Multiple systematic reviews (SR) have been performed on the effects of proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), often providing conflicting findings. This overview and network meta-analysis (NMA) aimed to summarize SR findings on the efficacy and safety of PCSK9i and provide an updated NMA.
Materials and methodsMEDLINE (Pubmed), Scopus, Cochrane, Epistemonikos and Google Scholar were searched from inception to September 21, 2023 for SRs of randomized controlled trials (RCTs) and from January 1, 2020 to September 21, 2023 for additional RCTs. Double-independent study selection, data extraction and quality assessment were performed. Qualitative analysis was performed for SRs and a frequentist random-effects model NMA was performed for RCTs.
ResultsTotally, 86 SRs and 76 RCTs were included. Alirocumab (77/86 [90%]) and evolocumab (73/86 [85%]) were mostly analyzed. Associations from SRs (35/42 [83%]) and the updated NMA indicated PCSK9i benefit on major adverse cardiovascular events (MACEs). Reductions were also noted for cerebrovascular events (47/66 [71%]), coronary revascularization (29/33 [88%]) and myocardial infarction (41/63 [65%]). Alirocumab was associated with reductions on all-cause mortality (RR=0.82, 95%CI [0.72,0.94]). Data on any CV event reduction were conflicting (7/16 [44%]). Inclisiran appeared effective only on MACEs (RR=0.76, 95%CI [0.61,0.94]). No reductions in heart failure were observed (0/16). No increases were identified between PCSK9i and any (0/35) or serious adverse events (0/52). However, PCSK9i were associated with injection-site reactions (20/28 [71%]).
ConclusionPCSK9i appeared to be effective in CV outcomes and their clinical application was generally safe.
Las revisiones sistemáticas (RS) sobre los efectos de los inhibidores de la proproteína convertasa subtilisina/kexina tipo 9 (PCSK9i), presentan resultados contradictorios. Esta revisión general y metaanálisis en red (MER) tiene como objetivo resumir los hallazgos sobre la eficacia y seguridad de los PCSK9i.
Materiales y métodosSe realizaron búsquedas en MEDLINE (PubMed), Scopus, Cochrane, Epistemonikos y Google Scholar desde sus inicios hasta el 21 de septiembre de 2023 para las RS de ensayos controlados aleatorios (ECA) y desde el 1 de enero de 2020 hasta 21 de septiembre de 2023 para los ECA adicionales. La selección de estudios, extracción de datos y evaluación de calidad se llevaron a cabo de manera doble e independiente. Se realizó un análisis cualitativo de las SR y un modelo de efectos aleatorios frecuentistas MER para los ECA.
ResultadosEn total, se incluyeron 86 SR y 76 RCT. Alirocumab (77/86 [90%]) y evolocumab (73/86 [85%]) fueron los más analizados. Se reconocieron beneficios de los PCSK9i en eventos cardiovasculares adversos mayores (ECVAM), reducción de eventos cerebrovasculares (47/66 [71%]), revascularización coronaria (29/33 [88%]) e infartos de miocardio (41/63 [65%]). Alirocumab redujo la mortalidad por todas las causas (RR: 0,82; IC del 95%: 0,72-0,94). Los resultados sobre la reducción de cualquier evento cardiovascular (CV) fueron contradictorios (7/16 [44%]). Inclisiran pareció ser efectivo solo en la reducción de ECVAM (RR: 0,76; IC del 95%: 0,61-0,94). No se observaron reducciones en insuficiencia cardíaca (0/16) o relación con eventos adversos serios (0/52). Sin embargo, se asociaron con reacciones en el lugar de la inyección (20/28 [71%]).
ConclusiónLos PCSK9i parecieron ser generalmente efectivos, y su aplicación clínica fue segura.
Despite substantial effort to reduce its incidence, cardiovascular disease (CVD) remains the leading cause of morbidity and mortality worldwide,1 causing an extreme financial burden on healthcare systems.2 Nonetheless, much of this impact is amenable to change. It is estimated that approximately 70% of incident CVD cases and deaths are associated with well-known and modifiable risk factors (e.g. dyslipidemias, hypertension, diabetes mellitus).3 Consequently, tackling these factors constitutes a crucial step towards reducing CVD, an intervention further supported by recent epidemiological evidence.4
Dyslipidemias, especially increased low density lipoprotein cholesterol (LDL-C) constitute well established modifiable CVD risk factors, affecting many individuals worldwide.5 A wealth of evidence stemming from randomized controlled trials (RCTs),6 Mendelian randomization7 and observational studies8 have elucidated the causal effect9 and the log-linear relationship10 between LDL-C levels and CVD risk. Various pharmacological therapies have been developed thus far to lower LDL-C,11 yet a considerable percentage of patients remains above the thresholds of current guideline recommendations.12 Additionally, statins, which constitute the primary pharmacological intervention may be associated with adverse events (AEs), which can lead to drug intolerance, discontinuation and treatment failure.13 To address these issues, proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), were introduced in our pharmacological armamentum.
Hitherto, PCSK9i are recommended as an adjuvant therapy in patients already on statins who remain undertreated or for statin-intolerant patients.14 From their inception to the their establishment in clinical practice, many RCTs have been undertaken to evaluate their efficacy and safety. To summarize this evidence, multiple systematic reviews (SRs) have been performed, frequently providing contradictory conclusions.10,15–17 Overviews of reviews (overviews) or umbrella reviews constitute a relatively new evidence synthesis form integrating SRs as their analytical unit of information synthesis,18,19 while network meta-analyses (NMA) examine interventions’ comparative effectiveness. Considering the above, the primary objectives of this overview and NMA were to examine the efficacy and safety of PCSK9i on CV outcomes and provide an updated NMA on critical outcomes. Secondary objectives were to quantify the degree of evidence overlap from SRs and provide guidance for future research agenda.
MethodsThis overview and NMA was conducted according to a registered pre-defined protocol (https://osf.io/v79z6/) and following Cochrane guidance.20 Protocol amendments are described in Table S.1. Reporting was based on the Preferred Reporting Items for Overviews of Reviews (PRIOR) statement21 and the extension of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement for NMAs.22 A PRIOR checklist is presented in Table S.2.
Information sources and search strategyA comprehensive search was performed for SRs in MEDLINE (via Pubmed), Scopus, the Cochrane database and Epistemonikos from inception up to September 21, 2023 without language restriction. Keywords and MeSH terms of the eligible interventions and study type were combined using Boolean operators. An updated search for primary studies was performed from January 1, 2020 up to September 21, 2023 (partial overlap with the search date of most recent SRs) in MEDLINE and Scopus to identify any additional RCTs for inclusion into the updated NMA (full search strategy at Table S.3). Google Scholar was also hand-searched.
Eligibility criteriaPatients with dyslipidemia or at increased CVD risk receiving injectable PCSK9i compared to any control type (active or non-active) were eligible. The primary efficacy outcomes were all-cause mortality and major adverse cardiovascular events (MACEs) as defined in each SR or primary study. Secondary efficacy outcomes included any cardiovascular event (CVE), CV mortality, cerebrovascular events, heart failure (new-onset or worsening), coronary revascularization (bypass graft surgery, percutaneous intervention) and myocardial infarction (MI).
Primary safety outcomes were any AE and serious AEs. Secondary safety outcomes included allergies, creatine kinase (CK) elevation, diabetes mellitus (DM) (new-onset or worsening), drug discontinuation, liver enzymes elevation, musculoskeletal AEs, myalgias, neurocognitive AEs, headache, haemorrhagic stroke, injection site reactions, allergies, atrial fibrillation, bronchitis, any cancer, cataract, ophthalmological AEs, and rhabdomyolysis. The definition of efficacy and safety outcomes was adopted as specified in each SR or primary study. Definitions used in SRs were extracted and presented in our results.
Regarding the search for SRs, eligible studies were SRs of RCTs with quantitative (meta-analysis [MA] or network meta-analysis [NMA]) or qualitative analysis and individual patient data (IPD) analyses of RCTs published in English. The Cochrane20 definition of SR was adopted. Regarding the updated search for primary studies, the same eligibility criteria were applied for the included RCTs with an additional restriction regarding the minimum number of participants (at least 100) and the minimum number of events (at least one event in total). Detailed eligibility criteria are found at Table S.4.
Study selectionInitially, all records were screened by title and abstract by two authors independently. Subsequently, the same authors also independently evaluated the remaining studies through full text screening. Disagreements were resolved through discussion or involvement of a third and more experienced author. Abstrackr and Mendeley Desktop were used for screening and reference management.
In case of overlapping SRs (SRs with similar research question including similar primary studies), all records were included in the analysis. To detect and visualize the degree of primary study overlap, a citation matrix was constructed.20 To quantify overlap degree, the corrected covered area (CCA) was calculated adjusted for chronological structural missingness.23 The CCA is a percentage taking values in the range of 0–100% with higher values indicating higher overlap (proposed cut-offs are 0–5%: slight overlap; 6–10%: moderate overlap, 11–15%: high overlap; >15%: very high overlap24).
Data extractionInitially, a draft extraction form was designed and tested on three studies through pilot extraction. After discussion and calibration exercises, a final extraction form was created. Extraction was performed by two authors independently and discrepancies were resolved through discussion or involvement of a third author. All extracted variables are presented in Table S.5. To retrieve missing evidence, primary study reports were reviewed and if retrieval was not feasible, the study was excluded. In case reporting discrepancies were identified (data from the same primary study being reported differently across SRs), primary study reports were also reviewed. If not feasible, prioritization was given to the reports provided by the SR with the highest methodological quality. Regarding the updated NMA performed, primary study data on the events and number of participants for each group were extracted from the SRs with the highest methodological quality. If not available, then primary study reports were used.
Methodological quality/risk of bias assessmentsMethodological quality assessment of SRsTwo authors independently assessed the methodological quality of SRs using A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR-2) statement.25 Ratings on the overall confidence in SR results (“High”, “Moderate”, “Low”, “Critically Low”) were calculated based on authors’ recommendations. Disagreements were resolved upon discussion or involvement of a third and more experienced author.
Methodological quality/risk of bias assessment of primary studiesMethodological quality assessments of the primary studies included within SRs were collected from SRs. To maximize comparability and uniformity, the assessments using the first version of Cochrane Risk of Bias tool26 were prioritized. In case of reporting discrepancies across SRs on the quality assessments of the same primary studies, the assessments from the SR with the highest quality were extracted. If assessments were missing in any primary study, they were not assessed anew.
Data analysisAll data were analyzed and graphs were produced using the R Statistical Software (v. 4.2). Categorical variables are presented as frequencies with percentages (%), while continuous variables as mean with standard deviation (SD) when normally distributed, otherwise as median with interquartile range (IQR). In line with Cochrane recommendations,18 data from SRs were analyzed using a qualitative (narrative) synthesis. Effect estimates with 95% confidence intervals (CIs) were categorized and stratified based on the intervention, comparator and outcome characteristics to present unique associations for each outcome. Associations for each outcome are presented in forest plots with tables. The adjusted CCA was calculated using the package “ccaR”.23 Regarding the updated frequentist NMA, direct (pairwise) effect estimates and 95% CIs were calculated using random effects DerSimonian–Laird models. In case of zero events identified in a treatment arm, a continuity correction was applied to include them in the analysis. Transitivity assumption was qualitatively evaluated through the distribution of potential effect modifiers across different comparisons. Statistical consistency couldn’t be evaluated due to lack of head-to-head comparisons between PCSK9i. A common heterogeneity measure was assumed for the whole network and quantified using the I-squared. To rank interventions for each outcome, the p-score was used. Small study effects (including publication bias) for each outcome was assessed visually with comparison-adjusted funnel plots and formally tested with Egger's test. For treatment effect estimates, a two-tailed p-value less than 0.05 was considered statistically significant. The NMA was performed using the R package “netmeta”.
Certainty (quality) of evidenceCertainty of evidence assessments was extracted from SRs when available. When the Grading of Recommendations, Assessment, Development and Evaluations (GRADE)27,28 approach was used, two authors independently extracted ratings (“Very low”, “Low”, “Moderate”, or “High” certainty of evidence).
ResultsStudy selectionInitially, 1070 unique records were screened. After excluding 914 records, 156 were considered for full text screening. From these, nine records were non-retrievable and 61 were excluded with rationale (Table S.6). Finally, 86 SRs and 76 primary studies (75 identified from SRs and one from the updated search) were analyzed (Fig. S.1).
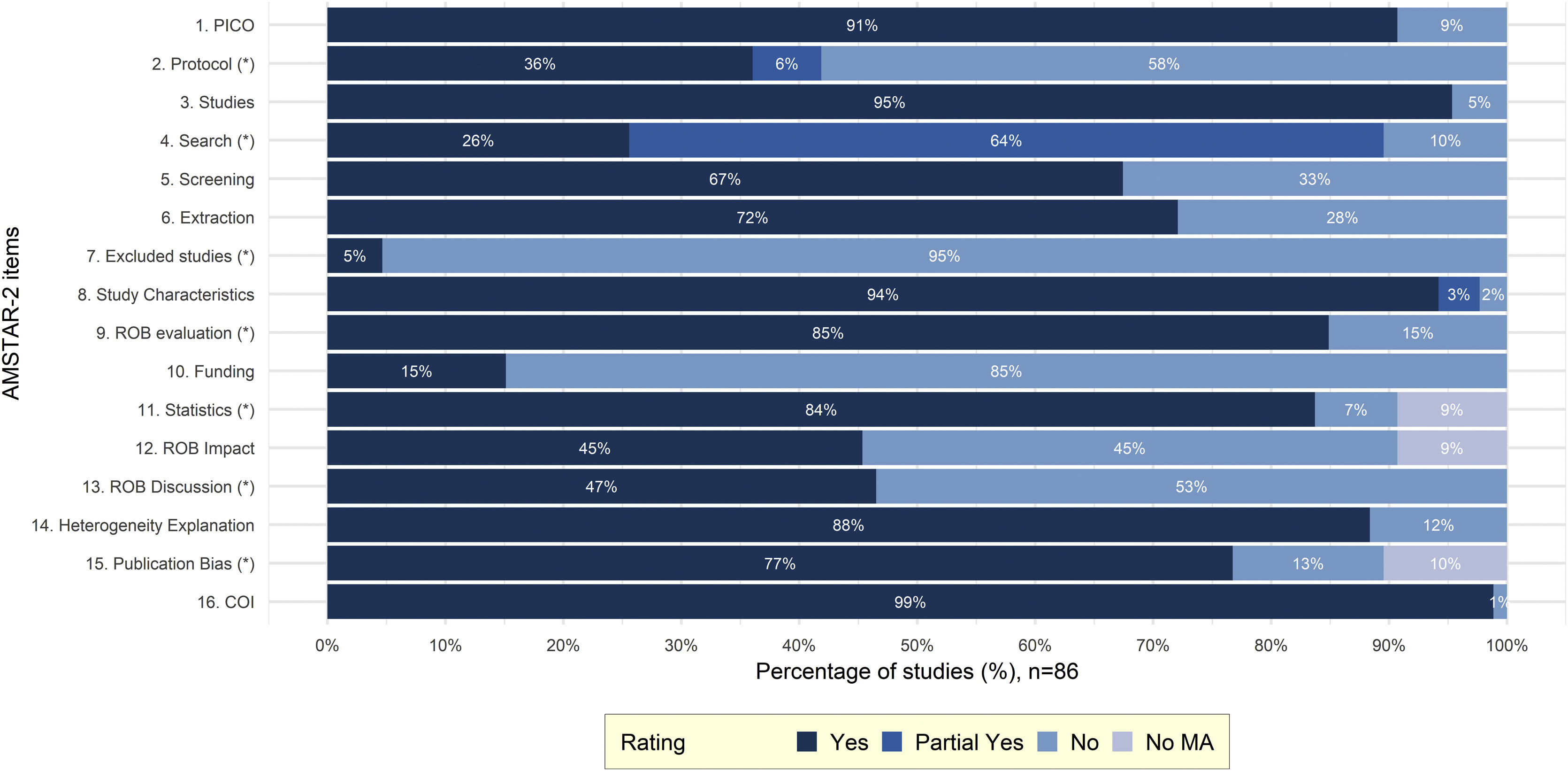
Study characteristicsCharacteristics of included SRs are presented in Table S.7. In total, 86 SRs analyzed 75 primary studies. The most frequently analyzed PCSK9i was alirocumab (77/86 [90%]) followed by evolocumab (73/86 [85%]), bococizumab (25/86 [29%]) and inclisiran (15/86 [17%]). Most SRs (83/86 [97%]) performed a quantitative analysis (paired MA (63/86 [73%]), NMA (13/86 [15%]) and IPD MA (7/86 [8%]). The mean number of databases searched was 3 (SD=1), the mean number of included primary studies pertinent to PCSK9i was 15.4 (SD=11.9) and the median number of participants in PCSK9i trials was 51,391 (IQR [6983,63055]). Efficacy of PCSK9i was considered in 53/86 (62%) SRs, while safety in 69/86 (80%). Most SRs were of “Critically low” (64/86 [74%]) or “Low” quality (20/86 [23%]) and only two studies (2/86 [2%]) were of “High” quality. The lack of a study protocol (50/86 [58%]) and of a list of excluded studies (82/86 [95%]) were the most frequently missing critical AMSTAR-2 domains (Fig. 1). Detailed AMSTAR-2 assessments are presented in Table S.8. From the 75 primary studies within SRs, risk of bias (ROB) assessments using the first version of Cochrane ROB tool were available for 63/75 (84%) (Table S.9). Primary studies were generally reported to have low or unclear ROB in most domains. The domain of incomplete outcome data was most frequently at high ROB (14/63 [22%]) (Fig. S.2). Finally, regarding the updated NMA, in total 33 primary studies were analyzed for the outcomes of all-cause mortality and MACEs. For both outcomes, the distribution of potential effect modifiers across different comparisons was homogeneously distributed and hence, transitivity is a plausible assumption. Primary study and participants’ characteristics included in the NMA are presented in Table S.10.
Barplot representing the distributions of AMSTAR-2 ratings across every item (domain). Items considered to be critical in the overall evaluation of methodological quality are signed with an asterisk (*). PICO, Population, Intervention, Comparator, Outcome; ROB, Risk of Bias; COI, Conflict of Interest; MA, Meta-analysis; AMSTAR-2, A MeaSurement Tool to Assess systematic Reviews-2.25
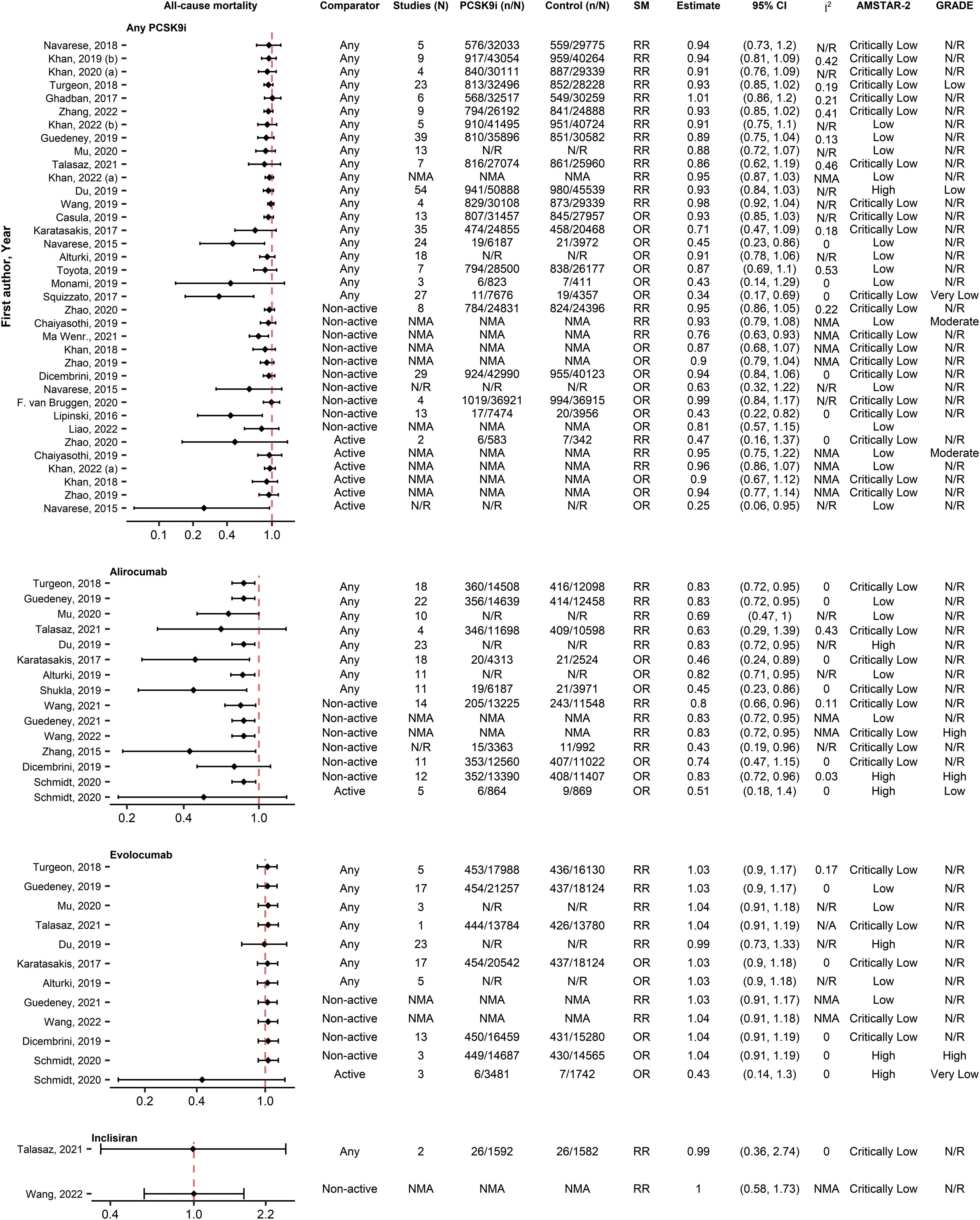
Totally, 65 unique associations from 35 SRs with MA were identified for all-cause mortality (Fig. 2). In the analysis of all PCSK9i, most SRs reported non-significant associations (31/36 [86%]) regardless of comparator type. The SR by Du et al.29 had the highest quality and the most analyzed patients and also didn’t report a significant difference. Nevertheless, examining each PCSK9i separately, 11/15 (73%) associations indicated a significant benefit of alirocumab on all-cause mortality. The SR by Guedeney et al. (2019)30 was the most comprehensive and the SR of Schmidt et al.31 and Du et al.29 had the highest quality. Du et al.29 and Guedeney et al. (2019)30 reported a significant 17% reduction on all-cause mortality while Schmidt et al.31 reported a significant 17% benefit of alirocumab compared with non-active comparators and a neutral effect versus active comparators. All associations were non-significant for evolocumab (12/12 [100%]) and inclisiran (2/2 [100%]). Evidence from IPD analyses were also in concordance with these results.
Forest plot and table presenting unique associations between proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) and comparators for the outcome of all-cause mortality. The plot is grouped according to PCSK9i type and further arranged according to comparators. Black diamonds and lines represent the summary measure (SM) with 95% confidence intervals (CI) in logarithmic scale. The dashed red line represents the relative risk (RR) or odds ratio (OR) value of no association (1). The column of Grading of Recommendations, Assessment, Development and Evaluations (GRADE)27,28 represents assessments on certainty of evidence for each unique association. The column A MeaSurement Tool to Assess systematic Reviews-2 (AMSTAR-2)25 represents the methodological quality assessment of systematic reviews. N/R, Not Reported; NMA, Network Meta-Analysis.
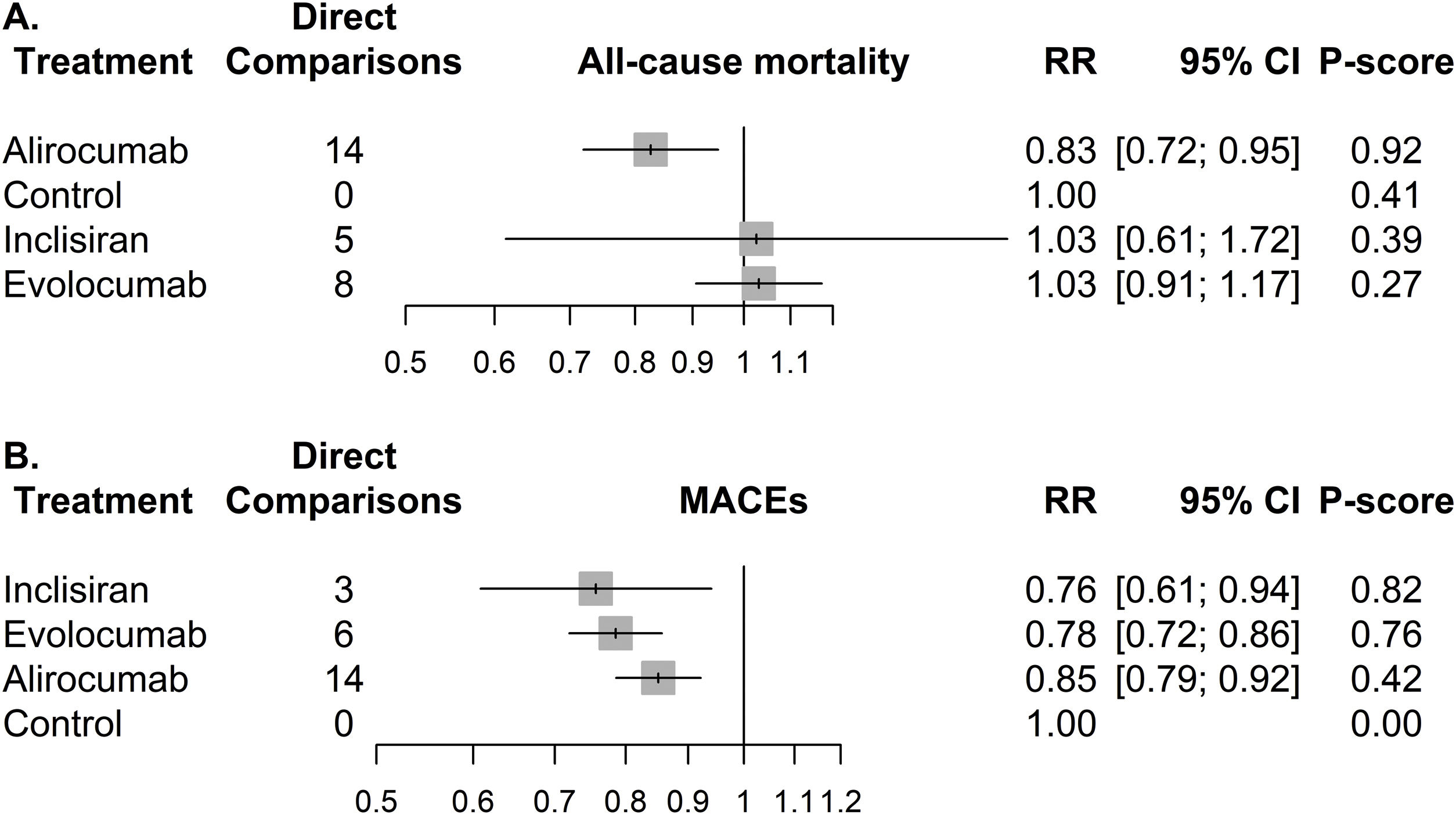
Regarding the updated NMA performed on all-cause mortality, 27 RCTs with 67,426 participants (Table S.11A) were analyzed (25 studies were excluded because of zero events). Detailed table of events for each study is shown in Table S.12. The network graph depicts a sparse network with no head-to-head comparisons between PCSK9i (Fig. S.3A) and considerably less data available for inclisiran compared to other PCSK9i. A contribution plot is provided in Fig. S.4. The results of the NMA were similar with the evidence from the analysis of SRs, with alirocumab being the only PCSK9i providing a significant reduction against control (RR=0.83, 95% CI [0.72, 0.95]) and having the highest p-score (0.92) (Fig. 3A). Alirocumab was also significantly better against evolocumab (Table S.13A) but not against inclisiran. Network's heterogeneity was small (I-squared=0%, p=0.850). The visual inspection of the comparison-adjusted funnel plot (Fig. S.5A) and the Egger's test (p=0.462) indicated no evidence of small study effects (including publication bias).
Forest plots representing the associations between different proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) versus control for the outcomes of (A) all-cause mortality and (B) Major Adverse Cardiovascular Events (MACEs). All associations derive from pairwise meta-analyses, since no head-to-head comparisons were identified. RR, risk ration; CI, confidence interval.
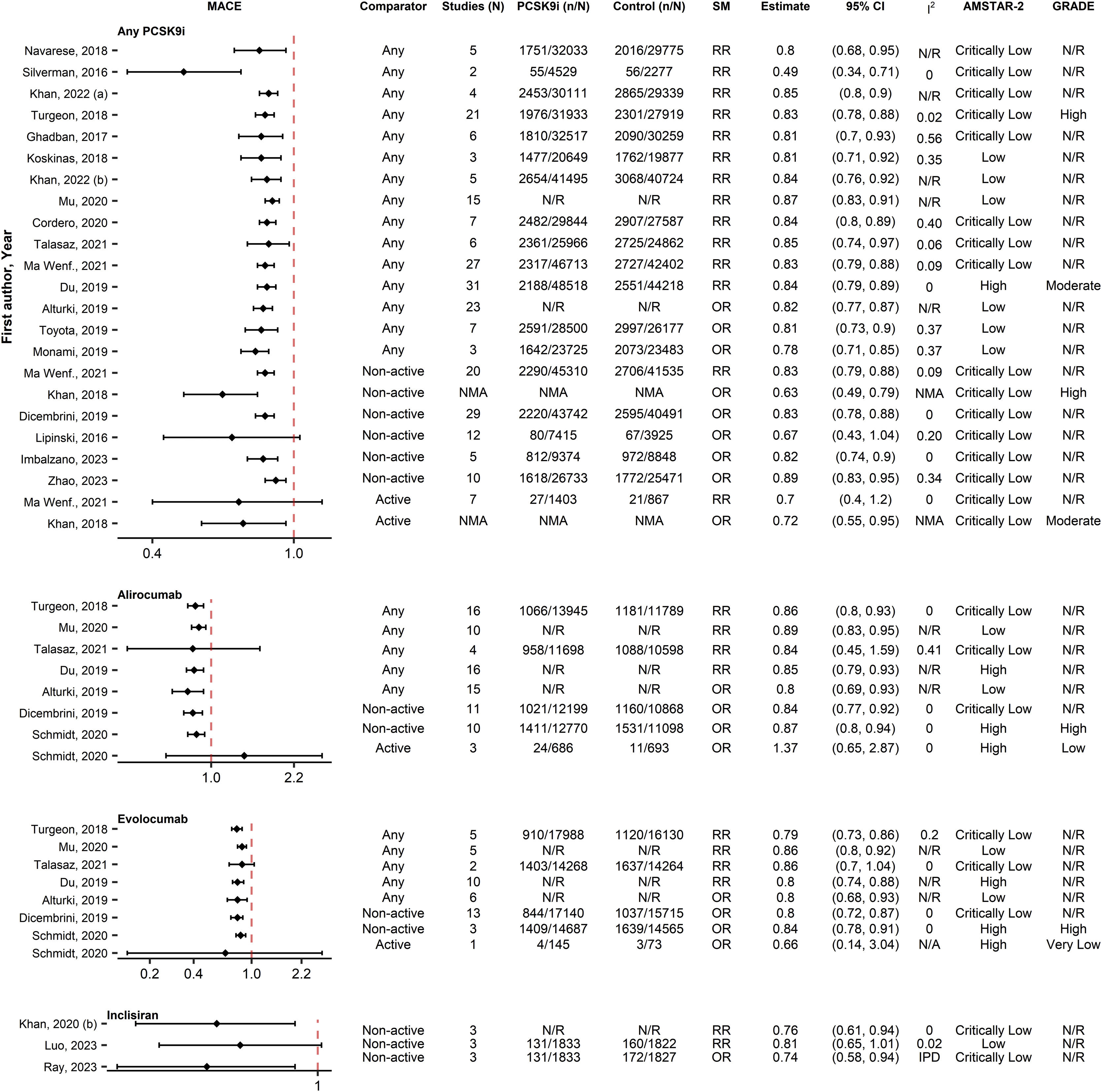
Regarding MACEs, 42 associations from 24 SRs were included (Fig. 4). Most SRs reported significant associations (35/42 [83%]) between PCSK9i and MACEs reduction, regardless of PCSK9i type. Three SRs32–34 of “Critically low” quality and the associations versus active controls of the “High” quality SR by Schmidt et al.31 reported a neutral effect. All these neutral associations had a relatively small number of participants. The SR of Du et al.,29 which had the largest number of participants and the highest quality, found a significant benefit reporting 16% reduction in the analysis of all PCSK9i, 15% reduction for alirocumab and a 20% reduction for evolocumab. The SR by Schmidt et al.,31 which was also of “High” quality reported significant effects for both alirocumab and evolocumab when compared with non-active controls. Evidence on the effectiveness of inclisiran (2/3 [67%]) were conflicting since the IPD analysis from Ray et al.35 reported a significant reduction of approximately 26%, while the most recent MA of Luo et al.36 reported a marginally non-significant benefit of 19%. Only one qualitative SR37 was identified on CV outcomes and provided similar conclusions for evolocumab and alirocumab.
Forest plot and table presenting unique associations between proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) and comparators for the outcome of Major Adverse Cardiovascular Events (MACE). The plot is grouped according to PCSK9i type and further arranged according to comparators. Black diamonds and lines represent the summary measure (SM) with 95% confidence intervals (CI) in logarithmic scale. The dashed red line represents the relative risk (RR) or odds ratio (OR) value of no association (1). The column of Grading of Recommendations, Assessment, Development and Evaluations (GRADE)27,28 represents assessments on certainty of evidence for each unique association. The column A MeaSurement Tool to Assess systematic Reviews-2 (AMSTAR-2)25 represents the methodological quality assessment of systematic reviews. N/R, Not Reported; NMA, Network Meta-Analysis; IPD, individual patient data.
Regarding the updated NMA performed on MACEs, in total, 23 RCTs encompassing 64,022 participants (Table S.11B) were analyzed (2 studies were excluded because of zero events). Detailed table of events for each study is shown in Table S.14. The network graph depicts a sparse network with no head-to-head comparisons between PCSK9i (Fig. S.3B) and considerably less data available for inclisiran compared to other PCSK9i. A contribution plot is provided in Fig. S.6. All PCSK9i were associated with significant reductions on MACEs against control (Fig. 3B). Inclisiran was ranked first (RR=0.76, 95% CI [0.61, 0.94], p-score=0.82) above evolocumab (RR=0.78, 95% CI [0.72, 0.86], p-score=0.76) and alirocumab (RR=0.85, 95% CI [0.79, 0.92], p-score=0.42). There was no significant difference among different PCSK9i (Table S.13B). Network's heterogeneity was small (I-squared=0%, p=0.543). The visual inspection of the comparison-adjusted funnel-plot (Fig. S.5B) and the Egger's test (p-value=0.116) indicated no evidence of small study effects (including publication bias).
Other efficacy outcomesRegarding any CVE (16 associations, 10 SRs), most associations (9/16 [56%]) indicated no benefit of PCSK9i (Fig. S.7). However, in the SR of Wang et al.,38 which included the largest sample size in the analysis of all PCSK9i, authors reported a significant 6% reduction. Analyzing each PCSK9i separately, evidence from the most updated meta-analyses suggested benefit for both alirocumab (Wang et al.39) and evolocumab (Zhu et al.40). All associations versus active comparators were non-significant, nonetheless, they contained a small number of participants.
Totally, 62 associations from 33 SRs were observed for CV mortality (Fig. S.8). Almost all associations in the analyses containing all PCSK9i (42/43 [98%]) indicated no benefit. In the separate analyses, most associations didn’t indicate a significant effect for alirocumab (11/13 [85%]) or inclisiran (2/2 [100%]). Contrariwise, most associations (3/4 [75%]), including those from the SR with the most patients (Bai et al.41) and the SR with the highest quality (Schmidt et al.31) indicated a significant reduction around 25% for evolocumab. All associations versus active controls were non-significant.
Regarding cerebrovascular events (66 associations, 35 SRs) most associations suggested efficacy in the all PCSK9i analysis (24/33 [73%]) and in alirocumab (9/15 [60%]) (Fig. S.9). Du et al.29 analyzed the most participants and had the highest quality, reporting significant 25% reduction both for alirocumab and in the all PCSK9i analysis. Evolocumab was consistently (14/14 [100%]) associated with reductions around 21–23%. No benefit was reported for inclisiran (0/4 [0%]).
On coronary revascularization (33 associations, 17 SRs with MA) the effect of PCSK9i appeared to be generally beneficial (29/33 [88%]) (Fig. S.10). Evolocumab was more consistently (8/8 [100%]) associated with greater reductions compared to alirocumab (4/8 [50%]). Data on MI (63 associations, 32 SRs with MA) mostly indicated benefit of PCSK9i (41/63 [65%]), especially in the subgroup of evolocumab (11/14 [79%]) (Fig. S.11). The study by Du et al.29 had the highest quality in all intervention groups and authors reported a significant 17% reduction in the combined PCSK9i analysis and a significant 14% reduction for alirocumab and evolocumab. Guedeney et al.30 included the largest number of patients and provided similar results, except for alirocumab where they reported a non-significant 24% reduction. Most associations (3/4 [75%]) reported non-significant benefit of inclisiran on MI, including the IPD analysis of Luo et al.36 Finally, no effect was observed on HF (16 associations, 7 SRs) regardless of intervention type or comparators (0/16 [0%]) (Fig. S.12).
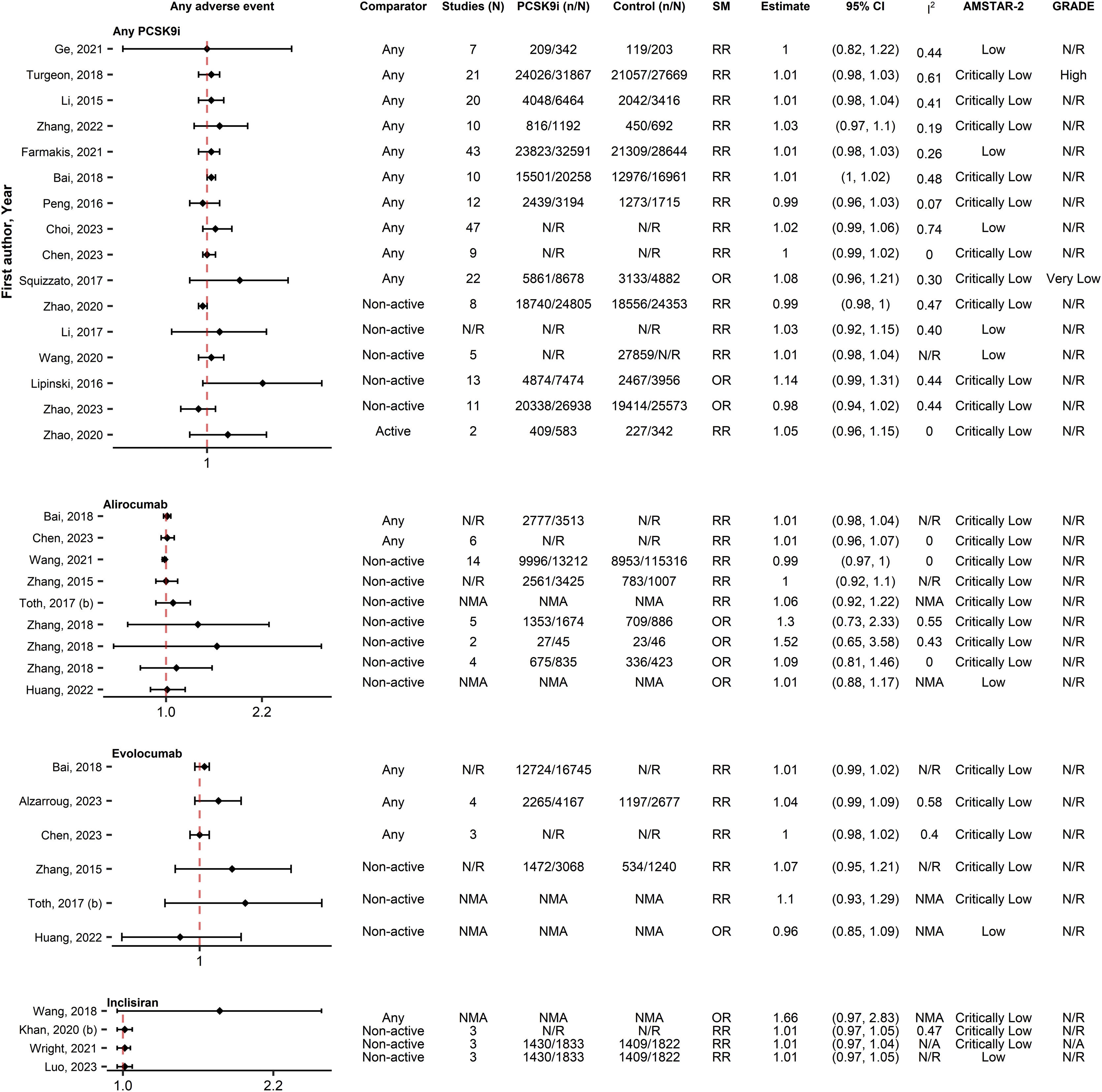
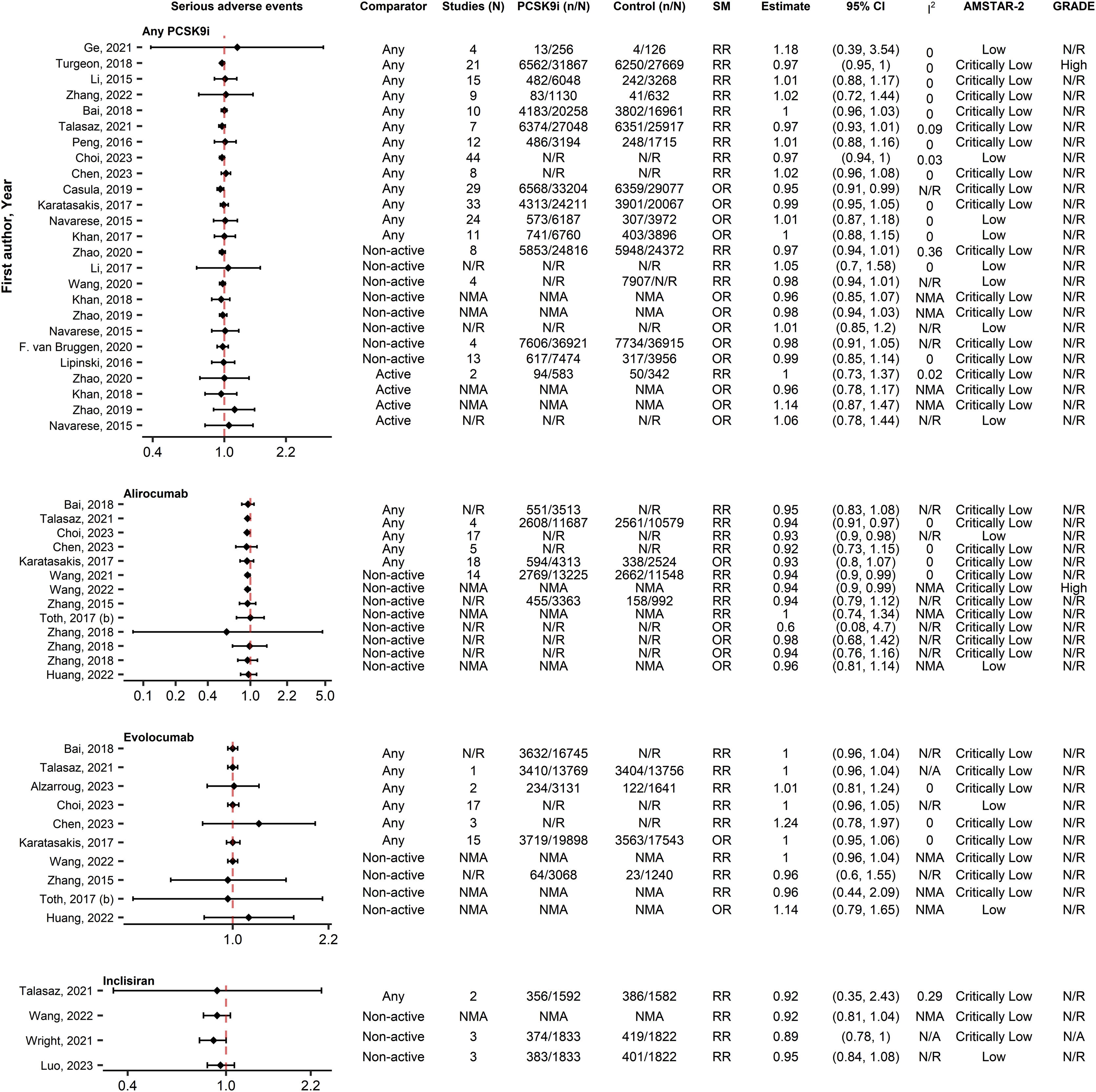
SafetyAny AE and serious AEsIn the analysis of any AE (35 associations, 25 SRs), no increases were observed from PCSK9i use (0/35 [0%]), regardless of PCSK9i type or comparator (Fig. 5). Similarly, no significant associations (0/52 [0%]) were reported between PCSK9i and serious AEs (52 associations, 29 SRs) (Fig. 6). Similar results were reported from the IPD analyses35,42–45 and the SRs with qualitative synthesis.37,46,47
Forest plot and table presenting unique associations between proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) and comparators for the outcome of any adverse event. The plot is grouped according to PCSK9i type and further arranged according to comparators. Black diamonds and lines represent the summary measure (SM) with 95% confidence intervals (CI) in logarithmic scale. The dashed red line represents the relative risk (RR) or odds ratio (OR) value of no association (1). The column of Grading of Recommendations, Assessment, Development and Evaluations (GRADE)27,28 represents assessments on certainty of evidence for each unique association. The column A MeaSurement Tool to Assess systematic Reviews-2 (AMSTAR-2)25 represents the methodological quality assessment of systematic reviews. N/R, Not Reported; NMA, Network Meta-Analysis.
Forest plot and table presenting unique associations between proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) and comparators for the outcome of serious adverse events. The plot is grouped according to PCSK9i type and further arranged according to comparators. Black diamonds and lines represent the summary measure (SM) with 95% confidence intervals (CI) in logarithmic scale. The dashed red line represents the relative risk (RR) or odds ratio (OR) value of no association (1). The column of Grading of Recommendations, Assessment, Development and Evaluations (GRADE)27,28 represents assessments on certainty of evidence for each unique association. The column A MeaSurement Tool to Assess systematic Reviews-2 (AMSTAR-2)25 represents the methodological quality assessment of systematic reviews. N/R, Not Reported; NMA, Network Meta-Analysis.
The use of any PCSK9i was unanimously reported safe on the outcomes of creatine kinase elevation (22 associations, 14 SRs) (Fig. S.13), musculoskeletal AEs (17 associations, 10 SRs) (Fig. S.14), myalgias (4 associations, 3 SRs) (Fig. S.15), rhabdomyolysis (5 associations, 3 SRs) (Fig. S.16), liver enzymes elevation (23 associations, 15 SRs) (Fig. S.17), nasopharyngitis (7 associations, 6 SRs) (Fig. S.18), allergies (4 associations, 3 SRs) (Fig. S.19), atrial fibrillation (1 association, 1 SR) (Fig. S.20A), cancer (4 associations, 3 SRs) (Fig. S.20-C), headache (6 associations, 6 SRs) (Fig. S.21), haemorrhagic stroke (2 associations, 2 SRs) (Fig. S.20-E), any ophthalmological AE (2 associations, 1 SR) (Fig. S.20-F) and cataract (1 association, 1 SR) (Fig. S.20-D). Safety was consistent regardless of comparator type.
Regarding diabetes mellitus (46 associations, 21 SRs) (Fig. S.22) and neurocognitive AEs (38 associations, 22 SRs) (Fig. S.23) all SRs indicated neutral effects except the study of Ma et al.48 for diabetes and Lipinski et al.32 for neurocognitive AEs. Drug discontinuation (23 associations, 14 SRs) (Fig. S.24) didn’t differ between PCSK9i and controls, except for one marginally significant comparison by Wang et al.49 Regarding injection-site reactions (28 associations, 15 SRs) (Fig. S.25), most comparisons (20/28 [71%]) including the IPD analysis by Wright et al.50 reported increased incidence regardless of PCSK9i type or comparator. Inclisiran was the PCSK9i associated more commonly (4/5 [75%]) and more strongly (RR ranging from 4.7 to 7.5) with injection-site reactions. Finally, inclisiran was also associated with bronchitis in the IPD analysis of Wright et al.50 (Fig. S.20-B).
Primary study overlapThe citation matrix is available at (https://osf.io/v79z6/). The adjusted CCA index was estimated to be 21%, indicating a very high degree of primary study overlap. The most frequently analyzed primary studies were ODYSSEY LONG TERM51 (68/86 [79%] SRs) and FOURIER52 (55/86 [64%] SRs). The inclusion of the above studies in most SRs has resulted in giving substantial more weight in their results, thus overstating their findings in our analysis.
DiscussionSummary of main findings in the context of other evidenceIn the present overview and NMA we have summarized a large amount of data on the efficacy and safety of PCSK9i on clinical outcomes and provided an updated analysis on the primary outcomes. Evidence suggested consistent associations between the use of PCSK9i and reductions in MACEs, cerebrovascular events, coronary revascularizations and MI. Data on CVE were conflicting, however, the most updated meta-analyses suggested efficacy. Despite their similar mechanism of action and number of analyzed patients, alirocumab appeared to reduce all-cause mortality, while evolocumab was effective only in CV mortality. Results for alirocumab and evolocumab mainly stemmed from the ODYSSEY LONG TERM and FOURIER trials respectively, which have been included in most SRs. Hence, their findings may have been overstated in our analyses and readers should account for this when interpreting our results. Our analysis on inclisiran indicated efficacy only on MACEs, nonetheless, there were substantially less data compared to other PCSK9i, mainly due to the absence of large trials with long follow-up. Consequently, readers are encouraged to interpret the results of inclisiran's analysis as hypothesis-generating, rather than hypothesis-testing. In general, most significant associations were observed versus any or non-active controls, while associations versus active controls contained the smallest number of participants and were mostly non-significant.
Regarding safety, the use of PCSK9i was found to be relatively safe as no significant increases were observed in any AE or serious AEs. Out of many safety outcomes, only injection-site reactions were consistently associated with PCSK9i. Nonetheless, these AEs are mostly mild, usually subside and rarely lead to drug discontinuation.53 Antidrug antibodies associated with injection-site reactions have been infrequently reported in alirocumab treatment54 without affecting LDL-C lowering effect. Notably, safety results within our overview stem from RCTs with a maximum follow-up duration of five years. Open-label studies with extended follow-up55,56 have provided similar safety results.
It is discouraged to draw conclusions on the comparative effectiveness of interventions from overviews, which is preferably done in the context of a NMA. In line with our findings, the NMA by Wang et al.57 examining PCSK9i efficacy on CV outcomes reported that alirocumab ranked above evolocumab on the outcome of all-cause mortality. The authors also noted that alirocumab was superior in CV mortality and stroke reductions. In turn, evolocumab ranked first on MI and inclisiran was the PCSK9i with the most injection-site reactions. No other significant differences were reported between these agents in terms of safety. Another NMA by Guedeney et al.58 compared alirocumab and evolocumab and confirmed alirocumab's superiority on all-cause mortality without significant differences on CV mortality, MI, stroke or coronary revascularization. The authors also reported a similar safety profile between these PCSK9i, except for slightly increased incidence of injection-site reactions with alirocumab. Our updated NMA on MACEs indicated a potential benefit of inclisiran over other PCSK9i. Inclisiran however lacks data form large trials compared to the other PCSK9i and thus, our results should be treated with caution and seen more as indicative rather than definitive. Of note, no head-to-head comparisons were identified between PCSK9i in our or any other NMA.
Implications for practice and researchCurrent guidelines recommend PCSK9i in patients at high CVD risk who are undertreated despite optimal medical treatment or are statin-intolerant.12,14 This overview and NMA provides substantial evidence to further strengthen current recommendations. Apart from hard cardiovascular outcomes, evidence also suggests that PCSK9i produce substantial lipoprotein(a)59 reductions. Statin therapy is associated with increases in plasma PCSK960 and lipoprotein(a),61 which constitutes an excellent biological substrate for PCSK9i to exert their action. Mendelian randomization studies also suggest that loss-of-function mutations are associated with reduced LDL-C levels and lower CVD risk62 while gain-of-function mutations have opposing effects.63 Additionally, various pleiotropic actions have been reported for PCSK9i, including anti-atherosclerosis, atherosclerotic plaque stabilization and anti-aggregation which could partly explain their cardiovascular benefits beyond lipid lowering.64,65
Evolocumab and alirocumab are subcutaneously injected once or twice every month while inclisiran is administered more sparsely. Generally, PCSK9i seem to have good safety profile and adherence rates and patients are willing to self-inject.66 To further facilitate their usage, a new oral form is under investigation with promising results on effectiveness and safety.67 Despite all PCSK9i advantages, statins constitute the mainstay of dyslipidemias’ management and a well-known class of drugs with established benefit on hard CV outcomes. Furthermore, evidence thus far suggests that they might be superior to PCSK9i in clinical outcomes like all-cause and CV mortality.68 Long-term efficacy and safety of PCSK9i must be explored and cost-effectiveness issues need to be addressed before considering their broader use.
Considering the substantial primary study overlap, the conduct of more SRs using the current primary data seems futile. Contrariwise, future research should focus on the conduct of RCTs with longer follow-ups to elucidate the impact of PCSK9i on all-cause and CV mortality and their long-term safety. Inclusion of frequently excluded patient subgroups like ethnic minorities, patients with moderate-severe kidney disease, severe heart failure or immunosuppression69 could further clarify effectiveness and safety in these populations. Furthermore, effectiveness of PCSK9i versus active controls should also be more thoroughly explored and head-to-head comparisons should be evaluated on hard cardiovascular outcomes to inform clinical practice. Most evidence from RCTs on PCSK9i stem from patients at high or very high CVD risk. Hence, effectiveness and cost-effectiveness issues need to be more thoroughly addressed for patients at low or moderate CVD risk. Finally, mechanistic insights should be explored regarding the potential benefits of alirocumab beyond the CV system, as it has been consistently associated with reductions on all-cause mortality, not fully explained by reductions on CV mortality.
Strengths and limitationsThe present study constitutes the first systematic overview and NMA evaluating the efficacy and safety of PCSK9i on clinical outcomes. Among its advantages is the large number of synthesized SRs and primary studies, focusing our presentation on the most comprehensive, updated and high quality SRs. Methodological advantages are the strict adherence to pertinent methodological and reporting guidelines and on a pre-defined protocol. Additionally, the implication of two independent reviewers at all steps increases the validity and reproducibility of our results.
Nevertheless, certain limitations should be acknowledged. Notably, we have only included SRs published in English, which may have led to information loss. Furthermore, the validity of our results is hindered by the poor methodological quality of the analyzed SRs as most were of “Low” or “Critically low” quality. Moreover, we didn’t assess anew the methodological quality of the primary studies included within SRs or perform certainty of evidence assessments when missing. Additionally, given that we followed the outcome definitions of each SR, definition heterogeneity could have also impacted the validity and interpretability of our results. Nevertheless, effect estimates were generally consistent for most outcomes. Readers are encouraged to interpret the results of our NMA cautiously, since inclisiran lacked data from large RCTs compared to the other PCSK9i. Finally, populations underrepresented in trials are unavoidably underrepresented in our analysis.
ConclusionIn conclusion, based on the results of this overview and NMA, PCSK9i seemed effective on reducing MACEs, cerebrovascular events, coronary revascularizations and MI. Alirocumab was associated with reductions on all-cause mortality, while evolocumab on CV mortality. Inclisiran was only associated with reductions on MACEs. No evidence of reduction in heart failure was observed, while data on any CVE were conflicting. Use of PCSK9i was generally reported to be safe. Only injection-site reactions were associated with their use. Our results further strengthen current guideline recommendations on the use of PCSK9i. Future research should focus towards the conduct of RCTs with longer follow-ups, inclusion of patient subgroups frequently excluded from PCSK9i trials and cost-effectiveness studies.
FundingThis study received no funding.
Conflict of interestsThe authors declare that they have no conflict of interest.