To describe the clinical characteristics, the reasons for initiating therapy, and the effects of treatment in the initial phase of evolocumab availability in Lipid/ Internal Medicine Units in Spain.
MethodsRetrospective, observational study, based on the medical records of consecutive patients initiating treatment with evolocumab (from February 2016 to July 2017), in 20 Internal Medicine Units in Spain. A review was made of the demographic and clinical characteristics of the patients, the lipid lowering treatment, and the evolution of the lipid profiles between 12 weeks pre-initiation and 12 ± 4 weeks post-initiation of evolocumab.
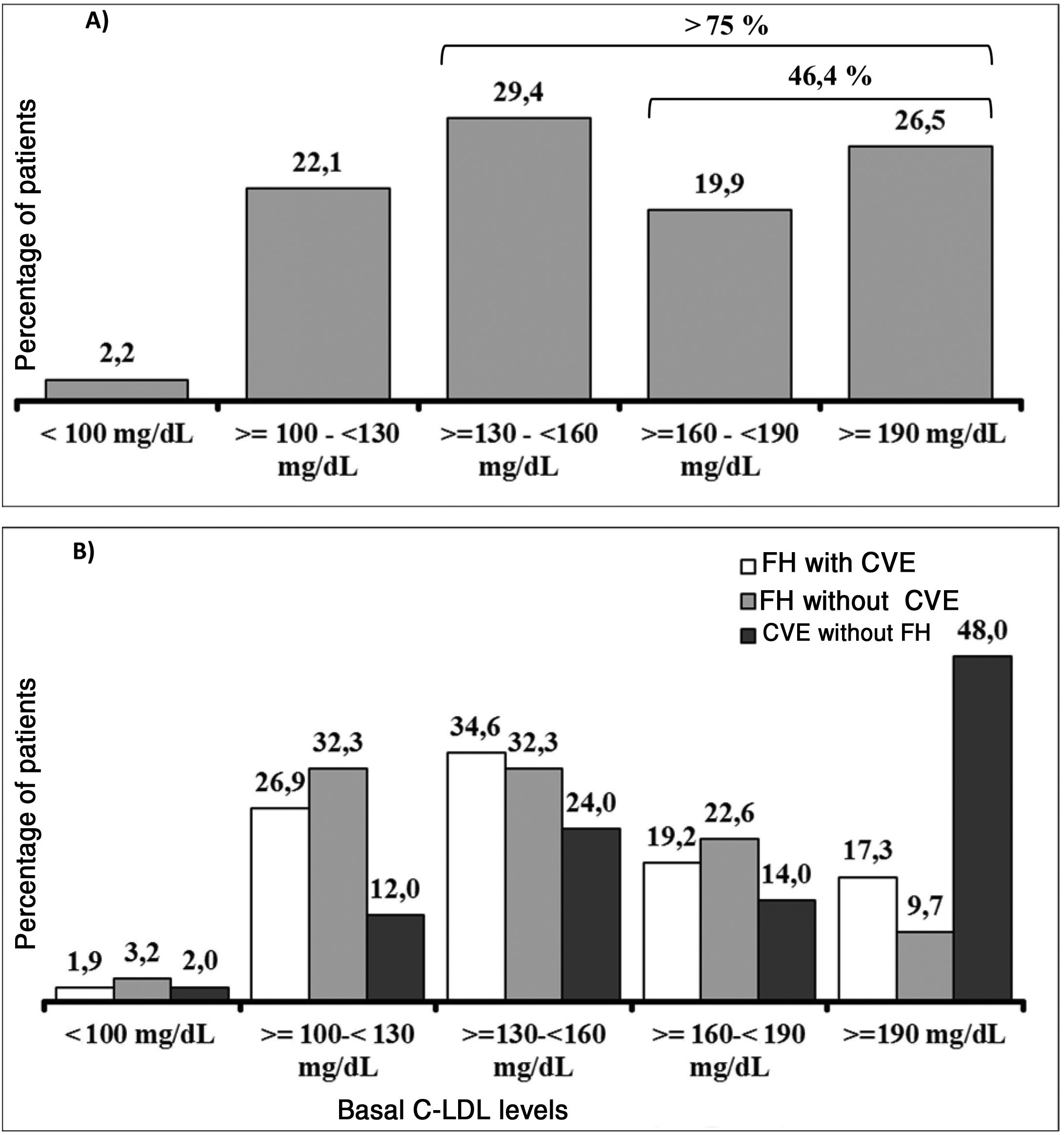
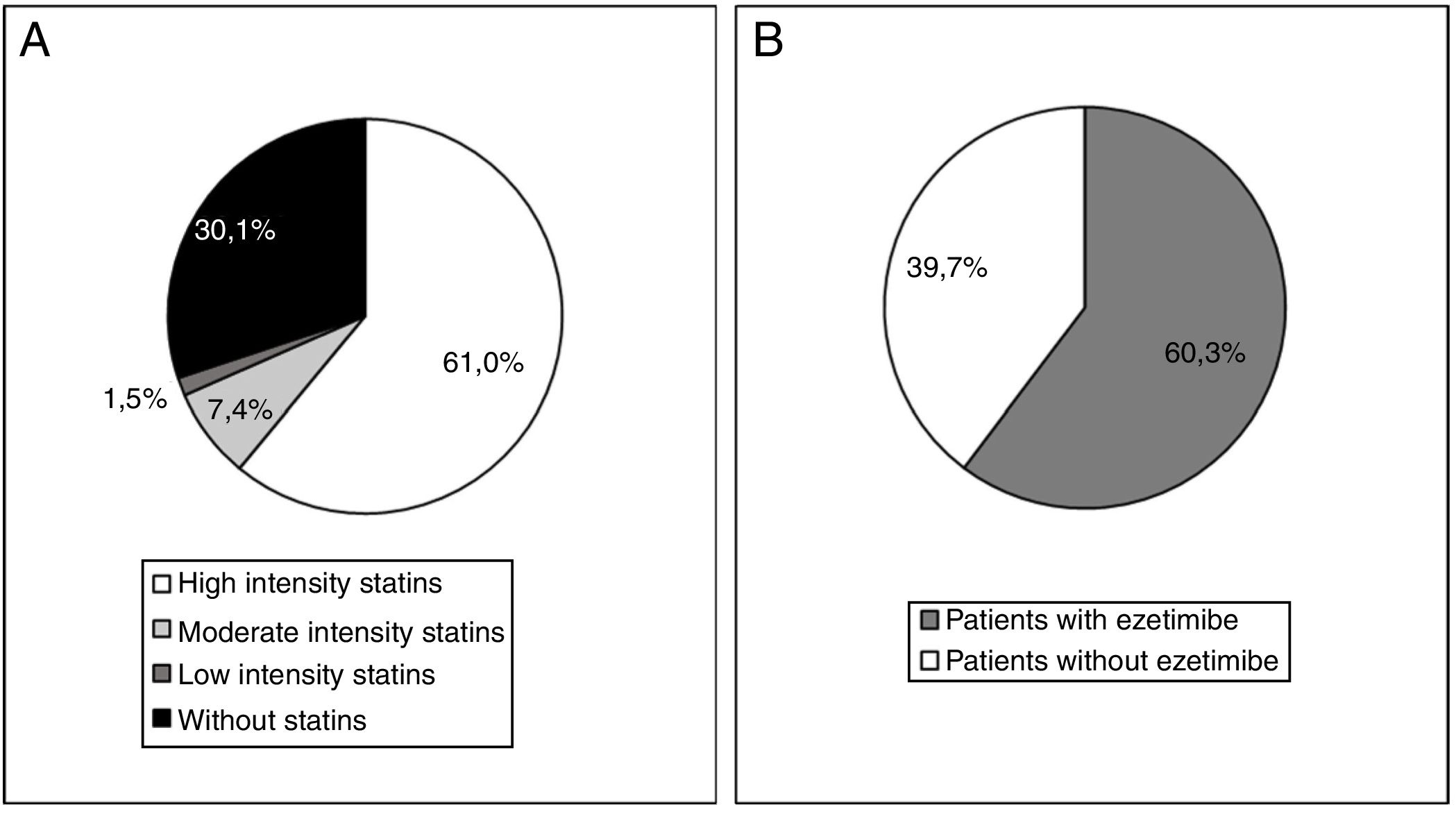
ResultsA total of 136 patients were analysed, of whom 64.0% were men, and the mean age (standard deviation, SD) was 56.6 (11.5) years. The large majority (75%) had familial hypercholesterolaemia (FH) (4 homozygous), and 51.0% of them had suffered at least one cardiovascular event. Atherosclerotic cardiovascular disease (ASCVD) was present in 61% of all patients. At initiation of evolocumab, 61.0% of the patients were taking high-intensity statins, and 60.3% were receiving ezetimibe. The mean (and SD) of LDL-c levels at initiation of evolocumab was 169.1 (56.6) mg/dL. The LDL-c was greater than 160 mg/dL in 46.4% of patients, and ≥190 mg/dL in 26.5%. During the observation period, evolocumab produced significant reductions in LDL-c of 55.7% (p < .0001), achieving mean values of 74.3 mg/dL. At week 12, more than half (53.8%) of patients achieved LDL-c levels <70 mg/dL, and 26.9% <50 mg/dL.
ConclusionsIn the Lipid/Internal Medicine Units, evolocumab was mainly prescribed in patients with FH, with or without ASCVD. The initial use of evolocumab was in accordance with the guidelines of the Spanish Society of Arteriosclerosis (SEA) of 2016, with LDL-c levels being well above the recommended thresholds for treatment initiation. Evolocumab treatment in clinical practice reduced LDL-C levels by about 55%, a similar reduction to that reported in clinical trials. Most patients achieved LDL-c goals.
Describir las características clínicas, las razones del inicio de la terapia y los efectos del tratamiento en la fase inicial de disponibilidad de evolocumab en las unidades de lípidos/medicina interna de España.
MétodosEstudio retrospectivo, observacional, a partir de las historias clínicas de pacientes consecutivos que iniciaron tratamiento con evolocumab (de febrero 2016 a julio 2017), en 20 unidades de Medicina Interna en España. Se revisaron las características demográficas y clínicas de los pacientes, el tratamiento hipolipemiante y la evolución de los perfiles lipídicos entre 12 semanas antes y 12 ± 4 semanas después del inicio de evolocumab.
ResultadosSe analizaron 136 pacientes: el 64,0% eran hombres, la edad media (desviación estándar, DE) fue de 56,6 (11,5) años. El 75,0% tenía hipercolesterolemia familiar (HF) (4 homocigotos) de los que el 51,0% habían sufrido al menos un evento cardiovascular (CV). El 61,0% del total de pacientes presentaban enfermedad cardiovascular aterosclerótica (ECVA). Al inicio de evolocumab, 61,0% de los pacientes tomaban estatinas de alta intensidad y el 60,3% estaban recibiendo ezetimiba. La media (DE) de los niveles de c-LDL al inicio de evolocumab fue de 169,1 (56,6) mg/dL. En 46,4% de los pacientes el c-LDL fue superior a 160 mg/dL y en el 26,5% ≥190 mg/dL. Durante el período de observación, evolocumab produjo reducciones significativas de c-LDL del 55,7% (p < 0,0001), alcanzando unos valores medios de 74,3 mg/dL. En la semana 12, el 53,8% de los pacientes alcanzó niveles de c-LDL <70 mg/dL y el 26,9% <50 mg/dL.
ConclusionesEn las Unidades de Lípidos/Medicina Interna, evolocumab se prescribió predominantemente en pacientes con HF con o sin ECVA. El uso inicial de evolocumab se ajustó a las pautas de la Sociedad Española de Arteriosclerosis (SEA) de 2016, estando las concentraciones de c-LDL claramente por encima de los umbrales recomendados para inicio de tratamiento. El tratamiento con evolocumab en la práctica clínica redujo los niveles de cLDL en torno al 55%, cifra comparable a los ensayos clínicos permitiendo alcanzar los objetivos terapéuticos en la mayoría de los casos.
Low density lipoprotein-vehiculated cholesterol (cLDL) is a causal factor of cardiovascular arteriosclerotic disease (CVAD),1 which originates a high level of morbimortality in our population.2 As a whole, the set of cardiovascular diseases (CVD) cause approximately one of every three deaths in Spain, slightly more so for women than men.3,4 Treatment with hypocholesterolemiants has helped to reduce cardiovascular (CV) mortality.5 More specifically, the use of statins (which became widespread in the final decade of the previous century) has given rise to an improved prognosis for CVAD. In the past 30 years, several controlled and randomised studies have supplied very solid scientific evidence for the benefit of hypocholesterolemiant treatment in case of cardiovascular risk (CVR).1,5
The development and clinical availability of PCSK9 inhibitors (PCSK9i) have led to a major advance in the management of hypercholesterolaemia. Evolocumab in particular has shown in multiple phase 2 and 3 clinical trials following its pharmacological development that it reduces cLDL by an average of more than 60%, regardless of the basal hypolipemiant treatment.6 In the FOURIER study, which included more than 27,500 patients with cerebral and peripheral atheramatous vascular cardiac disease, evolocumab reduced the number of events significantly over a period of 2.2 years, confirming that the fall in cLDL induced by the use of evolocumab leads to a lower risk of CV complications.7 These results are fully compatible with those foreseen by the Cholesterol Treatment Trialist Collaboration (CTTC).8 Similar data were obtained in the ODYSSEY OUTCOMES study, which used alirocumab9 in patients with recent acute coronary syndrome. Likewise, the SPIRE studies with bococizumab10 gave similar results, although they were interrupted due to the loss of efficacy associated with the development of anti-bococizumab antibodies. The efficacy of the PCSK9i in preventing CV is in line with their hypolipemiant effect and the duration of treatment, according to the results of the CTTC. In the FOURIER study the patients treated with evolocumab attained average cLDL concentrations of 30 mg/dl, so that it also showed that lower figures than those obtained in previous studies were associated with a greater clinical benefit without increased side effects. These results show that in the prevention of CV the lower the cLDL achieved the better, without to data having been able to set a minimum level of cLDL at which this benefit attenuates or disappears.11
In spite of the robust scientific evidence about the benefits of reducing cLDL levels to improve the prognosis of patients with high CVR, very few of them attain their therapeutic objectives. Recent fifth edition EUROASPIRE12 registry data show that 71% of the patients who have suffered a coronary event do not achieve the target cLDL level below 70 mg/dl, and in 37% of cases they have levels above 100 mg/dl. Additionally, hypolipemiant treatment in patients with familial hypercholesterolaemia (FH) is a long way from achieving its therapeutic objectives, even in patients followed-up in specialised lipid units.13 The beneficial effects of evolocumab have been demonstrated in population groups at high and very high CVR, achieving very high percentages of patients who attained their therapeutic objectives and even achieved lower figures, associated with greater clinical benefit.
In spite of the unquestionable hypolipemient efficacy of the PCSK9i drugs, their prescription has been restricted in Spain due to a supposedly better cost-benefit balance. It is only dispensed in hospitals and its use has been restricted to patients with secondary prevention, or those with FH and cLDL levels higher than 100 mg/dl following treatment with high intensity statins or with the maximum tolerated dose of the same. Additionally, these indications have been modulated by scientific societies such as the Sociedad Española de Arteriosclerosis (SEA). In 201614 this society issued recommendations that were recently updated on the basis of new scientific evidence that shows the efficacy of PCSK9i drugs in reducing CV events in different groups of patients.15 The European Medicines Agency recently approved the indication for PCSK9i to reduce CV complications, over and above simply reducing cholesterol, although the Spanish Medicines Agency has not yet updated the report on therapeutic positioning.16–19
The chief aim of this study was to evaluate which type of patients have been treated with evolocumab in the first phase of its clinical availability, in the internal medicine departments that most lipid units in Spain belong to, and its hypolipemiant efficacy within this context.
Patients and methodsThis multicentre, retrospective and observational study reviewed the clinical histories of patients who had received evolocumab as part of the usual clinical treatment of hyperlipidaemia in a lipids unit /internal medicine department in Spain. Patients aged ≥ 18 years were included who had started treatment with evolocumab prescribed by a doctor in a lipids unit /internal medicine department (regardless of study protocol) from 1 February 2016 to 31 July 2017, who had received at least one dose of evolocumab and who had at least one determination of cLDL within the 12 weeks prior to the start of treatment with evolocumab. Patients who had taken part in a study or had received treatment with a PCSK9i in the 12 previous weeks and/or who had taken part in a clinical trial during the study period were excluded. 20 hospitals took part based on their geographical distribution and level of care.
The protocol was approved by an ethics committee in each hospital, and all of the patients signed the informed consent document. Clinical data were recorded anonymously from 12 weeks prior to the start of treatment with evolocumab until 12 ± 4 weeks after this start. The last analytical parameters measured within the 12 weeks prior to starting treatment with evolocumab were considered to be basal values.
The indications for starting treatment with evolocumab were recorded: the presence of FH and/or CVD established in the 12 weeks prior to starting the study. The main efficacy variable measured the variation between the previous cLDL levels (during the previous 12 weeks) and subsequent ones (the final determination during the following 12 weeks) after commencing with evolocumab. The following variables were also recorded: age, sex, professional situation, weight, height and body mass index at the start of treatment with evolocumab, family medical history (FH or any other dyslipidaemia, death due to a CV event, diabetes mellitus or hypertension), personal CVAD (ischemic cardiopathy, cerebrovascular accident, peripheral arteriopathy), a personal history of dyslipidaemia (FH, combined familial hyperlipidaemia, other types of hypercholesterolaemia and mixed or combined hyperlipidaemia), diabetes mellitus, hypertension, a history of chronic nephropathy, liver failure, hypothyroidism and smoking. Their previous analytical parameters were determined (the last available figure in the 12 weeks prior to starting treatment with evolocumab and the values subsequent to starting treatment available in the patient’s clinical history and corresponding to the planned follow-up visits according to clinical practice): lipid profile (total cholesterol, cLDL, cHDL, triglycerides, non-HDL cholesterol and lipoprotein (a) [Lp(a)]. Additionally, analysis took place to detect whether the therapeutic objectives set by the clinical guides in force at the time (ESC/EAS 2016) for managing hyperlipidaemia had been achieved.20
Finally, the use of other hypolipemient treatments was recorded, together with any statin intolerance before starting treatment with evolocumab and all of the hypolipemient treatment in the 12 subsequent weeks. Statin intolerance was defined as intolerance to an initial statin at maximum tolerable dose and to a second statin at any dose with an adverse effect attributed to the drug that was resolved when it was withdrawn.
In an exploratory way the hospital and speciality of the doctor who referred the patient to the hospital internal medicine department to start treatment with evolocumab were also determined, together with the number of planned visits in the following months during the first year of treatment and other medical specialities consulted by the patients in the hospital during the study, the hospital in which treatment with evolocumab started and the speciality of the doctor who diagnosed hypercholesterolaemia.
Statistical analysisA descriptive statistical analysis was performed for all of the variables. The quantitative variables were described using central tendency and dispersion measurements (average, standard deviation [SD], minimum, 25% quartile [Q1], median, 75% quartile [Q3] and maximum for non-parametric variables). For qualitative variables frequency tables and parentages over the total number of responses that could be evaluated were used. In all cases 95% confidence intervals were used (CI 95%).
The demographic and clinical variables, medical family and personal histories, CVR factors and lipid profile evolution during the study were compared between the following patient subgroups: presence and type of FH, presence and type of diabetes, statin intolerance, basal cLDL ranges, primary and secondary prevention and age groups.
The change in cLDL levels was compared in different visits versus basal levels using the Student t-test for repeated measurements. Levels < .05 were considered to be statistically significant.
An analysis was also carried out of the cLDL levels at the last available measurement after starting treatment with evolocumab.
All of the statistical analyses were performed using version 9.4 of the SAS® System for Windows.
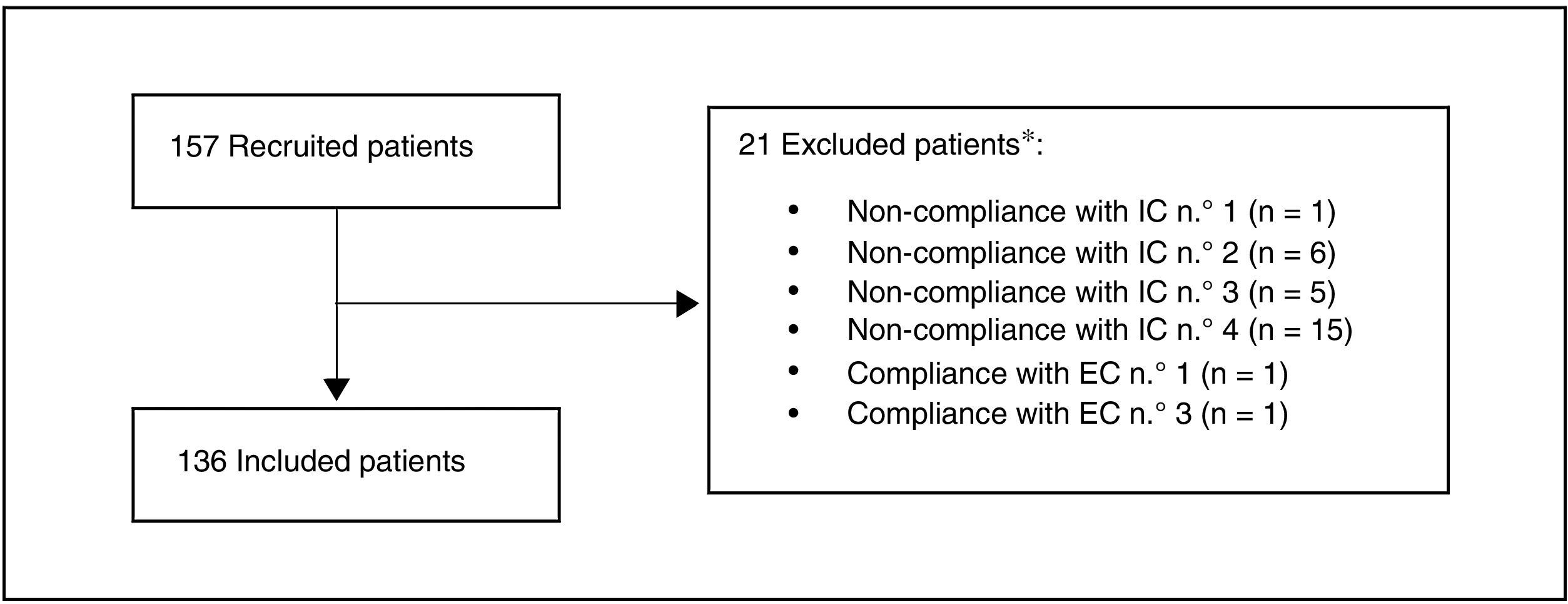
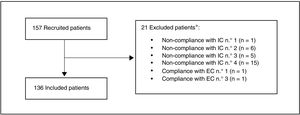
ResultsA total of 157 patients were recruited, of whom 21 were excluded as they did not meet the selection criteria. The most common reason for exclusion (71.4% of the patients) was that the date their final determination of cLDL was obtained was before the pre-established margin of 12 weeks (Fig. 1). Finally, 136 patients were included in the evaluation.
Study population.
*A patient may be excluded for more than one reason
Inclusion criteria (IC):
1. Adults (≥ 18 years) at moment of starting treatment with evolocumab.
2. Who started treatment with evolocumab based on medical criteria, regardless of study protocol, from 1 February 2016 and 31 July 2017.
3. Who received at least one dose of evolocumab (based on medical criteria)from a specialist in an internal medicine unit in Spain.
4. With at least one available determination of cLDL in the 12 weeks prior to starting to receive evolocumab (last available level in the 12 previous weeks).
Exclusion criteria (EC):
1. Having taken part in a study with a PCSK9 inhibitor in the 12 weeks before starting treatment with evolocumab.
2. Having received a PCSK9 inhibitor in the 12 weeks before starting treatment with evolocumab.
3. Having participated in a clinical trial during the retrospective observational period, i.e., 12 weeks before starting treatment with evolocumab or up to 12 weeks after it commenced.
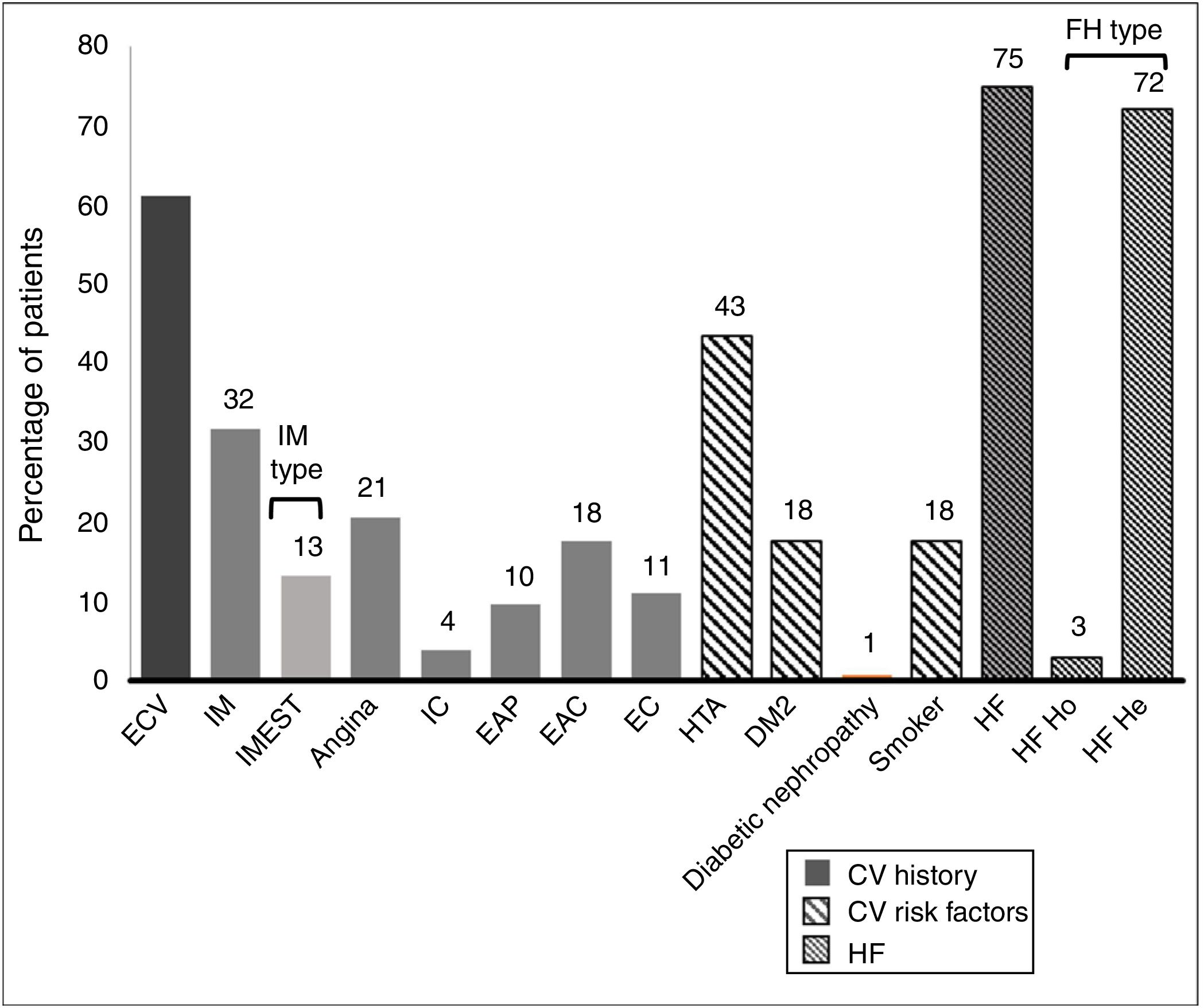
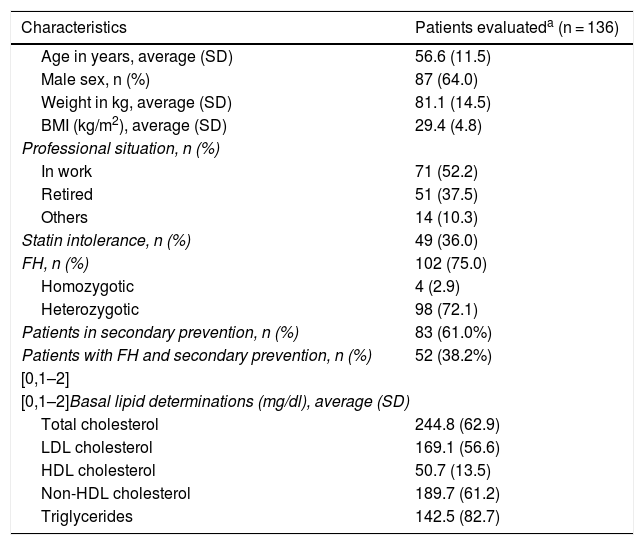
The average age (SD) of these 136 patients was 56.6 (11.5) years. 64.0% were men, and their average body mass index was 29.4 (4,8) kg/m2. The average cLDL level (SD) before starting treatment with evolocumab was 169.1 (56.6) mg/dl. Their sociodemographic, anthropometric and clinical data are shown in Table 1. 75.0% of the patients included were diagnosed as FH, of whom 4 were homozygotic. 51.0% of the patients with FH had previous CVAD, and 36.0% of all the patients were considered to be statin intolerant. These were mainly intolerant of atorvastatin (81.6% of the intolerant patients), followed by rosuvastatin (57.1%) and simvastatin (38.8%). The median(Q1-Q3) time from detection of intolerance was 4.0 (2.0-7.9) years.
Sociodemographic, anthropometric and basal clinical characteristics.
| Characteristics | Patients evaluateda (n = 136) |
|---|---|
| Age in years, average (SD) | 56.6 (11.5) |
| Male sex, n (%) | 87 (64.0) |
| Weight in kg, average (SD) | 81.1 (14.5) |
| BMI (kg/m2), average (SD) | 29.4 (4.8) |
| Professional situation, n (%) | |
| In work | 71 (52.2) |
| Retired | 51 (37.5) |
| Others | 14 (10.3) |
| Statin intolerance, n (%) | 49 (36.0) |
| FH, n (%) | 102 (75.0) |
| Homozygotic | 4 (2.9) |
| Heterozygotic | 98 (72.1) |
| Patients in secondary prevention, n (%) | 83 (61.0%) |
| Patients with FH and secondary prevention, n (%) | 52 (38.2%) |
| [0,1–2] | |
| [0,1–2]Basal lipid determinations (mg/dl), average (SD) | |
| Total cholesterol | 244.8 (62.9) |
| LDL cholesterol | 169.1 (56.6) |
| HDL cholesterol | 50.7 (13.5) |
| Non-HDL cholesterol | 189.7 (61.2) |
| Triglycerides | 142.5 (82.7) |
BMI: body mass index; CVE: cardiovascular event; FH: familial hypercholesterolaemia; SD: standard deviation.
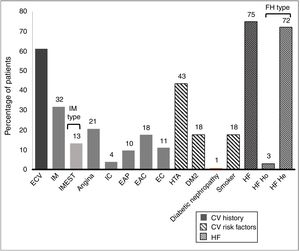
61.1% of the patients had an established history of CVAD. Their histories and basal CVR factors are shown in Fig. 2.
History and cardiovascular risk factors.
AHT: arterial hypertension; CAD: carotid arteriosclerotic disease; CD: cerebrovascular disease; CI: cardiac insufficiency; CV: cardiovascular; CVE: cardiovascular event; DM2: type 2 diabetes mellitus; FH: familial hypercholesterolaemia; He: heterozygote; Ho: homozygote; IMEST: myocardial infarct with raised ST segment; MI: myocardial infarct; Neph: nephropathy; PAD: peripheral arterial disease.
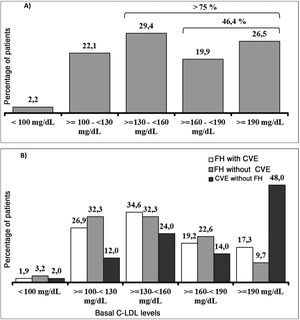
It should be underlined that 75.8% of the patients had cLDL levels higher than 130 mg/dl, while in 46.4% it was higher than 160 mg/dl and approximately one of every four patients (26.5%) had levels above 190 mg/dl (Fig. 3A). Their highest basal cLDL levels generally corresponded to patients with FH in primary prevention, and they were lower in patients without FH in secondary prevention. The basal levels of cLDL according to the clinical characteristics of the patients are shown in Fig. 3B.
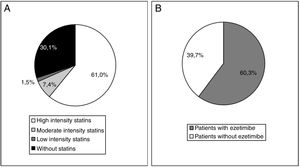
At the start of treatment with evolocumab 61.0% of the patients were taking high intensity statins, 60.3% were taking ezetimibe and 49.3% were taking both. The median (Q1-Q3) duration of treatment prior to starting with evolocumab was 3.9 (1.5-7.0) years for the high intensity statins and 4.7 (1.1-10.0) years for ezetimibe. Fig. 4 shows statin treatment intensity distribution (A) and with ezetimibe (B) before starting treatment with evolocumab.
Treatment with evolocumab produced an average fall of cLDL at 12 weeks of 55.7%, achieving average values of 79.6 mg/dl. More specifically, 26.9% of the patients attained cLDL levels below 50 mg/dl, 53.8% reached levels below 70 mg/dl and 74.4% reached levels below 100 mg/dl.
The results of the analysis of cLDL levels in patients who had data for their last observation after the start of treatment with evolocumab (n = 110) showed an average fall from basal level of 60.8%, achieving an average value of 74.3 mg/dl after a median follow-up time of 10.9 weeks.
Likewise, evolocumab also induced falls in non-HDL cholesterol (an average reduction of 49.9%), without finding a significant change in cHDL levels. There were too few patients with Lp(a) determinations after starting treatment to enable evaluation of the same.
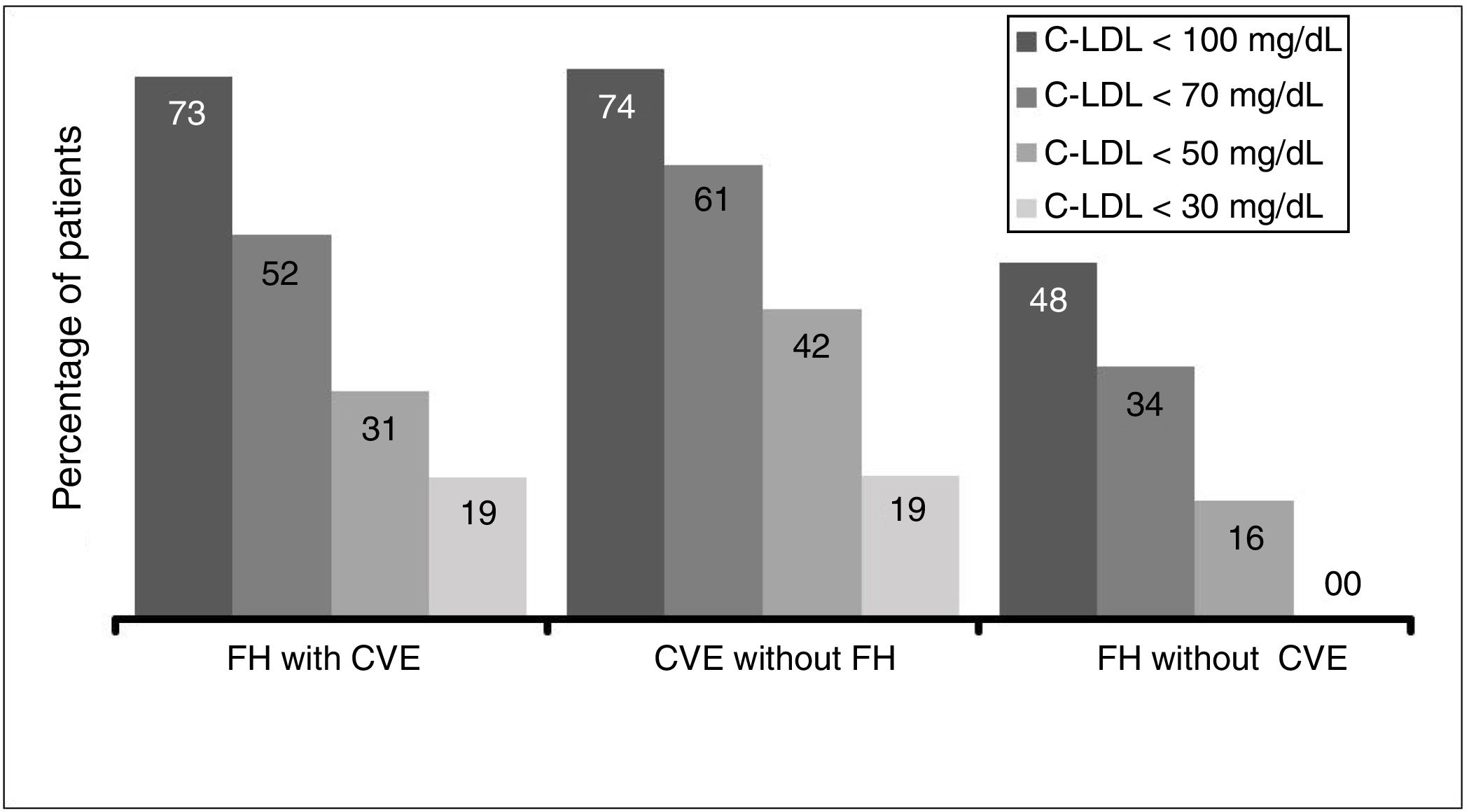
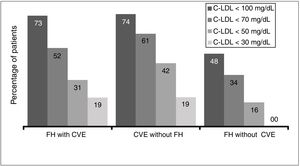
Due to the inclusion criteria, no patient was at target cLDL levels according to their clinical condition. The therapeutic objectives in the study populations (defined as cLDL levels < 70 mg/dl or cLDL < 100 mg/dl by the 2016 ESC/EAS guides) depending on the CVR situation were achieved by 51.9% of the patients with FH and a CV event, 61.3% of patients with a CV event and no FH and 48.0% of the patients with FH and no previous CV event (Fig. 5).
Basic hypolipemiant treatment was kept stable in the majority of the patients, and only 10.8% suspended their treatment with ezetimibe. The treatment with evolocumab was not suspended in any patient in the studied population, and only one patient increased the interval between doses due to discomfort at the point of injection.
The majority of the patients included in the study were already being managed by lipid units/internal medicine (68.4%), while 19.1% were detected by primary care and 8.1% were detected by cardiology and referred to the said units. Treatment with evolocumab involved an average of two visits per year for control and renewal of the prescription.
DiscussionThis work describes the clinical characteristics of the patients who were prescribed treatment with evolocumab in specialised lipid and CVR units in internal medicine departments in Spain during the initial phase of product commercialisation. As may be expected, the majority of the patients treated in these units had FH (75% of the sample), bases on two criteria: a very high level of cLDL in the case of primary prevention, or patients who had already suffered an event (51% of the patients with FH). The second group is composed of patients in secondary prevention without FH. As a whole, 61% of all treatments were prescribed for patients with established CVAD, and for 38% of patients with established CVAD and FH. The fact that the work centred on internal medicine units, the majority of which have SEA accredited lipid units, explains the high proportion of patients with FH. Moreover, as the study took place at the start of availability of the drug, many patients were included who had been monitored historically by the said units who had not attained their objectives and for whom no effective alternative treatment had been available. A clear sign of this is the high percentage of statin-intolerant patients who were included (36%). It also has to be said that 39% of the patients were not receiving high intensity statins (practically all of them were statin-intolerant), as well as the high percentage of patients treated with ezetimibe (60%). It is clear that patients in secondary prevention without FH represent a more numerous group of candidates for therapy with evolocumab. Nevertheless, and unlike the situation with FH, which is usually controlled and followed up in specialised dyslipedaemia units, subjects who have suffered an ischemic CV event are treated by cardiology, neurology or vascular surgery units. Subsequently, these patients are often followed up by primary care doctors. This circumstance may lead to a not insignificant group of these patients not receiving a suitable prescription for PCSK9i, as this drug is dispensed in hospital. Cardiac rehabilitation units, or otherwise lipid, vascular risk or secondary prevention units, play an especially important role, as these are units that determine the moment when PCSK9i is indicated.
It is striking that the average cLDL level at the moment of indication (169 mg/dl) was clearly above the authorised level for financing of the drug, which stands at 100 mg/dl, showing that the patients who started treatment clearly fulfilled the indications of the therapeutic positioning report. The fact that the starting point in this cohort is clearly above the recommended treatment thresholds, with almost half of the subjects with cLDL levels > 160 mg/dl and 26% > 190 mg/dl, may indicate that the first patients who were prescribed evolocumab were patients who had exhausted all of the other therapeutic options in attempting to achieve their cLDL objectives. It also has to be taken into account that the majority of this cohort are patients with FH and, therefore, with very high basal cLDL levels. In fact, the basal cLDL of the patients without FH in secondary prevention was clearly lower. Additionally, given the administrative barriers against prescribing PCSK9i, it is probable that in this initial phase patients with an unquestionable therapeutic indication were selected.
The SEA recently published its latest recommendations for the use of PCSK9i, where it clearly identified the patient profiles which will gain the greatest clinical benefit with these therapies.15 This may imply the indication of these drugs at lower cLDL levels in patients at especially high risk.
This study in the context of habitual clinical practice confirms the hypolipemiant efficacy of evolocumab, which produced an average fall in initial average cLDL concentrations of 56%, a similar result to that of the clinical trials. In this way, the average levels of cLDL fell below 80 mg/dl in spite of their high starting point (only 2.2% of the patients had basal levels lower than 100 mg/dl). Additionally, 54% attained cLDL levels below 70 mg/dl, and 27% attained levels below 50 mg/dl. One highly important datum is the high percentage of patients who attained their therapeutic objectives according to whether or not they had a previous CV event and/or FH. According to the inclusion criteria, no patient had complied with their therapeutic cLDL objectives; the majority of them were a long way from doing so. In spite of this starting point, approximately half of the patients with FH (51.9% with a CV event and 48% without a previous CV event) and 61% of the ones with a CV event without FH achieved their respective therapeutic objectives as set in the 2016 ESC/EAS guides20 after 12 weeks of treatment with evolocumab.
However, these treatment guides have been updated very recently, and they now specify much stricter therapeutic objectives for the control of cLDL. Patients with CVAD (with or without FH) should attain cLDL concentrations below 55 mg/dl, while in patients with FH without CVAD the therapeutic objective is to achieve cLDL levels below 70 mg/dl. Taking these new objectives into account, and in spite of the severity of the patients who started treatment with evolocumab at the start of its commercialisation, 30.8% and 41.9% of the patients with FH and a CV event or a CV event without FH obtained levels below 50 mg/dl, respectively. On the other hand, of the patients with FH without a previous CV event, 34% of them obtained levels below 70 mg/dl, indicating that at least one of every 3 patients would accomplish the stricter new objectives in the 2019 ESC/EAS guides.21
These data show the utility of treatment with evolocumab in achieving therapeutic objectives in high risk patients. Taking into account the recent data from the EUROASPIRE 512 registry, the possibility of increasing hypercholesterolaemia control to this degree in secondary prevention by using evolocumab is unprecedented. Moreover, the results of the FOURIER study, in which more than 87% of the patients were found to achieve cLDL levels ≤ 70 mg/dl after 48 weeks of treatment with evolocumab, also emphasises the usefulness of this drug in achieving therapeutic targets.7
Although the objectives of this study did not include monitoring pharmacological safety, the high level of adherence to the treatment should be mentioned, given that only one patient discontinued therapy with evolocumab temporarily.
This study has certain limitations: it is a retrospective study limited in observation time, so that it cannot offer data on the impact of the therapy over the longer term. The sample size is small, too, although it is based on strict selection criteria and exhaustive quality control of the data obtained. On the other hand, it centres on lipid units/internal medicine departments, with their particularities: a high frequency of severe dyslipidaemia and statin intolerance. It is probable that the power of evolocumab to attain therapeutic objectives in other contexts would be even greater. In connection with other lipid parameters such as Lp(a), given the observational nature of the study it was not possible to have sufficiently large samples at different points of the study to be able to evaluate its effects.
There are other analyses of clinical experience with evolocumab in Spain, such as the twin study of this one, the RETOSS-CARDIO study which was undertaken in cardiology units. The efficacy of evolocumab was comparable, as it gave rise to significant falls in cLDL of 58%, although with differences in population type, with more patients with CVD and without FH.22 Cordero and his collaborators recently published a retrospective multicentre analysis of the use of PCSK9i in 5 Spanish hospitals. They found significant falls in cLDL in approximately 68% and of 41% in comparison with the basal level after using evolocumab and alirocumab, respectively.23
This is the first study to report on the clinical profile of the patients treated with evolocumab in lipid units /internal medicine departments in Spain. In this first phase of availability of the therapy patients have been included with a marked diagnosis of severity, very high CVR and very high cLDL levels. Evolocumab has to offer clinical benefits to a population with less extreme indications and therapeutic financing that the one covered by this work, in agreement with the data of the scientific evidence now available.
FinancingThis study was financed by Amgen through an unrestricted subvention.
Conflict of interestsL. Masana has received fees for conferences or scientific consultancy services from Amgen, Sanofi-Regeneron, Mylan, MSD, Daiichi-Sankyo, Danone and Servier.
J. López Miranda has received fees for conferences or scientific consultancy services from Amgen, Sanofi-Regeneron, Ferrer, Esteve and Boeringher.
F. Civeira has received fees for conferences or scientific consultancy services from Amgen, Sanofi-Regeneron, MSD and Ferrer.
L. Reinares participated in the ODYSSEY study as head researcher in the Hospital Clínico.
C. Guijarro has received fees for talks, training and consultancy from Amgen, Ferrer, MSD, Pfizer and Sanofi.
N. Plana has received fees for conferences from Amgen, Sanofi-Regeneron and MSD.
R. Cuenca has received fees for conferences from Amgen and Esteve.
J.L. Hernández has received fees for talks or conferences and courses from Amgen, MSD, Sanofi and Esteve, together with research grants from Amgen.
R. Andrés has received fees for talks or conferences from Amgen and Sanofi.
A. Blanco has received fees for conferences or scientific consultancy services from Amgen, Sanofi-Regeneron and Servier.
S. Villamayor is an Amgen S.A. employee.
D. Sánchez has no conflict of interests to declare.
We would like to thank the medical department of Amgen for the design of the study in collaboration with the national coordinator of the same and the main researchers (scientific committee) in each hospital.
The manuscript was prepared by the scientific coordinator of the study and validated by the scientific committee, with the help of TFS S.L., which monitored the study, undertook the statistical analysis and revised the text of the manuscript.
Please cite this article as: Masana L, López Miranda J, Civeira F, Reinares L, Guijarro C, Plana N et al. Perfil clínico de los pacientes tratados con evolocumab en unidades de lípidos/medicina interna en España. Estudio observacional (RETOSS-IMU). Clin Investig Arterioscler. 2020;32:183–192.