The textbook outcome (TO) is a multidimensional measure to assess the quality of healthcare practice. This is reflected as the “ideal” surgical result, attending to a series of indicators or established reference points that are adapted depending on the surgical disease that we want to analyze. There are few references and series published about TO, all of them very recent.
ObjectiveWe present a series of gastric surgery from the TO perspective and we analyze its impact on survival.
MethodRetrospective observational study of all gastric neoplasms operated on in our center. Period: January 2015–December 2020. The criteria for TO were: margins R0, >15 lymph nodes in the histological study, no Clavien–Dindo complications > IIIa, hospital stay < 21 days, no mortality or readmission in the 30 postoperative days. A comparative analysis was performed between the TO group versus the non-TO group.
Results91 patients were operated on. We reached the TO in 34.1% of the patients. The variable >15 lymph nodes was the one that most affected to achieve a TO. When performing the survival analysis, we obtained that the group in which the TO was obtained had a greater survival (p < 0.008).
ConclusionIn our series, obtaining the TO has an impact on survival which 34,1% of degree of compliance.
El textbook outcome (TO), o resultado de libro, es una medida multidimensional para evaluar la calidad de la práctica asistencial. Ésta viene reflejada como el resultado quirúrgico «ideal», atendiendo a una serie de indicadores o puntos de referencia establecidos que se adaptan en función de la patología quirúrgica que queramos analizar. Son pocas las referencias bibliográficas y las series publicadas al respecto, todas ellas muy recientes.
ObjetivoValorar el grado de cumplimiento del TO y su impacto sobre la supervivencia.
MétodoEstudio observacional retrospectivo de todas las neoplasias gástricas intervenidas en nuestro centro. Periodo: desde enero del 2015 hasta diciembre del 2020. Se determinaron los siguientes criterios TO: márgenes R0, >15 ganglios linfáticos en el estudio histológico, sin complicaciones mayores (Clavien–Dindo > IIIa), estancia hospitalaria < 21 días, no presentar mortalidad en los 30 días posoperatorios ni readmisión durante esos 30 días. Se realizó un análisis comparativo entre el grupo de TO vs. grupo no TO.
ResultadosSe intervinieron 93 pacientes. Alcanzamos el TO en un 34,1% de los pacientes. La variable > 15 ganglios linfáticos fue la que más afectó a conseguir un TO Al realizar el análisis de supervivencia, observamos que el grupo en que se obtuvo el TO presentó mayor supervivencia (p < 0,008).
ConclusiónEn nuestra serie, la obtención del TO tiene impacto sobre la supervivencia con un grado de cumplimiento del 34,1%.
According to the World Health Organization (WHO) definition from 2000, quality of care is the achievement of intrinsic objectives to improve health by healthcare systems as well as responsiveness to the legitimate expectations of the population1. Its determination involves a series of dimensions, such as efficiency, effectiveness, access, technical competence, equity, adequacy, availability and opportunity. In short, to use a colloquial term, quality of care could be defined as the classic ‘good practice’ applied to healthcare2,3.
The field of medicine has progressed a great deal over the last century, and today’s globalized, interconnected and digitized society searches the Internet for hospital-related results when dealing with a specific pathology, seeking hospitals that provide ‘exemplary’ care and results.
New quality indicators include the textbook outcome (TO), proposed by Kolfschoten in 20134. Initially used in the context of colorectal oncology surgery, several groups have shown interest in this quality indicator, and in recent years TO models have been developed for the fields of esophagogastric, hepatobiliary and pancreatic surgery, etc5–12.
In the field of esophagogastric surgery, two recent articles have shown how achieving TO is independently associated with greater survival after adjusting for confounding variables4,6.
TO is a measurement of quality of care that encompasses a series of indicators, used as criteria to establish what is considered the ‘ideal’ surgical result4. To achieve this ideal result, it is necessary to analyze patient characteristics, surgical variables, complications, and the oncological quality of resections in an attempt to identify indicators or reference points that groups of expert surgeons consider ‘textbook’7. From the standpoint of patient information, the TO has the advantage of being an easily understood summary statistic, unlike other more complex quality indicators. It is easier for the patient to understand that a hospital has a percentage of patients with ideal results “who are doing well” than, for example, volume/mortality or mean hospital stay of a procedure which, although useful as quality assessment tools, may not be relevant information for patients6. We present a gastric surgery series from the perspective of TO, conducted at a tertiary hospital over a five-year period, with the aim of evaluating the level of compliance and its impact on survival.
MethodsA retrospective observational study was conducted from January 2015 to December 2020 including all gastric cancers treated surgically at our hospital. The administration (or not) of neoadjuvant therapy was determined by a multidisciplinary clinical committee on a weekly basis. The inclusion criteria were: patients with gastric cancer who had undergone elective surgery with curative intent. Data on patient, tumor and treatment characteristics were obtained from the database of the Esophagogastric Surgery Unit, written records, and electronic medical records. Comorbidity was evaluated according to the American Society of Anesthesiology (ASA) classification, Charlson index, and age8–10. Tumors were staged according to the TNM classification (8th edition)11. Postoperative complications were determined using the Clavien–Dindo classification12. Based on the Kalff et al. definition13, the reference points to establish TO were the following: R0 margins, >15 lymph nodes in the histological study, no Clavien–Dindo complications > IIIa. In addition to the above, the patients included in the TO group had to have a hospital stay of <21 days, no mortality within 30 postoperative days, and no readmission during those 30 days7,13. Survival time was calculated from the date of surgery to the date of all-cause mortality or the last follow-up visit.
Statistical analysisThe log-rank test was used to compare the survival of patients with or without TO. Patient, tumor, and treatment characteristics were compared between patients with and without TO. Mean quantitative variables and standard deviation (SD) were determined, and the variables were compared using the chi-squared test and the Mann–Whitney U test for continuous variables. COX multivariate regression models were used to analyze the association between TO and patient-adjusted survival, considering a P < .05 statistically significant in the analysis. The analysis was performed with SPSS v.25® (IBM Corp, Armonk, NY, USA).
ResultsDuring our study period, 93 gastrectomies were performed with curative intent. Out of the total of 93 gastrectomies, surgical resection was achieved in 85 patients (8 were unresectable), yielding a resectability rate of 91.3%. Mean patient age was 68.1 years; 65.9% were males and 34.1% females. The mean Charlson Comorbidity Index score of the series was 5.8. The most frequent tumor locations were the gastric body (44.7%) and antrum (45.9%). 67.1% of the patients did not receive preoperative neoadjuvant therapy. The ASA distribution was: 35.2% ASA I, 42.4% ASA II, 21.2% ASA III, and 1.2% ASA IV. Most of the gastrectomies performed were total (60%; 40% subtotal).
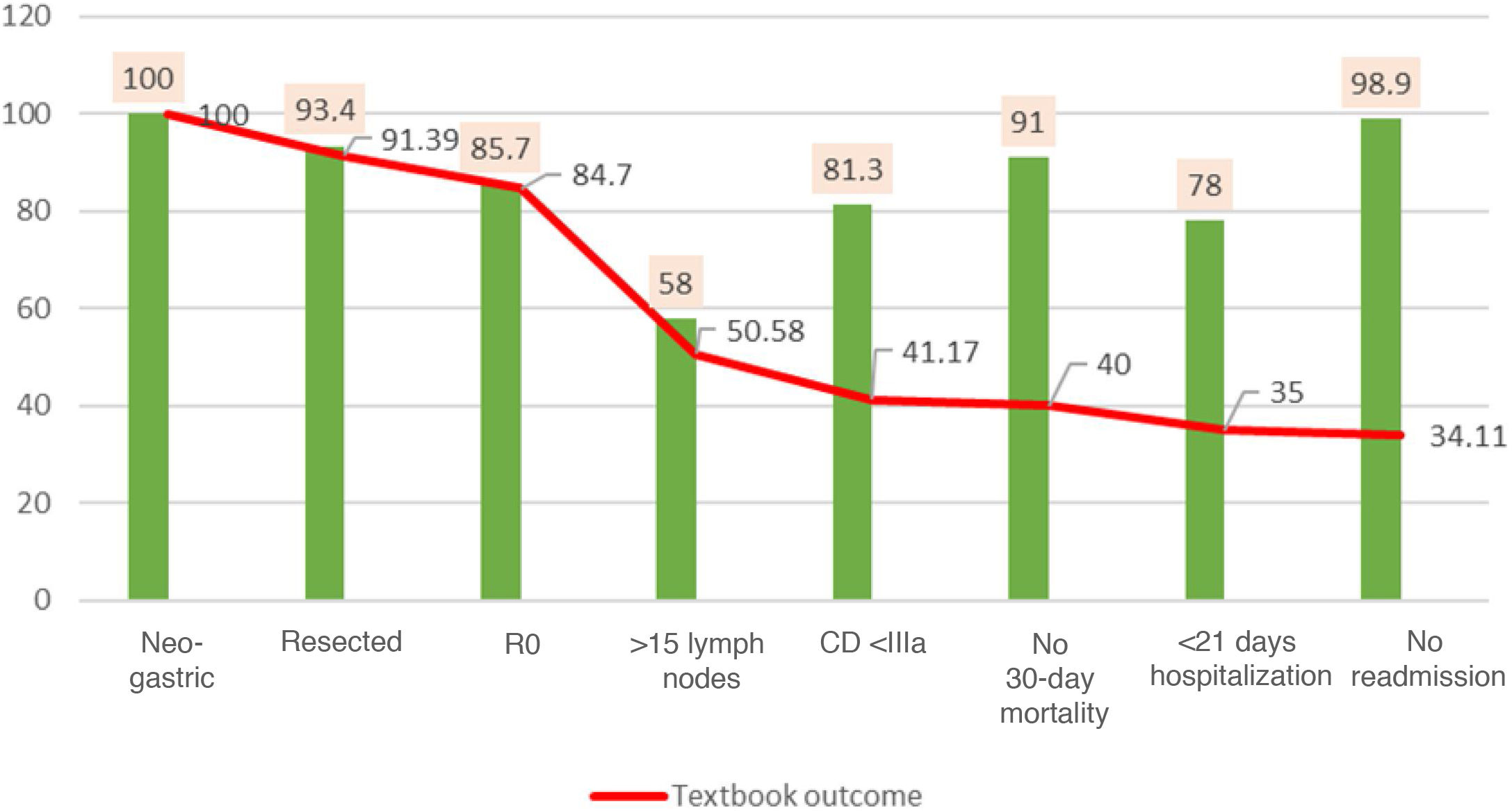
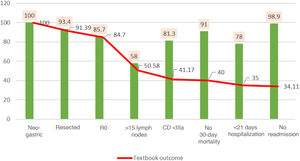
The search for TO criteria began with 100% of the patients treated with resection. After histological analysis of the specimens, 84.7% were found to be R0, meaning that obtaining R0 reduced the volume of patients by 15%. This figure dropped significantly to 50.5% when the >15 lymph nodes variable was included; this indicator represented by itself a reduction in TO in 1/3 of the series, which most reduced the gross number of patients, and only 58% of the total reached this requirement. When we included complications according to Clavien–Dindo, the previous percentage was reduced by 9.4%, with only 41.1% TO patients remaining. The percentage of patients was reduced by one point to 40% when we added no 30-day mortality. When we included the indicator of hospitalization <21 days, the percentage was reduced to 35%. Finally, when we included no readmissions within the 30 days of discharge, we obtained a final TO of 34.1% (Fig. 1).
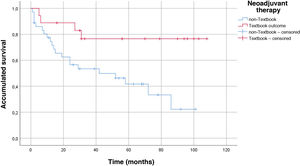
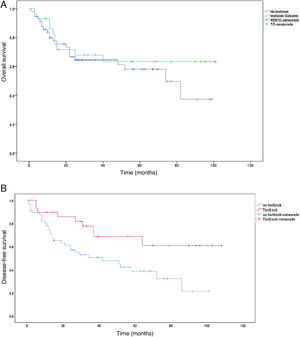
The main demographic characteristics for comparison between groups are summarized in Table 1. Mean age of the TO group was almost 4 years younger than the age of the non-TO group (65.7 vs 69.3 years). As for ASA, there were no differences in ASA I and II patients for obtaining TO, but there were differences in ASA III patients, as only 17.2% of these achieved TO. We did not obtain any textbook results in ASA IV patients. The distribution of patients who obtained TO vs non-TO according to the Charlson comorbidity index was 34.1% in the TO group vs 65.9% in the non-TO group. The histology of adenocarcinoma (intestinal or tubular) was diagnosed in the largest group of TO patients vs ‘other histologies’ (diffuse and signet ring cells), where the textbook result was 13.8%. There were no significant differences in the type of gastrectomy (total or subtotal) in either group, and this did not influence TO. There were no differences in the neoadjuvant group in reaching TO 34 vs 32%. We did find differences in terms of survival in the neoadjuvant group, and patients with previous treatment who also achieved TO lived longer (P = .008) (Fig. 2). The mean hospital stay was 16.2 days ± 12.7. The percentage of patients where >15 lymph nodes were obtained was higher in the TO group (23.9 ± 8.8 vs 13.8 ± 9.8), and this result had a P < .001.
Main characteristics of the series.
| Total n = 85 | Textbook outcome n = 29 | No textbook outcome n = 56 | P value | |
|---|---|---|---|---|
| n% | n% | n% | ||
| Age (mean ± SD) | 68.1 (12.7) | 65.7 (12.6) | 69.3 (12.8) | 0.212 |
| Sex % | 0.572 | |||
| Male | 65.9 | 65.5 | 66.1 | |
| Female | 34.1 | 34.5 | 33.9 | |
| ASA-score % | ||||
| I | 35.2 | 37.9 | 33.9 | 0.725 |
| II | 42.4 | 44.8 | 41.1 | |
| III | 21.2 | 17.2 | 23.2 | |
| IV | 1.2 | 0 | 1.8 | |
| Charlson score (mean ± SD) | 5.87 (2.58) | 34.1 (2.5) | 65.9 (2.5) | 0.072 |
| Histology % | ||||
| Adenocarcinoma (intestinal) | 89.4 | 86.2 | 91.1 | 0.478 |
| Other (diffuse and signet cell) | 10.6 | 13.8 | 8.9 | |
| Lymph nodes examined (mean ± SD) | 17.2 (10.6) | 23.9 (8.8) | 13.84 (9.8) | <0.001 |
| Affected lymph nodes (mean ± SD) | 5.1 (8.2) | 5.1 (5.5) | 5.1 (9.3) | 0.978 |
| Days of stay (mean ± SD) | 16.2 (12.7) | 10.7 (3.5) | 19.1 (14.7) | 0.004 |
| Survival in months (mean ± SD) | 38 (29.5) | 50.5 (31.5) | 31.5 (26.5) | 0.004 |
| Disease-free time in months (±) | 40.3 (32.1) | 50.5 (36.8) | 35.1 (28.2) | 0.006 |
| Neoadjuvant % | ||||
| No | 67.1 | 65.5 | 67.9 | 0.507 |
| Yes | 32.9 | 34.5 | 32.1 | |
| Gastrectomy type % | ||||
| Subtotal | 40 | 29.4 | 70.5 | 0.455 |
| Total | 60 | 37.2 | 62.7 | |
| Location % | ||||
| EGJ | 3.5 | 3.4 | 3.6 | 0.580 |
| Fundus | 5.9 | 3.4 | 7.1 | |
| Body | 44.7 | 48.3 | 42.9 | |
| Antrum/pylorus | 45.9 | 44.8 | 46.4 | |
| Stage pT % | ||||
| T0 | 2.4 | 6.9 | 0 | 0.126 |
| T1 | 7.1 | 3.4 | 8.9 | |
| T2 | 17.6 | 13.8 | 19.6 | |
| T3 | 29.4 | 24.1 | 32.1 | |
| T4a | 38.8 | 48.3 | 33.9 | |
| T4b | 3.5 | 0 | 5.4 | |
| Stage pN % | ||||
| Nx | 2.4 | 0 | 3.6 | 0.228 |
| N0 | 38.8 | 41.4 | 37.5 | |
| N1 | 15.3 | 3.4 | 21.4 | |
| N2 | 20 | 24.1 | 17.9 | |
| N3a | 17.6 | 20.7 | 16.1 | |
| N3b | 4.7 | 6.9 | 3.6 | |
| Stage pM % | ||||
| M0 | 89.5 | 89.7 | 85.7 | 0.662 |
| M1 | 10.5 | 6.9 | 12.5 | |
| Lymphovascular invasion % | 47.1 | 44.8 | 48.2 | 0.565 |
| Perineural invasion % | 42.4 | 34.5 | 46.4 | 0.177 |
ASA: American Society of Anesthesiology; EGJ: esophagogastric junction; SD: standard deviation.
pM, Pn, pT: TNM stage after the pathology study.
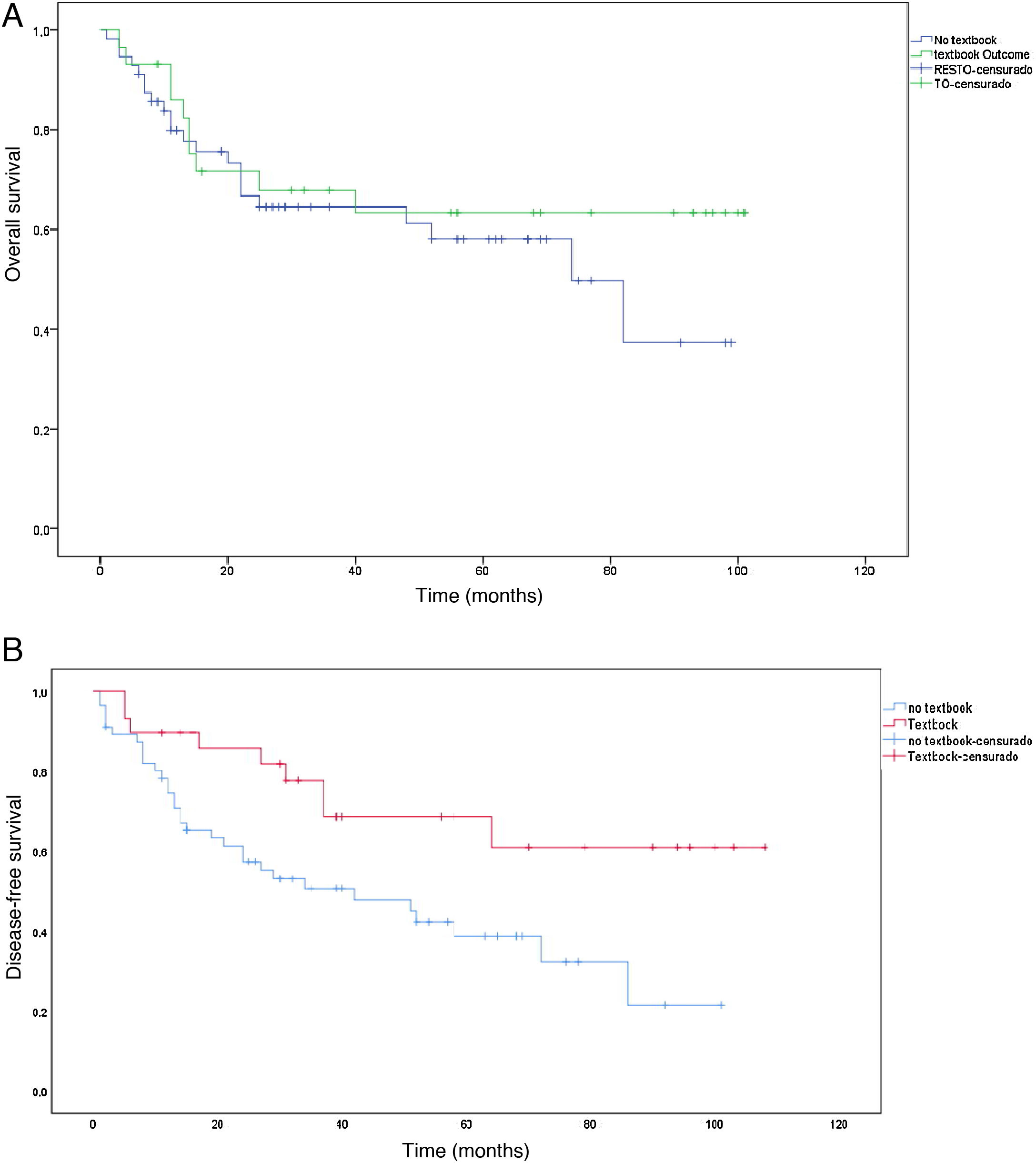
The median survival was 38 months, with an SD ± 29.5 months. Survival in the TO group (50.5 months ± 31.5 months) was longer than in the non-TO group (31.5 months ± 26.5 months) (P = .004). The same occurred with disease-free time, which was higher in the TO group (50.5 months ± 36.8 months) vs non-TO (35.1 months ± 28.2), but this result was not significant (P = .379). When we performed the survival analysis using the non-parametric Kaplan-Meier formula, comparing TO patients vs non-TO, we found an increase in survival in patients with TO (P < .008) (Fig. 3a). The same occurred with disease-free time, although without reaching statistical significance in this case (P = .379) (Fig. 3b).
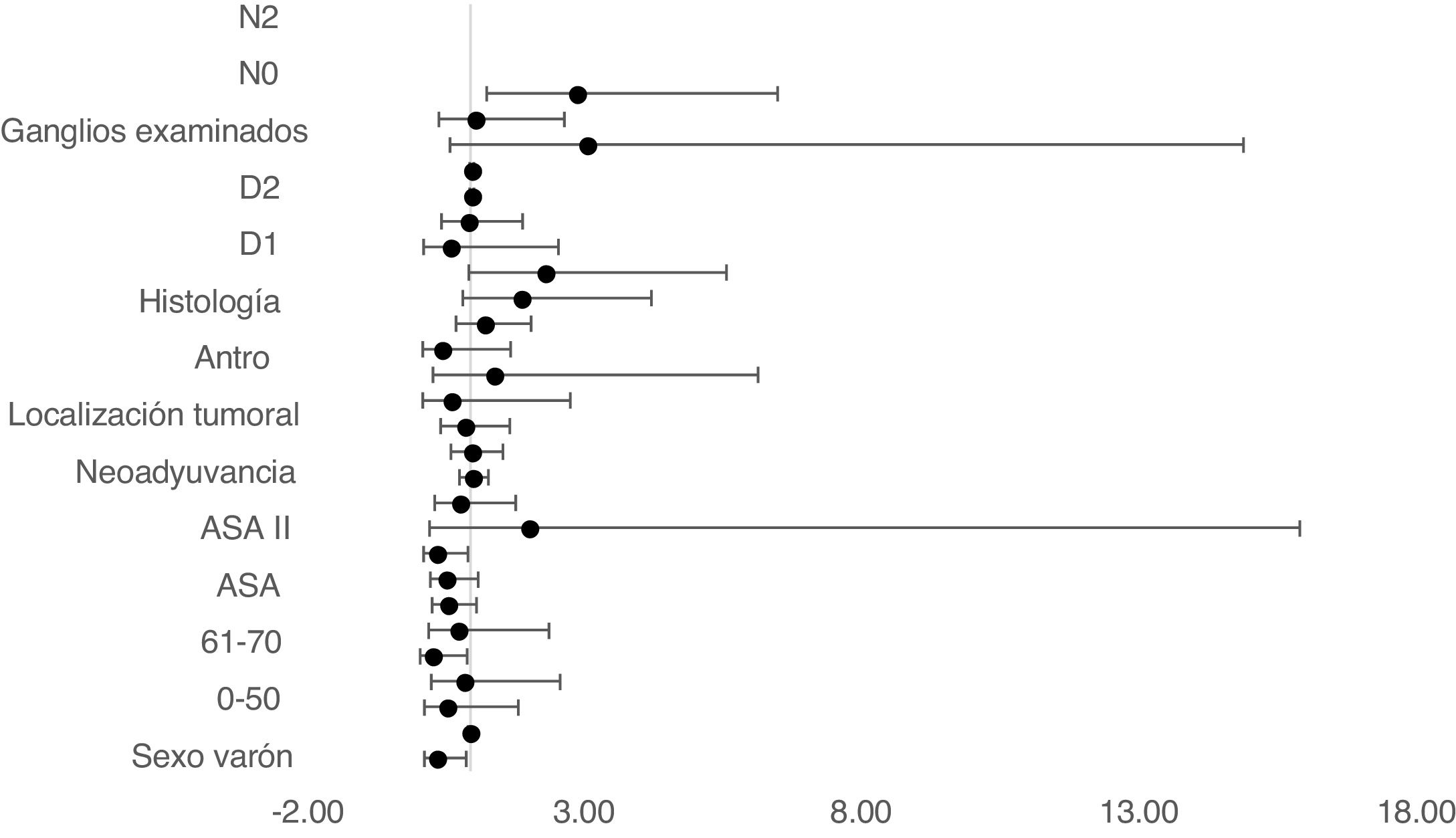
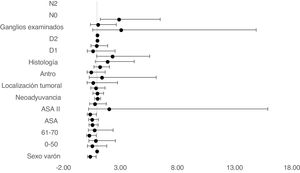
The multivariate Cox proportional hazards model adjusted for TO showed that age between 61–70, ASA II, D1 and D2 lymphadenectomy and no lymph node involvement in N0 were independent factors associated with survival (Table 2) (Fig. 4).
Association between textbook outcome and survival; Cox regression.
| Variable | HR | 95% CI inf | 95% CI sup | P-value |
|---|---|---|---|---|
| Sex (male vs female) | 0.394 | 0.169 | 0.919 | 0.031 |
| Age at diagnosis | 0.982 | 0.960 | 1.004 | 0.111 |
| 0–50 | 0.567 | 0.173 | 1.86 | 0.349 |
| 51–60 | 0.887 | 0.301 | 2.614 | 0.828 |
| 61–70 | 0.307 | 0.1 | 0.939 | 0.038 |
| 71–80 | 0.77 | 0.245 | 2.415 | 0.653 |
| >80 | – | – | – | – |
| ASA | 0.589 | 0.315 | 1.104 | 0.099 |
| ASA I | 0.562 | 0.278 | 1.136 | 0.109 |
| ASA II | 0.389 | 0.158 | 0.959 | 0.04 |
| ASA III | 2.053 | 0.264 | 15.977 | 0.492 |
| ASA IV | – | – | – | – |
| Neoadjuvant therapy (yes vs no) | 0.809 | 0.360 | 1.819 | 0.609 |
| Charlson score | 1.028 | 0.798 | 1.324 | 0.830 |
| Tumor location | 1.017 | 0.650 | 1.593 | 0.940 |
| Gastric body | 0.892 | 0.466 | 1.71 | 0.731 |
| Gastric antrum | 0.646 | 0.148 | 2.812 | 0.56 |
| Fundus | 1.421 | 0.326 | 6.19 | 0.64 |
| Esophagogastric junction | – | – | – | – |
| Histology (intestinal vs other) | 0.486 | 0.137 | 1.723 | 0.264 |
| Lymphadenectomy | 1.246 | 0.742 | 2.092 | 0.406 |
| D1 | 1.917 | 0.860 | 4.275 | 0.112 |
| D1+ | 2.347 | 0.980 | 5.618 | 0.055 |
| D2 | 0.63 | 0.15 | 2.59 | 0.007 |
| Gastrectomy (total vs other) | 0.961 | 0.474 | 1.949 | 0.912 |
| Nodes examined | 1.025 | 0.989 | 1.062 | 0.171 |
| Affected lymph nodes | 1.023 | 0.984 | 1.063 | 0.254 |
| N0 | 3.10 | 0.64 | 14.96 | 0.160 |
| N1 | 1.083 | 0.434 | 2.704 | 0.864 |
| N2 | 2.909 | 1.294 | 6.541 | 0.010 |
| N3 | 0.000 | 0.000 | – | 0.981 |
ASA: American Society of Anesthesiology; HR: hazard ratio; 95%: confidence interval.
Our initial objective for performing this study was to assess the degree of compliance with TO at our hospital compared to other similar publications. In addition, our other objective was to see whether textbook outcomes were associated with greater survival.
TO is a tool used to measure quality of care by means of a series of parameters that, as a whole, are considered ‘ideal’. One of the most controversial aspects of TO is that the defining indicators have been selected by groups of experts from hospitals with high volumes of esophagogastric surgery. In the series by Busweiler et al. of 1772 gastric cancers treated during the period analyzed, there was a change in the criteria that defined R0, which could have altered the results13–15. Thus, an international definition of TO indicators is essential to be able to compare results.
Another consideration about TO is that it is an all-or-nothing measurement that can be very strict when it comes to classifying patients and outcomes. In addition, it does not distinguish between low-risk and high-risk patients. In the original Kolfschoten series, the percentage of low-risk patients who achieved a textbook result was only 60%, so the risk stratification of each patient may be an important parameter to analyze4.
One of the main applications of TO is that it is useful to compare results between hospitals or even between surgeons (those with excellent results could be mentors), while also assessing whether the volume of cases is related to obtaining TO. However, Levy et al compared this parameter in their multicentric series, finding no clear differences16.
In the few published series on TO in gastric surgery, the figures obtained range from 22%–45%16,17. In our series, we obtained 34.1% TO. We must emphasize that TO obtained in our patients with Charlson >6 or ASA IV was minimal. In our series, there were no differences in terms of sex when obtaining TO. One of the parameters that most influenced our series in obtaining TO was >15 lymph nodes analyzed in the surgical specimen, so the percentage of TO reduced by this parameter was 34.12%. In a very recent article, Levy et al also observed how the parameter >15 lymph nodes was one of the indicators that they achieved less frequently. As in colorectal cancer, it has been shown that specimen processing and surgeon-pathologist collaboration can increase the quality of the studied specimen16,18,19. The use of indocyanine green (ICG) has become popular in the last decade, and recent studies support its use for lymph node identification in gastric cancer20,21. Using this method, the Baiocchi series obtained an average of 37.9 lymph nodes per patient20. Therefore, we believe that improving surgeon-pathologist collaboration and the recent acquisition of ICG equipment at our hospital can help us improve this TO criterion. Our average patient hospital stay was 16.2 days. In our opinion, a stay of 21 days is too long to be considered an ideal result, especially since the increasingly generalized application of multimodal rehabilitation protocols reduces hospital stay22.
In terms of survival, we have observed increased survival in patients who achieved TO. The median survival of the TO group was 50.5 months vs the 31.5 months reached by the non-TO group (P = .004). It seems reasonable that patients who present severe postoperative complications usually delay subsequent chemotherapy treatment, which affects survival. However, the overall survival functions show a clear separation in the unadjusted survival curves from the beginning, which increases during the remainder of the observation period (Fig. 3a), indicating a long-term effect of TO on patient survival that is similar to other published series4,14,17,23. Regarding the proportional hazards model, although we obtained some significant results, our sample is so small that we do not consider these data suitable for drawing definitive conclusions.
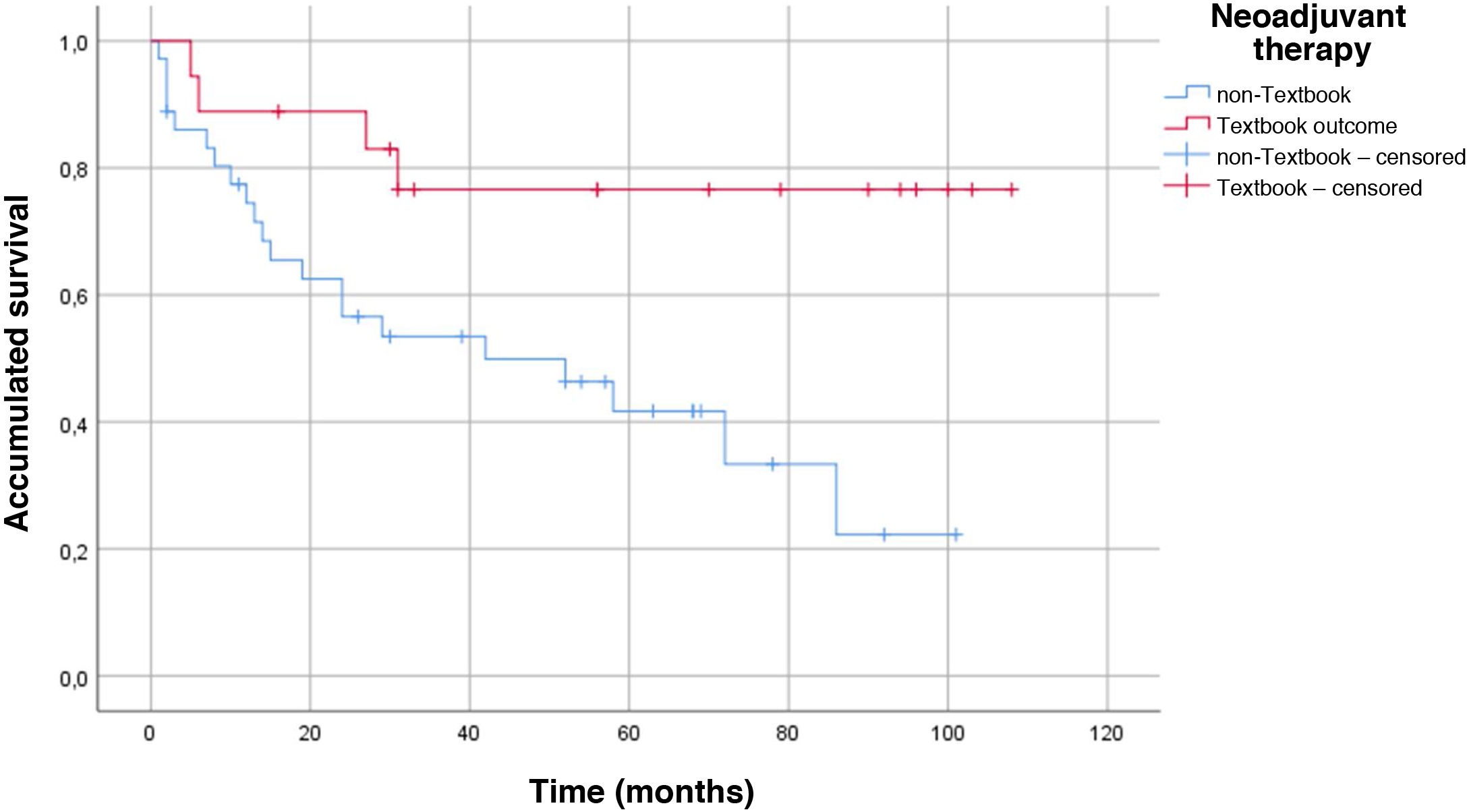
One datum that has not been previously evaluated is TO in patients who have received neoadjuvant therapy. The proportion of patients who received neoadjuvant therapy in our series is 32.7%. Following the protocol of our hospital, neoadjuvant therapy is administered to all T3 or N + after staging laparoscopy. The most widely used neoadjuvant regimen is 5Fu + leucovorin + oxaliplatin + docetaxel (FLOT) × 4 before surgery + another 4 cycles of FLOT after surgery24. Although one would expect that patients undergoing neoadjuvant therapy would obtain a lower rate of ideal results as they are in more advanced stages, this datum was not confirmed in our series, and we obtained the same percentage in TO vs non-TO patients (34% vs 32%; P = .507). Despite not finding significant differences when we analyzed the survival curve by neoadjuvant treatment and TO, we did obtain an increase in survival in the group with the ideal result and neoadjuvant treatment (P = .008) (Fig. 2).
Another parameter that has shown an impact on survival is the minimally invasive approach, and this is another interesting parameter whose inclusion in TO criteria has been controversial14. In our series, we have not included this approach since it is not part of the TO, although a percentage of our cases were performed by laparoscope.
One of the limitations of our study is that it is a limited, single-center sample. However, our team of surgeons is specialized in esophagogastric surgery, and the members of the multidisciplinary team have not varied during the period studied, which are factors that strengthen our data.
We believe that TO is a valuable benchmark for assessing gastrectomy quality that should be used in clinical research and healthcare quality improvement programs.
In conclusion, TO is a multidimensional measurement that is easy to perform and interpret for patients, healthcare professionals, and administrators who manage quality of care. It is necessary to reach a consensus regarding the parameters that define TO and to carry out multicenter studies to validate this quality analysis tool. In our series, TO was obtained in 34% of patients and is associated with greater survival and longer disease-free time.
Conflict of interestsThe authors have no conflict of interests to declare.