laboratory requires specific standardization. Carotid arteries were sensitive to atherosclerosis in these mice, and progression was exponential. In conclusion, morphometric data show the importance of the choice of the duration of treatment, the appropriate controls, and the age at which to begin the experiments.
El objetivo de este estudio es explorar la progresión longitudinal de la aterosclerosis y la correlación entre los métodos para medir la lesión en los ratones deficientes en apolipoproteína E. La progresión de la aterosclerosis se evaluó mediante mediciones de deposiciones ricas en células espumosas en las aortas proximales y/o en las arterias extirpadas quirúrgicamente para evaluar histológicamente el estrechamiento luminal. Se realizó un estudio longitudinal, y los valores para la carótida, la aorta, las arterias femorales e ilíacas se compararon mediante técnicas histológicas comunes. No hubo diferencias significativas en la progresión entre las diferentes arterias, pero la correlación con la medición clásica de la aterosclerosis en la raíz aórtica era pobre. Cada laboratorio requiere su normalización específica. Las arterias carótidas fueron sensibles a la aterosclerosis en estos ratones y la progresión fue exponencial. En conclusión, los datos morfométricos muestran la importancia en la elección de la duración del tratamiento, los controles apropiados y la edad a la cual comenzar los experimentos.
Atherosclerosis is the single most important contributor to cardiovascular disease. Mechanistic studies are difficult in humans for obvious reasons and the number of unanswered questions is remarkable. However, emerging evidence, mainly obtained in the study of carotid atherosclerosis, suggests that regression and/or stabilization of atherosclerosis in humans are achievable goals.1 Promising procedures such as to increase the efflux of lipids from plaques or to facilitate the emigration of foam cells out of the arterial wall only can be currently envisioned in animal models but further progress will probably require better models and/or more conclusive interpretations.2,3 Current atherosclerosis-susceptible animal models have provided valuable insights in preclinical studies, but in this stage of knowledge, further achievements will require a complete understanding of the limiting factors.
Atherosclerosis in humans is a lifelong, insidious disease, in which it is difficult to explore the course and the effects of continuing management. To prove, discard or improve certain hypotheses, rodent models of atherosclerosis, and in particular knockout (KO) models, are extremely useful but they are highly susceptible to liver injury, and results are not interchangeable, poor reproducibility is common and limitations are difficult to appreciate under some circumstances.4,5 After the generation of Apolipoprotein E (Apo E) deficient mice, the generated prospects were high and have certainly provided useful data.6–8 The main advantage of these mice is likely the seemingly spontaneous development of atherosclerosis. Furthermore, as compared with other models, there is no need for dietary or surgical manipulations but a consensus view is difficult to achieve due to different opinions in the proper design of experiments.3,9,10 Extrapolations are also difficult. For example, the deficiency of Apo E is responsible of lipoprotein alterations not described in humans and it is unlikely that these modified mice could replicate the age-related distribution of lesions in humans.11
In this study, we explore a particular concern on how to reach decisions related to the duration of pharmacological treatments and the age at which to begin the experiments, which are troublesome factors in the design of preclinical studies. The longitudinal analysis of atherosclerosis progression in different sites and the correlation between methods to measure the lesion development may clarify important issues to improve validity of the conclusions.
Materials and methodsAll procedures were carried out in accordance with institutional guidelines (CEIA, 2014-237). We measured the atherosclerotic lesions in collected samples from experimental Apo E-deficient mice sacrificed at 10 and 24 weeks of age (8 males and 8 females at each time-point). This is the duration study commonly used in experiments according to available literature and we also used a common method to score lesions just beyond the aortic sinus.12,13 Values were compared to those obtained histologically in carotid arteries. Samples were embedded in paraffin and stained with haematoxylin and eosin. The area of the lumen was quantified by using AnaliSYS™ (Soft Imaging System, Münster, Germany). To avoid the influence of high-fat diets, mice in a C57BL/6J background obtained by inbreeding of mice purchased to Jackson Laboratory were housed under standard conditions and given a commercial, low-fat, mouse diet (14% protein rodent maintenance diet, Harlan, Barcelona, Spain). Results prompted us to design a longitudinal study with male mice allocated in 6 groups (n=5, each) and sacrificed at 16, 24, 36, 44, 50 and 60 weeks to remove aorta and main peripheral arteries to histologically analyze atherosclerotic lesions.
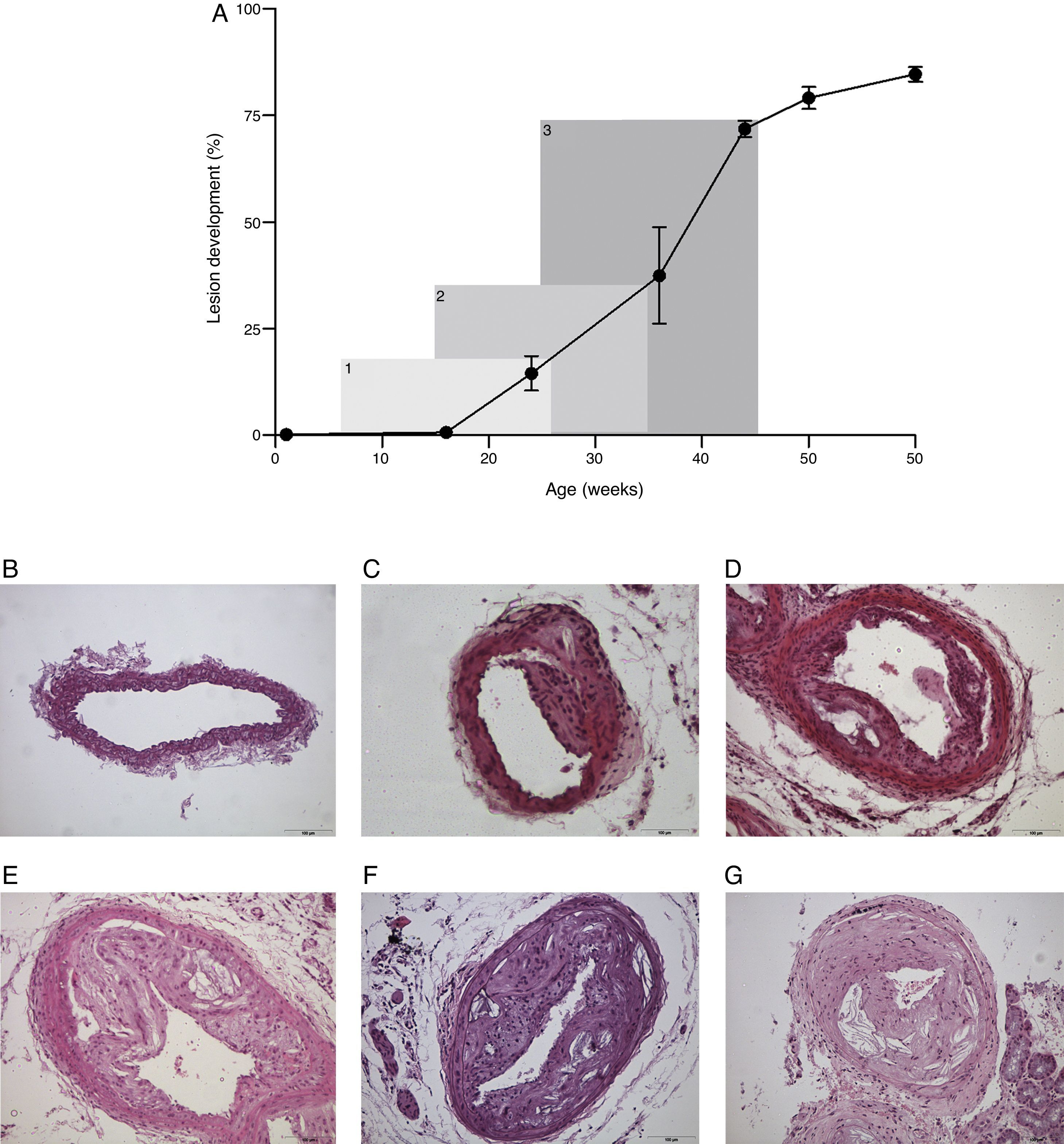
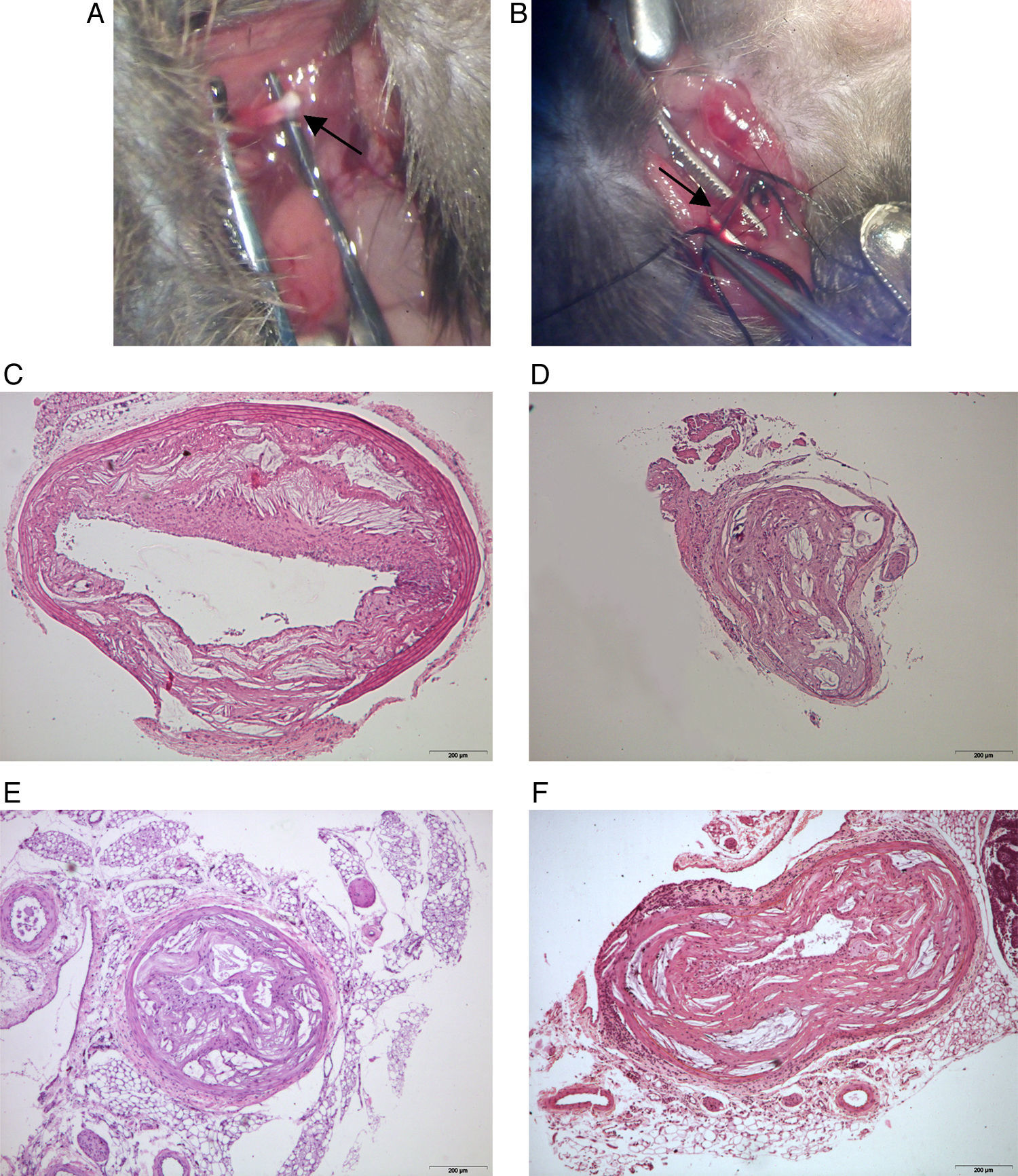
Results and discussionWe confirm that atherosclerosis progression in female mice is higher than observed in male mice in measurements beyond the aortic sinus. In contrast, this difference was not appreciable with serial histological sectioning of carotid arteries. Therefore, gender should be considered a confounding factor and, as such, it should be clearly stated in the description of results. When comparing the measurement of atherosclerosis progression in the aortic sinus and in carotid arteries, values of the Pearson's correlation coefficient were not significantly different from zero at the 0.05 levels for this sample size (r=0.267 and r=0.320 for female and male, respectively). This finding could be considered predictable in short experiments because progression of atherosclerosis in arteries is accompanied by remodelling leading to a significant absence of luminal narrowing. However, other measurements related to the shape and sizes of the arteries’ wall were also irrelevant. Of note, the effects in other models remain to be investigated but additional confounding factors should be expected.4 We found that morphometric data in a longitudinal analysis established exponential rather than lineal atherosclerosis progression in carotid arteries (Fig. 1A). Therefore, in order to design the experiments, the different rate of atherosclerosis progression should be considered to establish the hypothesis and to decide the duration of treatment and the age at which to begin the experiments (Fig. 1B) because this may severely affect the assessment in statistical evaluation and consistency of the data. There were no apparent differences among arteries of different size (Fig. 2). In contrast to humans, mice remained viable for over 60 weeks of age despite important decreases in the lumen size of important arteries. However, we have found an apparently secondary advantage in this model for training vascular surgeons in the management of small arteries, to develop reproducible models of peripheral artery disease, including stroke, and for investigating surgical procedures that may modify the process of the disease (Fig. 2A and B).
The progression of atherosclerosis in carotid arteries from Apo E-deficient mice is exponential rather than linear (A). In a typical treatment period of 20 weeks the assessment in the amount of lesion changes considerably (6–26 weeks, 19%; 15–35 weeks, 36%; 25–45 weeks, 73%). This may have implications in the aim and design of the experiments. Representative microphotographs of lesions are depicted for mice with 16 (B), 24 (C), 36 (D), 44 (E), 50 (F) and 60 (G) weeks of age. All sections were stained with haematoxylin and eosin and the scale bar of 100μm represents a magnification of 100×.
Manipulation of small arteries requires the use of microscopy but may be used to design experimental models related to the management of peripheral artery disease, including stroke (A, B). The blood flow of most arteries is compromised but mice remain viable over the 60 weeks of age. This is illustrated in aorta (C), carotid (D), femoral (E) and iliac (F) arteries. All sections were stained with haematoxylin and eosin and the scale bar of 200μm represents a magnification of 40×.
Most studies on atherosclerosis based on this model are performed when the burden of the disease is low. This approach is valid for research but not for preclinical studies designed to explore advanced lesions. We have already obtained disparate results in drug testing and beneficial effects assayed in the initial period were not replicated when the experiments were extended for a longer period.13–15 These and other previous studies suggest a failure in the full assessment of the “natural course” in the progression of atherosclerosis, which should be considered in most experiments. This study was not designed to provide guidelines but the extended assessment of atherosclerosis progression seems a mandatory procedure to explore the effects of drugs.3 It is also common to explore the effect of additional genetic manipulations (double KO) in this Apo E-deficient background. In these experiments, a longitudinal study on the development of atherosclerosis is likely mandatory to choose the appropriate control animals. This is especially important if the manipulation results in premature death.16
The method to assess the atherosclerotic lesion, and in particular in which arteries to proceed, also raises some other concerns in a model in which most important arteries are easily accessible. For the reasons mentioned above, serial histological sectioning of excised arteries yielded a more physiological approach than that provided by the assessment of foam cell-rich depositions in the proximal aorta. We found this procedure reproducible in carotid arteries. Lesion development in other arteries was similar but not necessarily equal strongly suggesting that laboratories should proceed to their own standardization. Further, the pattern of raised lesions in arterial branches in relation to flow may differ considerably.17 In humans, this might be particularly important to provide specific models to progress in the diagnosis and management of peripheral artery disease. The consensus favours that for the study of atherosclerosis, mice are not the ideal models and the number of limitations is considerable. Comparisons between rabbits and humans were considered more valuable and limitations derived from the dietary approaches. In this scenario, the recently developed techniques to manipulate genes in the rabbit and in particular the creation of the Apo E KO rabbit18 suggest that, once fully characterized, they may add value in the further investigation of atherosclerosis.
In conclusion, to design experiments with Apo E-deficient mice, alone or in combination with other genetic manipulations, is not a simple task and limitations should be clearly stated. The experiments with Apo E-deficient mice require assessment of the relationship between the development of lesion and age to calculate the duration of treatment and robustness in statistical inferences. We recommend the measurement of atherosclerosis progression in a significant number of arteries for which serial histological sectioning is likely the best method to procure relevant and reproducible data.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interest statementThe authors of this manuscript declare no conflict of interests.
The Unitat de Recerca Biomèdica is currently being supported by the program of consolidated groups from the Universitat Rovira i Virgili and grants from the Fondo de Investigación Sanitaria (PI08/1381 and PI11/00130). MR-B is the recipient of a fellowship from the Universitat Rovira i Virgili (2010PFR-URV-B2-58).