Robotic surgery has become a safe and effective approach for the treatment of pulmonary surgical pathology. However, the adoption of new surgical techniques requires the evaluation of the learning curve. The objective of this study is to analyze the learning curve of robotic anatomical lung resections.
MethodsRetrospective analysis of all robotic anatomical lung resections performed by the same surgeon between June 2018 and March 2020. The learning curve was evaluated using CUSUM charts to estimate trend changes in surgical time, surgical failure and the occurrence of post-operative cardiorespiratory complications throughout the sequence of cases.
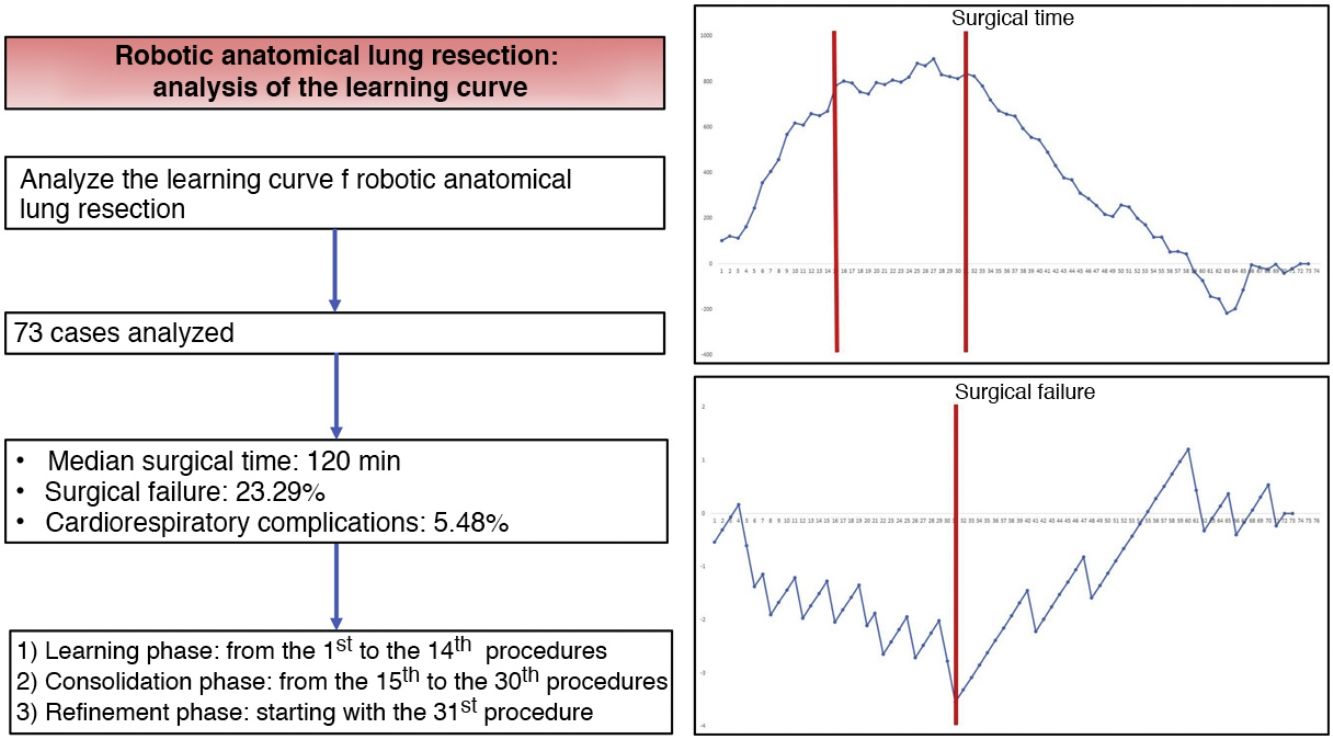
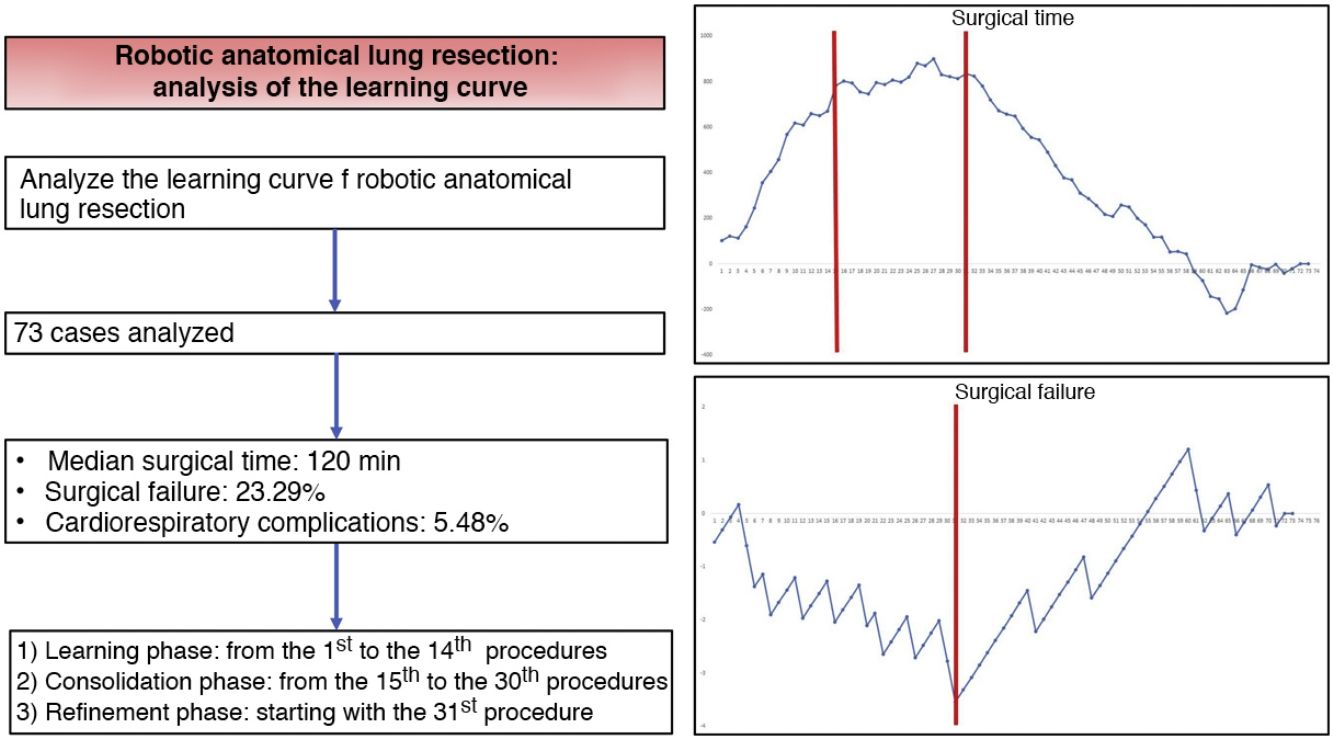
ResultsThe study included a total of 73 cases. The median duration of all complications was 120 min (interquartile range: 90−150 min), the prevalence of surgical failure was 23.29%, while 4/73 patients had any postoperative cardiorespiratory complication. Based on the CUSUM analysis, the learning curve was divided into 3 different phases: phase i (from the first to the 14th intervention), phase ii (between the 15th and 30th intervention) and phase iii (from the 31st intervention).
ConclusionsThe learning curve for robotic anatomical lung resections can be divided into 3 phases. The technical competence that guarantees satisfactory perioperative outcomes was achived in phase iii from the 31st intervention.
La cirugía robótica se ha convertido en una vía de abordaje segura y efectiva para el tratamiento de la patología quirúrgica pulmonar. Sin embargo, la adopción de nuevas técnicas quirúrgicas requiere de la evaluación de la curva de aprendizaje. El objetivo de este estudio es analizar la curva de aprendizaje de las resecciones pulmonares anatómicas por vía robótica.
MétodosAnálisis retrospectivo de todas las resecciones pulmonares anatómicas por vía robótica realizadas por un mismo cirujano entre junio de 2018 y marzo de 2020. La curva de aprendizaje se evaluó utilizando gráficas CUSUM para estimar los cambios en la tendencia del tiempo y los fallos quirúrgicos y la aparición de complicaciones cardiorrespiratorias postoperatorias a lo largo de la secuencia de casos.
ResultadosEl estudio incluyó un total de 73 casos. La mediana de duración de todas las intervenciones fue de 120 min (rango intercuartílico: 90−150 min), la prevalencia de fallo quirúrgico fue del 23,29%, mientras que 4/73 pacientes presentaron alguna complicación cardiorrespiratoria postoperatoria. Con base en el análisis CUSUM, la curva de aprendizaje fue dividida en 3 fases diferentes: fase i (desde la primera hasta la 14.a intervención), fase ii (entre la 15.a y la 30.a intervención) y fase iii (a partir de la 31.a intervención).
ConclusionesLa curva de aprendizaje para las resecciones pulmonares anatómicas por vía robótica puede dividirse en 3 fases. La competencia técnica que asegura resultados perioperatorios satisfactorios se consiguió en la fase iii, a partir de la 31.a intervención.
In recent years, robotic surgery has emerged as a new minimally invasive approach for the treatment of thoracic surgical pathology. Several studies have shown that it is a safe, feasible, and oncologically effective technique,1,2 capable of obtaining similar postoperative morbidity and mortality results to those achieved with video-assisted thoracoscopy surgery (VATS) when compared with the conventional open approach.3–5 In addition, some authors describe additional benefits in terms of better ergonomics, three-dimensional viewing, and optimized maneuverability thanks to the 360° rotation of the instruments.6
Although the first robotic lobectomies were described in 2003,7,8 the implementation of robotic technology in thoracic surgery is still limited. Recently, however, its use in lung resections has been increasing.
The implementation of new surgical techniques requires the evaluation of the surgeon’s learning curve. Although initial studies have shown that the learning curve for robotic anatomical lung resections ranges from 14 to 32 procedures,9–11 these studies have focused their analysis on the evaluation of surgical time and postoperative morbidity. However, since the occurrence of postoperative complications is mainly determined by patient characteristics,12 we consider that this variable is not a reliable reflection of the learning curve. On the contrary, the analysis of perioperative complications associated with the technique itself (surgical failure) could be considered a more precise tool for evaluating the acquisition of the technical skills necessary for satisfactory perioperative results.
The objective of this study is to analyze the learning curve of robotic anatomical lung resections by evaluating surgical time, surgical failure and cardiorespiratory morbidity using cumulative sum analysis (CUSUM) and risk-adjusted CUSUM.
MethodsFrom June 2018 to March 2020, 73 patients underwent robotic anatomic lung resection using the Da Vinci® system (Model X; Intuitive Surgical, Sunnyvale, CA, USA), performed by a single surgeon (MJ) at our hospital. Before starting to use the robot for lung resections, the surgeon had performed more than 200 anatomic VATS lung resections and 4 robotic thymectomies.
The selection criteria for patients who were candidates for robotic lung resection were based on the physiological evaluation of the patient recommended by current clinical practice guidelines13 and on the characteristics of the lesion to be resected. Patients who potentially required extended resection (associated with the chest wall, atrium, vena cava, diaphragm, vertebra, Pancoast tumors, sleeve resections, pneumonectomies or intrapericardial pneumonectomy) were not considered for this type of approach. The perioperative management of the patients was uniform throughout the study period.
The surgical technique is based on the use of the 4 robotic arms and an access port. First, we insert the camera through an 8-mm trocar at the 8th intercostal space on the mid-axillary line. The pleural cavity is analyzed with a camera at 0° angulation. Afterwards, we insert two 12-mm robotic trocars at the 8th intercostal space on the anterior axillary line at the insertion of the diaphragm and at the scapular line, respectively. The last robotic trocar is inserted into the 8th intercostal space at the triangle of auscultation and lung segment 6. Last of all, we insert an access port in the 9th intercostal space at the insertion of the diaphragm, just between the camera trocar and the 1st or 3rd trocar, creating an equilateral triangle. The position of this trocar depends on the lobe to be resected: between the camera trocar and the anterior port for the lower lobes, or between the camera trocar and the 3rd trocar for the upper lobes. We use CO2 insufflation at a pressure of 6−10 mmHg. The vessels, fissure and bronchus are dissected primarily with bipolar Maryland dissecting forceps, then divided with manual or robotic endostaplers. The surgical specimen is extracted with the help of a retrieval bag, lengthening the most anterior port. Lastly, we insert a 24 F tube through the camera incision. In all cases, a catheter for paravertebral analgesia is placed at the beginning of the procedure under endoscopic guidance.
The learning curve was evaluated based on the following results: surgical time, surgical failure, and postoperative cardiorespiratory complications. The surgical time was defined as the total duration of the procedure (from skin to skin), which includes both docking time and work time at the surgeon’s console. The perioperative adverse effects related with the technique (surgical failure) included: intraoperative complications, conversion, re-operation and postoperative complications associated with the technique (hemothorax, prolonged air leak, chylothorax, empyema, recurrent paralysis, wound hematoma, bronchial fistula). Postoperative cardiorespiratory complications included: respiratory failure, need for reintubation, need for mechanical ventilation >24 h, pneumonia, atelectasis requiring bronchoscopy, acute respiratory distress syndrome, arrhythmia requiring treatment, acute myocardial infarction, acute heart failure, cerebrovascular accident, and acute kidney failure. All complications were defined in advance following the recommendations published in the joint document of the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons.14
Statistical analysisBased on the results, the learning curve was analyzed using the CUSUM method for continuous variables (surgical time) and the standard CUSUM methods not adjusted for risk and risk-adjusted CUSUM for dichotomous variables (surgical failure and postoperative cardiorespiratory complications).
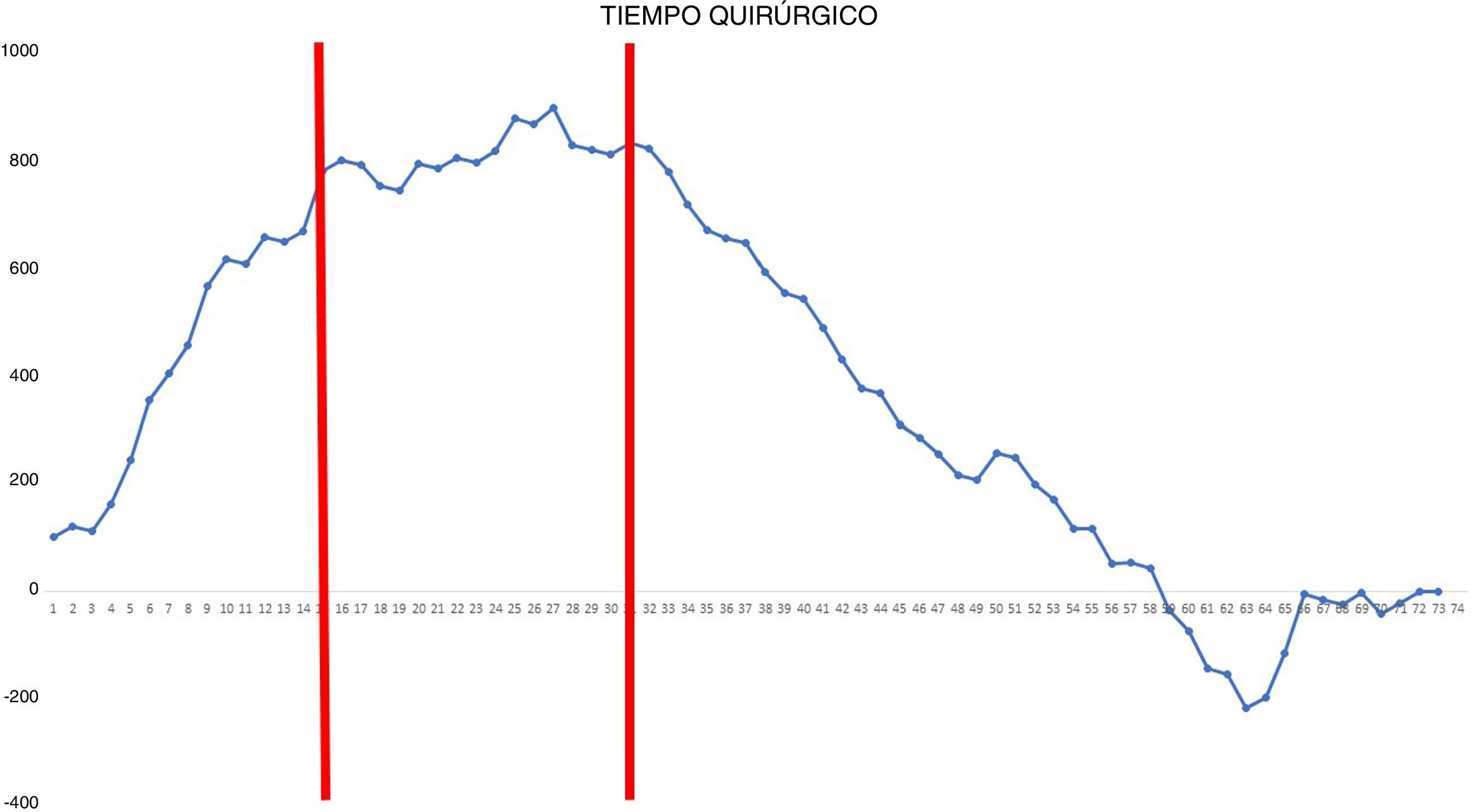
The surgical time was analyzed using the CUSUM method, which determines the differences of the accumulated total between the individual data and the mean of all the data.15 The patients were organized chronologically from the first patient in June 2018 to the last patient in March 2020. Subsequently, the difference was calculated between the result obtained and the mean of all the data for each patient. Finally, the accumulated sum of these differences was defined, and they were represented graphically. Line 0 of the chart represents the reference value that corresponds with the mean surgical time.
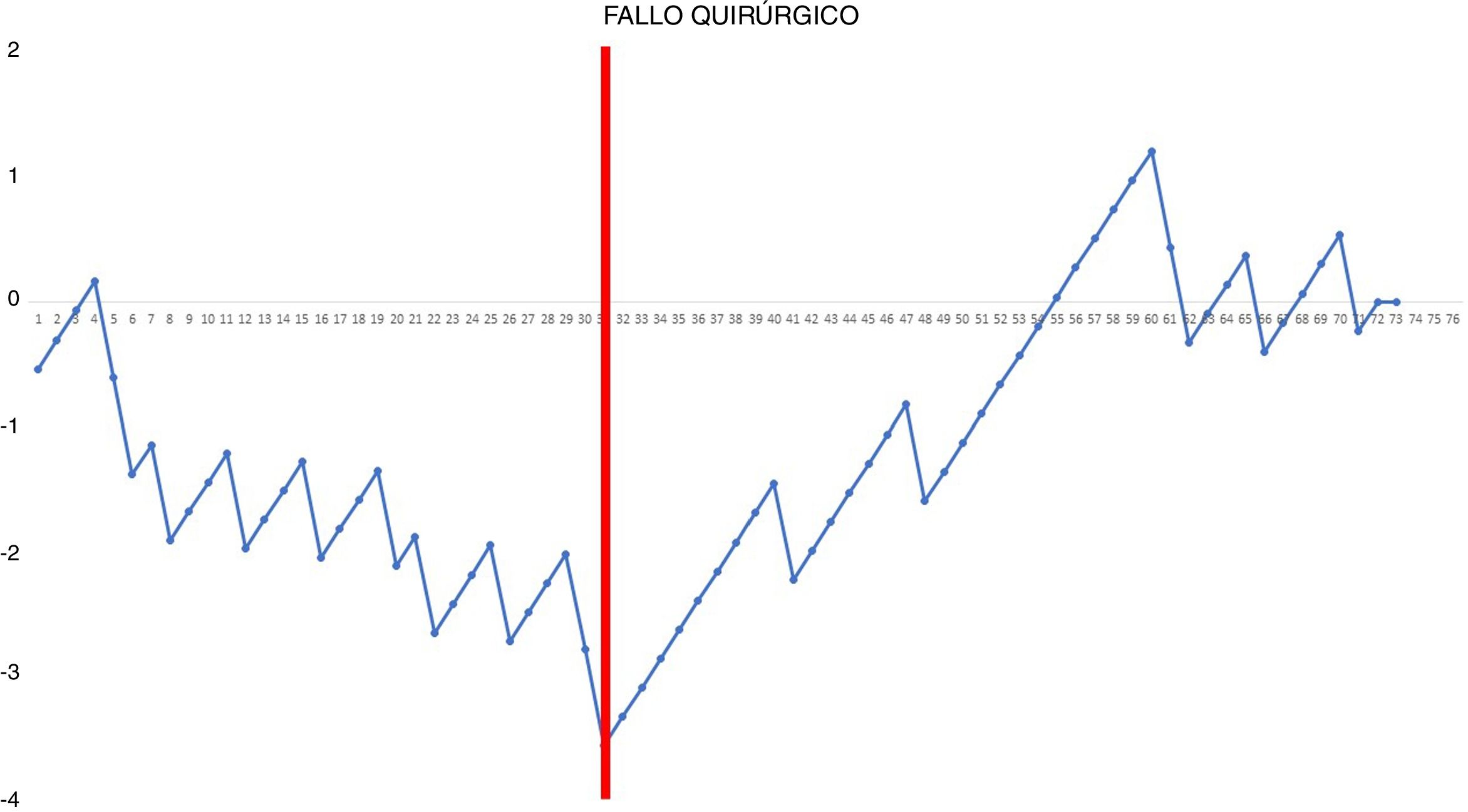
Surgical failure was analyzed using the standard CUSUM method (not adjusted for risk).15 Given that the basic principle of this type of analysis is to reward or penalize based on the risk of failure, which is constant for each case, before performing the analysis we calculated the risk of surgical failure from the global series. After organizing the patients chronologically according to their result (0 = no adverse effects and 1 = adverse effects), the difference between the result obtained (0 or 1) and the expected result (risk of surgical failure of the entire series) was calculated for each patient. Thus, when the patient did not present surgical failure, the reward obtained was equivalent to the risk of the global series of presenting failure: – (0 − risk of the global series). However, when a patient presented an adverse effect related to the technique, the penalization was –(1 − risk of the global series). Last of all, the accumulated sum of these differences was calculated and represented graphically. Line 0 of the chart represents the reference value that corresponds to the general prevalence of surgical failure.
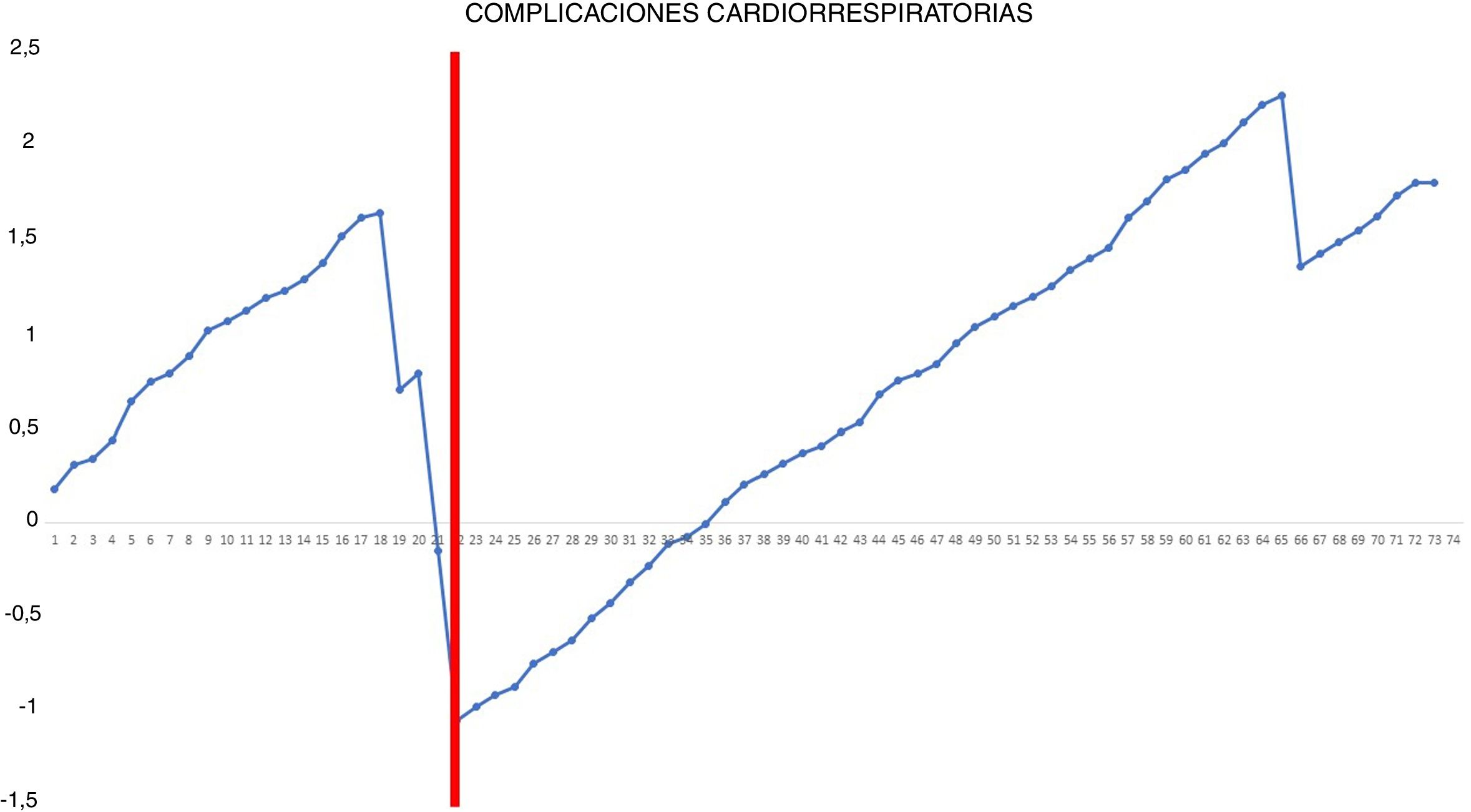
The occurrence of postoperative cardiorespiratory complications was analyzed using the risk-adjusted CUSUM method.15 Since this type of analysis considers the heterogeneity of the patients’ clinical characteristics, before performing the analysis, we calculated the individual risk of postoperative cardiorespiratory complications according to the Eurolung 1 risk model.16 After organizing the patients chronologically with their result (0 = no cardiorespiratory complications; 1 = cardiorespiratory complications), the difference between the result obtained (0 or 1) and the expected result (individual risk of complications according to the model) was calculated for each patient. Thus, when the patient did not present any postoperative cardiorespiratory complications, the reward obtained was equivalent to the individual risk of presenting it: – (0 − individual risk of complications according to the model). However, when the patient presented a complication of this type, the penalization turned out to be –(1 − individual risk of complications according to the model). Lastly, the accumulated sum of these differences was represented graphically.
The analysis of the demographic and clinical characteristics of the population was carried out using the SPSS® statistical software, version 26 (IBM Corp, Chicago, IL, USA, 2019), while the CUSUM charts were created with the Excel® program (Microsoft, Redmond, WA, USA).
ResultsThe study included a total of 73 cases. The demographic and clinical characteristics of the patients are listed in Table 1.
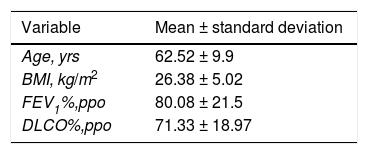
Clinical and demographic characteristics of the patients in the series.
| Variable | Mean ± standard deviation |
|---|---|
| Age, yrs | 62.52 ± 9.9 |
| BMI, kg/m2 | 26.38 ± 5.02 |
| FEV1%,ppo | 80.08 ± 21.5 |
| DLCO%,ppo | 71.33 ± 18.97 |
| N (%) | |
|---|---|
| Sex (male) | 32 (43.8) |
| Coronary disease | 2 (2.7) |
| Renal insufficiency | 1 (1.4) |
| Cerebrovascular disease | 0 (0) |
| Diabetes | 3 (4.1) |
| Hypertension | 18 (24.7) |
| Peripheral artery disease | 2 (2.7) |
| Previous neoplasm | 32 (43.8) |
| Type of resection | |
| Lobectomy | 56 (76.7) |
| Segmentectomy | 17 (23.3) |
| Diagnosis | |
| Lung carcinoma | 58 (79.5) |
| Metastasis of extrapulmonary origin | 8 (11) |
| Benign | 7 (9.6) |
DLCO%,ppo: percent predicted postoperative diffusing capacity for carbon monoxide; BMI: body mass index; FEV1%,ppo: predicted postoperative forced expiratory volume in one second.
The median duration of all the interventions was 120 min (interquartile range: 90−150 min). Seventeen of 73 patients had surgical failure. Table 2 lists the different perioperative adverse effects related to the technique of the series. The prevalence of cardiorespiratory complications was 5.48%. Two atrial fibrillations, one pneumonia and one cerebrovascular accident were registered. The mean risk of cardiorespiratory complications according to the Eurolung 1 model was 7.95%. No deaths were recorded in the series.
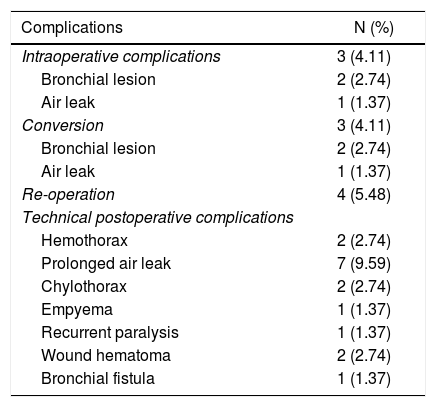
Perioperative adverse effects associated with the technique.
| Complications | N (%) |
|---|---|
| Intraoperative complications | 3 (4.11) |
| Bronchial lesion | 2 (2.74) |
| Air leak | 1 (1.37) |
| Conversion | 3 (4.11) |
| Bronchial lesion | 2 (2.74) |
| Air leak | 1 (1.37) |
| Re-operation | 4 (5.48) |
| Technical postoperative complications | |
| Hemothorax | 2 (2.74) |
| Prolonged air leak | 7 (9.59) |
| Chylothorax | 2 (2.74) |
| Empyema | 1 (1.37) |
| Recurrent paralysis | 1 (1.37) |
| Wound hematoma | 2 (2.74) |
| Bronchial fistula | 1 (1.37) |
CUSUM charts for surgical time, surgical failure, and postoperative cardiorespiratory morbidity can be seen in Figs. 1–3, respectively.
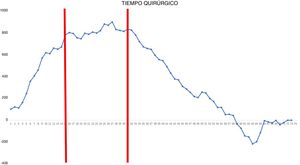
CUSUM chart for surgical time – The chart represents each procedure in the series, in chronological order from left to right. Two inflection points are observed in the curve: cases 15 and 31 (in red). This allowed us to identify 3 stages: stage I (from the 1st to the 14th procedures), in which the curve has an upward trend, indicating that the surgical time was greater than the mean of the series; stage II (between the 15th and 30th procedures), in which the curve remains relatively stable, indicating that the surgical time was similar to the mean time of the global series; and stage III (from the 31st procedure on), in which the curve has a downward trend, indicating that the surgical time was lower than the mean of the global series.
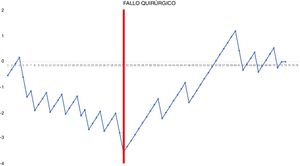
CUSUM chart not adjusted for risk for surgical failure with a constant risk of 20.55% – The chart represents each procedure in the series arranged in chronological order from left to right with the curve moving downward in the event of surgical failure and upward in the event of success. A single inflection point was identified at the 31st procedure (in red).
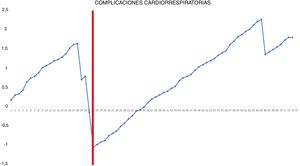
Risk-adjusted CUSUM chart for the occurrence of postoperative cardiorespiratory complications – The chart represents each procedure in the series, in chronological order from left to right, with the curve moving downward in the event of cardiorespiratory complications and upward in the absence of these. A single inflection point was identified at the 22nd procedure (in red).
In the surgical time chart, 2 inflection points were identified in which a change was observed in the trend of the duration of the intervention. The learning curve was divided into 3 stages: I (from the 1st to the 14th procedures), in which the curve has an upward trend, indicating that the surgical time was greater than the mean of the series; II (between the 15th and 30th procedures), in which the curve remains relatively stable, indicating that the surgical time was similar to the mean time of the global series; and III (from the 31st surgery on), in which the curve has a downward trend, indicating that the surgical time was lower than the mean of the global series. In the surgical failure chart, a single inflection point was identified at the 31st procedure, after which the curve showed an upward trend, indicating that the technical competence necessary to ensure satisfactory perioperative results was achieved from this procedure on. In the chart of cardiorespiratory complications, an inflection point was identified at the 22nd intervention, after which the curve showed a continuous upward trend, indicating the absence of complications after this procedure.
Based on the combined analysis of these charts, the learning curve was divided into 3 phases: initial learning (from the 1st to the 14th procedures), consolidation (between the 15th and the 30th procedures), and perfecting (from the 31st procedure on).
DiscussionRobotic surgery is a minimally invasive treatment strategy for thoracic surgical pathology and an alternative approach to VATS. Although several studies have shown that it is a safe and effective technique,2,3 its implementation requires the evaluation of the surgeon’s learning curve.
CUSUM charts are quality control charts that best adapt to the monitoring of clinical care processes.17 The main advantages of these charts are their simplicity, intuitive visual interpretation, and the ability to detect changes in trends regardless of sample size. Using CUSUM charts, it is possible to monitor the process in real time from its inception, making them useful for studying learning curves.18,19
In our study, the evaluation of surgical time using CUSUM charts allowed us to identify 3 different periods in the surgeon’s learning curve. However, the surgical time alone is not sufficient to conduct a multidimensional analysis of this curve. Technical competence should consider other surgical outcomes.20 The analysis of surgical failure, defined as the occurrence of perioperative adverse effects related to the technique, can be a more accurate indicator of the process of acquiring technical skills in robotic surgery.
In addition, our study shows that the occurrence of postoperative cardiorespiratory complications is not a useful indicator for evaluating the learning curve because of the low frequency of these events (only 4 in our series) and because they are more dependent on the intrinsic characteristics of patients than on the technical competence of the surgeon. Nonetheless, it is true that most of these complications occurred at the beginning of the second phase.
The results of our study are consistent with those obtained in previous analyses. Meyer et al.10 analyzed the robotic lobectomy learning curve in a series of 185 patients based on surgical time, mortality, and surgeon comfort, setting the learning curve at 15, 20 and 19 cases, respectively. Song et al.11 analyzed the learning curve of robotic lobectomy for lung cancer in a series of 208 patients using CUSUM analysis based on docking duration, console time and total procedure time, establishing the learning curve at 20, 34 and 32 cases, respectively. Toker et al.9 analyzed the results of 102 robotic anatomical resections, including lobectomies and segmentectomies, and established the duration of the learning curve at 14 cases. Meanwhile, Zhang et al.21 studied the learning curve of robotic segmentectomy, observing a decrease in surgical time after the 47th procedure, while the technical competence necessary to ensure satisfactory perioperative results was achieved after the 40th procedure.
The main limitation of this study is based on the possible heterogeneity of the operated patients in terms of surgical complexity, which is often not assessable preoperatively. Second, the sample size is relatively small to evaluate all levels of surgical complexity. Third, the surgeon had extensive experience in VATS lung resections and some degree of robotic experience, so the learning curve could be longer in surgeons without this type of prior experience.
In conclusion, our study demonstrates that the learning curve for robotic anatomical lung resections can be divided into 3 phases: the first 14 interventions were part of the initial learning period, the next 16 interventions were the consolidation phase, and the refinement period started at the 31st procedure. The technical competence that ensures satisfactory perioperative results was achieved in phase III, starting with the 31st procedure.
Conflict of interestsThe authors have no direct or indirect conflicts of interests related with the content of this manuscript.
Please cite this article as: Gómez Hernández MT, Fuentes Gago M, Novoa Valentín N, Rodríguez Alvarado I, Jiménez López MF. Resecciones pulmonares anatómicas por vía robótica: análisis de la curva de aprendizaje. Cir Esp. 2021;99:421–427.