Involvement of surgical resection margins is a fundamental prognostic factor in pancreatic oncological surgery. However, there is a lack of standardized histopathology definition. The aims of this study are to investigate the real rate of R1 resections when surgical specimens are evaluated according to a standardized protocol and to study its survival implications.
Patients and methodsOne hundred consecutive surgically resected patients with pancreatic ductal adenocarcinoma were included in the study. They were further divided into 2 groups: pre-protocol, evaluated before the introduction of the standardized protocol and post-protocol, analyzed with the standardized protocol.
ResultsR0 resection rate in the pre-protocol group was 78%, falling to 47% after the introduction of the standardized protocol (P=.003). The posterior retroperitoneal margin was the most frequently involved margin. In cases with tumors located at the pancreatic head and analyzed according to the standardized protocol R1 involvement negatively affected survival. Median survival in the R0 group was 22 months versus 16 in those with the margin involved (HR: 2.044; IC 95% 1.00–4.16; P=.043).
ConclusionsStandardized evaluation of the retroperitoneal margins in pancreatic cancer increases the rate of R1 patients. In cases with pancreatic cancer located at the pancreatic head involvement of posterior retroperitoneal margin significantly decreases survival.
La afectación microscópica de los márgenes de resección es un factor pronóstico fundamental en la cirugía del cáncer de páncreas. Sin embargo, su definición anatomopatológica no está estandarizada. Este estudio pretende identificar el porcentaje real de pacientes con resecciones R1 al analizar las piezas quirúrgicas con un protocolo estandarizado y evaluar sus implicaciones sobre la supervivencia.
Pacientes y métodosSerie de 100 pacientes consecutivos intervenidos por adenocarcinoma ductal de páncreas y resecciones macroscópicamente completas, divididos en 2 grupos: pre-y posprotocolo, según se intervinieran antes o después de la aplicación de un protocolo estandarizado de las piezas de resección.
ResultadosEn el grupo preprotocolo la tasa de resecciones R0 fue del 78%, mientras que tras la aplicación del mismo, se redujo al 47% (p = 0,003). El margen posterior retroperitoneal es el que se encuentra afectado con mayor frecuencia. En los casos con tumores localizados en cabeza de páncreas y analizados con el protocolo estandarizado, la detección del margen retroperitoneal afecto (R1) influye de forma negativa en la supervivencia. La mediana de supervivencia del grupo R0 fue de 22 meses frente a 16 meses en los que presentaban margen afecto (HR: 2,044; IC 95% 1,00-4,16; p = 0,043).
ConclusionesLa aplicación de un protocolo estandarizado para el estudio del margen retroperitoneal en el cáncer de páncreas incrementa la proporción de pacientes R1. En los pacientes con cáncer de cabeza de páncreas, la afectación del margen posterior retroperitoneal reduce significativamente la supervivencia.
Pancreatic ductal adenocarcinoma is a biologically very aggressive tumor. Less than 25% of the patients are eligible for surgical treatment with curative intent, the only option used to achieve long-term survival.
One factor that could have a greater impact on surgical oncology outcomes is the involvement of resection margins, both in microscopic (R1) and macroscopic (R2) forms. Therefore, it is surprising that, unlike other types of tumors, many pancreatic cancer patients with oncologic resections also have high local recurrence and poor survival rates, regardless of whether surgical resections are R0 or R1. The lack of clear and direct relationship between microscopic involvement of resection margins and survival that has been demonstrated in different case series and a meta-analysis1–7 could be a reflection of the great variability in the case series rate of margin involvement, ranging between 16% and 85%.6,8 This variability leads to the consideration that there is a lack of standardized protocols for the histopathological analysis of resected specimens, making it likely that the true rate of R1 resections is being underestimated.
This study aims to identify the actual percentage of patients with R1 resections when analyzing surgical specimens with a standardized protocol, and also to evaluate the prognostic effect of microscopic margin involvement on survival.
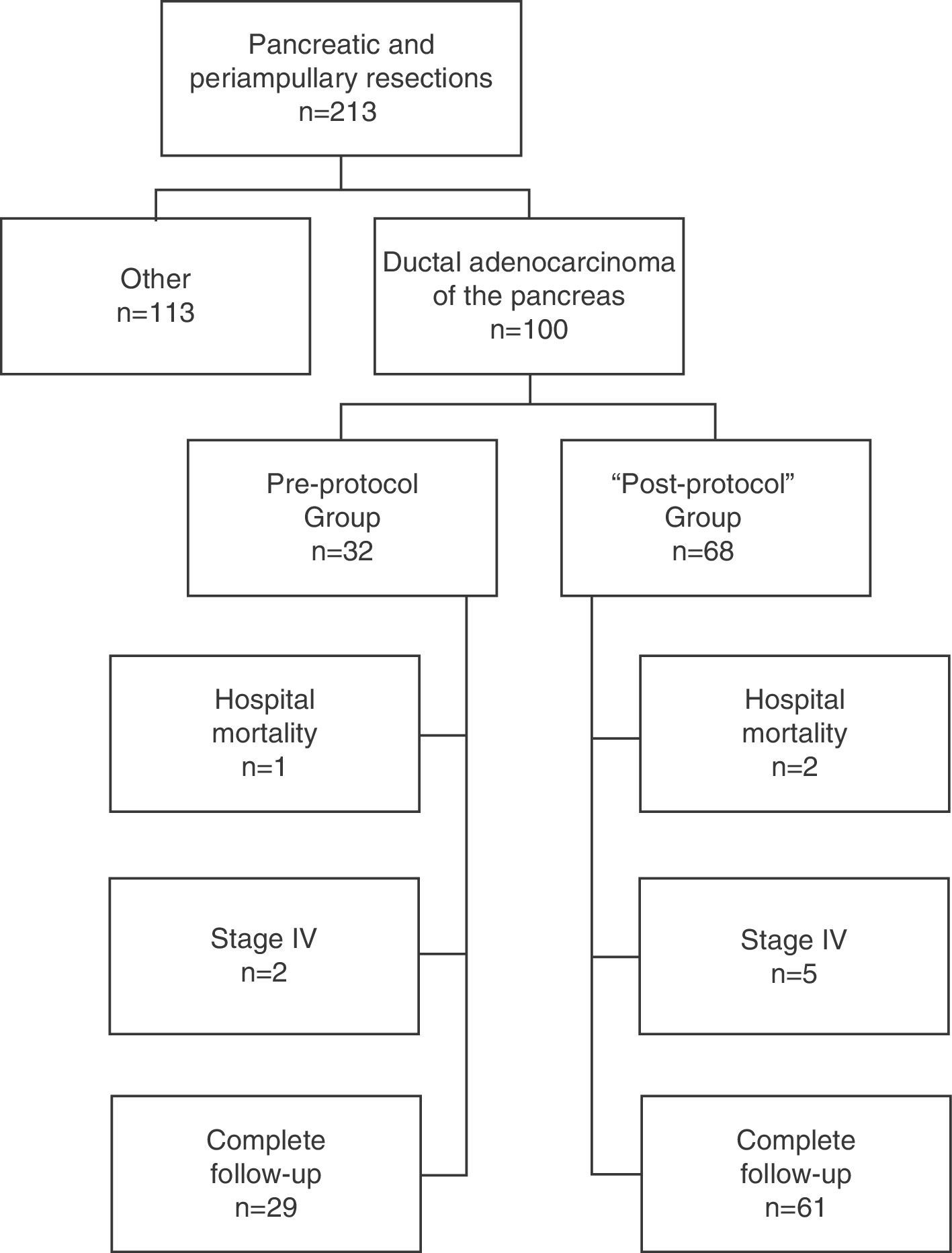
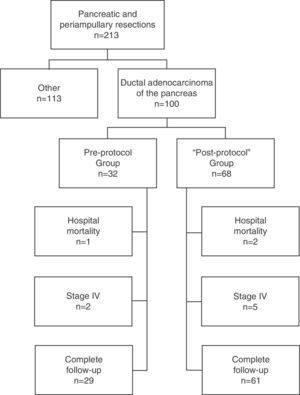
Patients and MethodSelection of PatientsAll patients were operated on consecutively at the Hepato-Biliary-Pancreatic Surgery Unit of the Hospital Clínico Universitario de Valencia [Valencia Clinical University Hospital] for a period of 14 years (January 1, 1999–December 31, 2012) and included prospectively in a database. Of the 213 pancreatic resections performed with curative intent, in 100 cases it was performed for ductal adenocarcinoma of the pancreas; these patients formed the study group. In late 2003, after we found a high rate of R0 resections in the pathological reports of patients operated until then, but the same low survival rate described in the literature, and in the context of a multidisciplinary team for the treatment of tumors in the hepatobiliopancreatic area, the procedure used until then for treating and preparing the specimens was updated, and a standardized protocol developed for resection specimens.9 After its development and implementation by the Department of Pathology, its application started in early 2004. Consequently, in our study, the patients included were divided into 2 groups: those operated before 2004, the “pre-protocol” group in which the specimens were analyzed without a standardized protocol, and those operated since 2004, the “post-protocol” group. The cohort observational flow chart of patients included in this study is shown in Fig. 1.
Surgical Management and Follow-upAll patients were previously evaluated by a multidisciplinary committee that decides the indications for surgery and assesses possible resectability. Indications, assessment of resectability and surgical techniques have been previously published.10 Patients were followed-up with regular appointments by the Department of Surgery or the Department of Medical Oncology. The date of death is identified by direct verification of patients dying in the hospital, in the Palliative Care Unit or in Home Hospitalization, and by telephone contact with relatives for all other cases.
Anatomopathological ProtocolFor patients in the pre-protocol group, analysis of the resected specimens was performed according to the personal opinion of the pathologist assigned to each case. For patients operated after implementation of the standardized protocol, i.e., the post-protocol group, 2 pathologists were in charge of all the specimens (AF and MCGM) who evaluated them according to a standardized preparing protocol, an anatomopathological study and report published by our group.9 Basically, this protocol involves, after the mandatory 24–48h for surgical specimen fixation, and based on a set code, the pathologist identifying and painting with different color inks the various resection margins, including: pancreatic transection margin and circumferential resection margin (CRM), which in turn comprises the medial circumferential or vascular margin and the posterior circumferential or retroperitoneal margin in cephalic pancreaticoduodenectomy ([CPD]), and the posterior margin of the body–tail pancreatic or total resection. Then, luminal margin samples are taken (bile, gastric or duodenal and jejunal), and any possible adenopathy is sought. Subsequently, a section perpendicular to the duodenal wall at the ampulla of Vater is performed, including the duodenal wall and pancreas, and then sections parallel to the former, separated by a distance of about 5mm toward the stomach (proximal) and the duodenum (distal), obtaining serial sections of the entire resected pancreas. The histopathological examination includes the tumor type, location, size, extent and differentiation degree, vascular, lymphatic and perineural invasion, total number of examined and positive lymph nodes. Our protocol defines R1 as the presence of microscopic residual tumor at a distance of ≤1mm, in any of the examined ranges.
Statistical AnalysisThe descriptive analysis of quantitative variables is shown as mean and standard deviation or median and range, and qualitative variables as percentages. The chi2 test was used to compare dichotomous variables and the Student t test for continuous variables. Kaplan Meier curves method for survival analysis was performed, and the log-rank test curve comparison was applied. A value of P<.05 was considered significant in all tests.
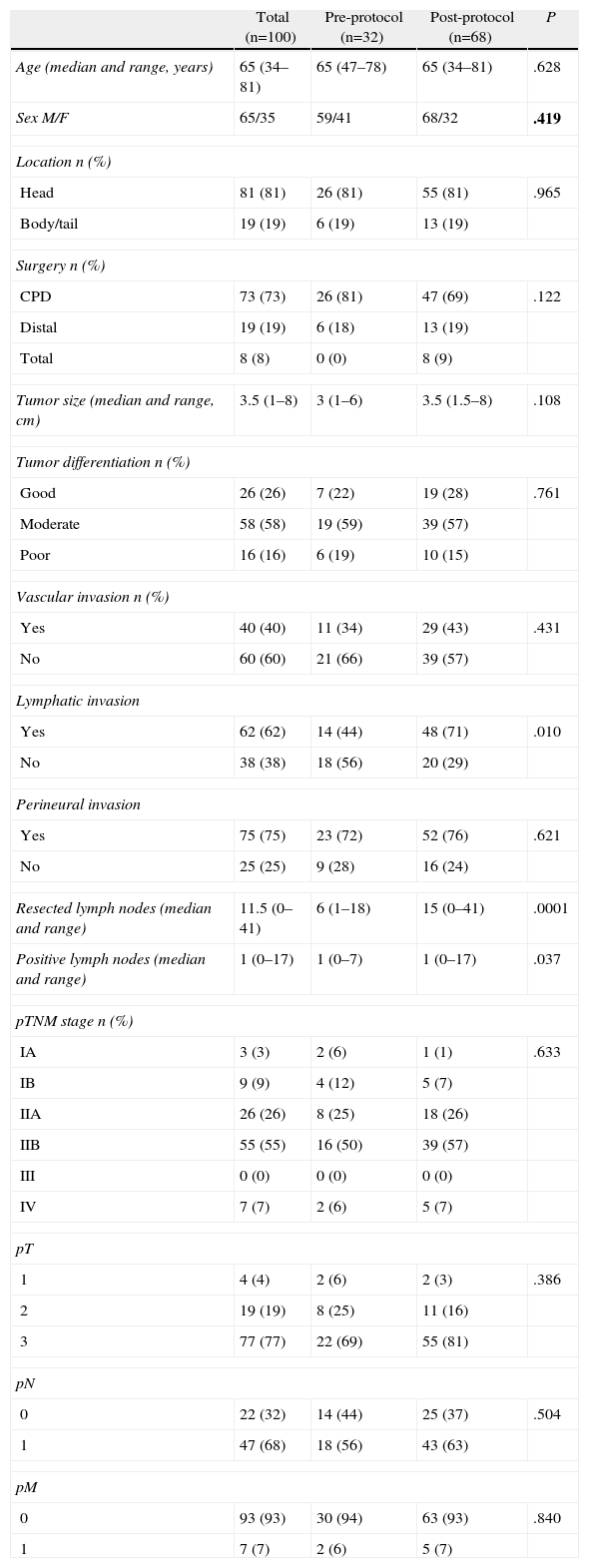
ResultsClinical and Histopathologic CharacteristicsOf the 100 patients included, 65% were male and 35% female, with a mean age of 64.6±8 years. The pre-protocol group consisted of 32 patients and the post-protocol group of 68 patients. The demographic characteristics, tumor location, type of intervention and histological study are shown in Table 1. Significant differences were observed in the post-protocol group, with a higher ratio of cases with lymphatic invasion, a larger number of lymph nodes examined and positive lymph nodes.
Clinical and Histopathological Characteristics of the Case Series and by Groups.
| Total (n=100) | Pre-protocol (n=32) | Post-protocol (n=68) | P | |
| Age (median and range, years) | 65 (34–81) | 65 (47–78) | 65 (34–81) | .628 |
| Sex M/F | 65/35 | 59/41 | 68/32 | .419 |
| Location n (%) | ||||
| Head | 81 (81) | 26 (81) | 55 (81) | .965 |
| Body/tail | 19 (19) | 6 (19) | 13 (19) | |
| Surgery n (%) | ||||
| CPD | 73 (73) | 26 (81) | 47 (69) | .122 |
| Distal | 19 (19) | 6 (18) | 13 (19) | |
| Total | 8 (8) | 0 (0) | 8 (9) | |
| Tumor size (median and range, cm) | 3.5 (1–8) | 3 (1–6) | 3.5 (1.5–8) | .108 |
| Tumor differentiation n (%) | ||||
| Good | 26 (26) | 7 (22) | 19 (28) | .761 |
| Moderate | 58 (58) | 19 (59) | 39 (57) | |
| Poor | 16 (16) | 6 (19) | 10 (15) | |
| Vascular invasion n (%) | ||||
| Yes | 40 (40) | 11 (34) | 29 (43) | .431 |
| No | 60 (60) | 21 (66) | 39 (57) | |
| Lymphatic invasion | ||||
| Yes | 62 (62) | 14 (44) | 48 (71) | .010 |
| No | 38 (38) | 18 (56) | 20 (29) | |
| Perineural invasion | ||||
| Yes | 75 (75) | 23 (72) | 52 (76) | .621 |
| No | 25 (25) | 9 (28) | 16 (24) | |
| Resected lymph nodes (median and range) | 11.5 (0–41) | 6 (1–18) | 15 (0–41) | .0001 |
| Positive lymph nodes (median and range) | 1 (0–17) | 1 (0–7) | 1 (0–17) | .037 |
| pTNM stage n (%) | ||||
| IA | 3 (3) | 2 (6) | 1 (1) | .633 |
| IB | 9 (9) | 4 (12) | 5 (7) | |
| IIA | 26 (26) | 8 (25) | 18 (26) | |
| IIB | 55 (55) | 16 (50) | 39 (57) | |
| III | 0 (0) | 0 (0) | 0 (0) | |
| IV | 7 (7) | 2 (6) | 5 (7) | |
| pT | ||||
| 1 | 4 (4) | 2 (6) | 2 (3) | .386 |
| 2 | 19 (19) | 8 (25) | 11 (16) | |
| 3 | 77 (77) | 22 (69) | 55 (81) | |
| pN | ||||
| 0 | 22 (32) | 14 (44) | 25 (37) | .504 |
| 1 | 47 (68) | 18 (56) | 43 (63) | |
| pM | ||||
| 0 | 93 (93) | 30 (94) | 63 (93) | .840 |
| 1 | 7 (7) | 2 (6) | 5 (7) | |
Distal, corporocaudal pancreatectomy; CPD, cephalic pancreaticoduodenectomy; Total, total pancreaticoduodenectomy.
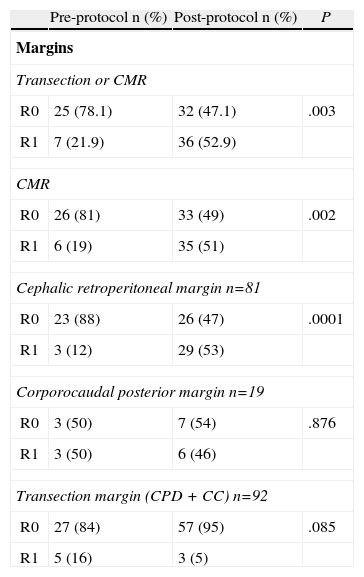
The resection margin analysis and R0/R1 rates are shown in Table 2. In the pre-protocol group, the R0 rate was 78%, changing to 47% after application of the standardized protocol (P=.003). In the evaluation of the various analyzed margins, involvement of the circumferential margin was significantly higher for the post-protocol group (51% compared to 19%, P=.002). This difference is obtained from the involvement of the retroperitoneal posterior margin in CPD, since neither the posterior margin of corporocaudal resections nor the transection margins in all types of resections are significantly different between the study groups.
Study of Resection Margins and R0/R1 Rates.
| Pre-protocol n (%) | Post-protocol n (%) | P | |
| Margins | |||
| Transection or CMR | |||
| R0 | 25 (78.1) | 32 (47.1) | .003 |
| R1 | 7 (21.9) | 36 (52.9) | |
| CMR | |||
| R0 | 26 (81) | 33 (49) | .002 |
| R1 | 6 (19) | 35 (51) | |
| Cephalic retroperitoneal margin n=81 | |||
| R0 | 23 (88) | 26 (47) | .0001 |
| R1 | 3 (12) | 29 (53) | |
| Corporocaudal posterior margin n=19 | |||
| R0 | 3 (50) | 7 (54) | .876 |
| R1 | 3 (50) | 6 (46) | |
| Transection margin (CPD+CC) n=92 | |||
| R0 | 27 (84) | 57 (95) | .085 |
| R1 | 5 (16) | 3 (5) | |
Cephalic, tumors located in pancreatic head; Corporocaudal, tumors located in the body/tail of pancreas; CPD+CC, pancreaticoduodenectomies+corporocaudal resections (total pancreatectomies are excluded); CMR, circumferential margin.
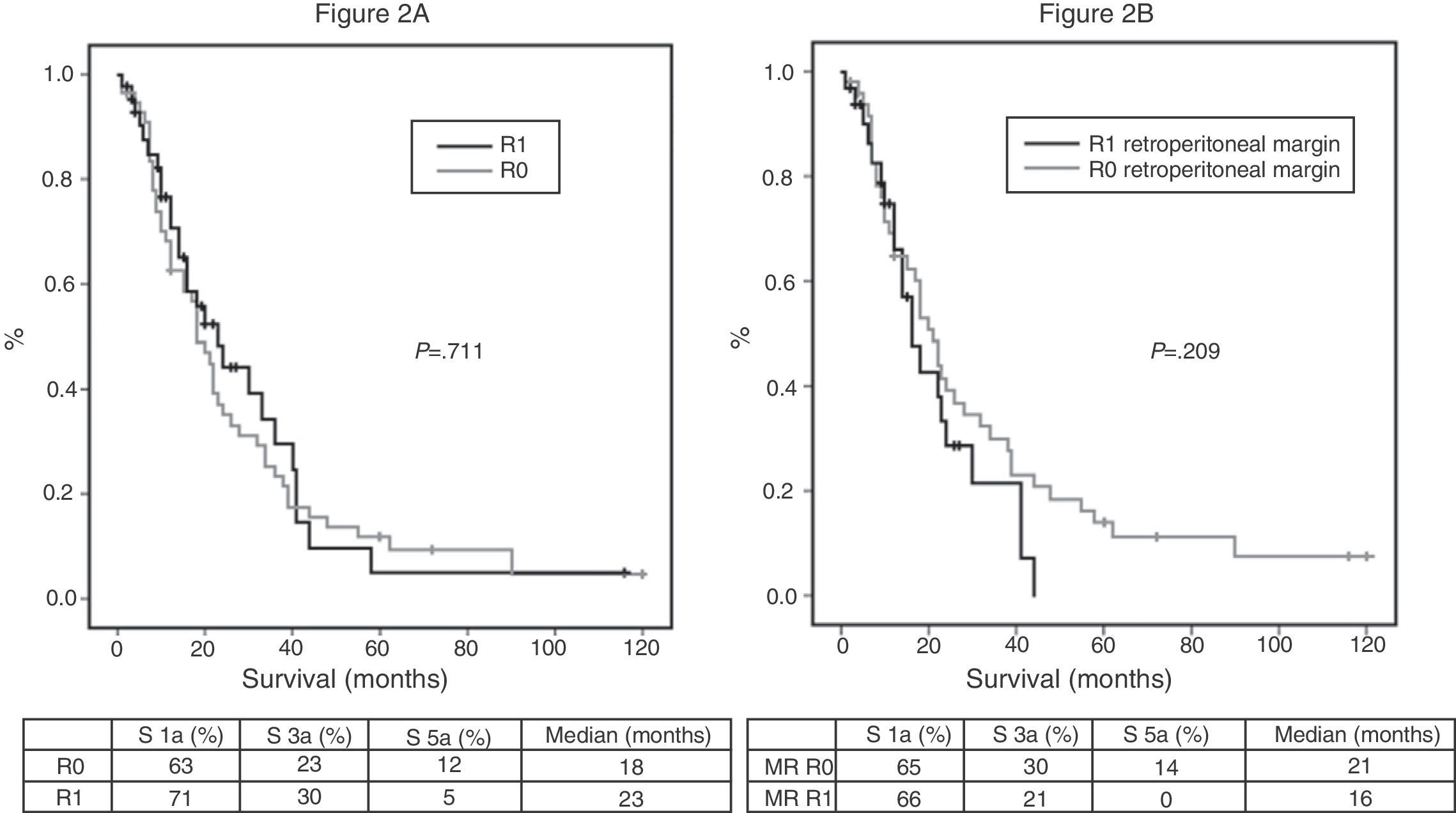
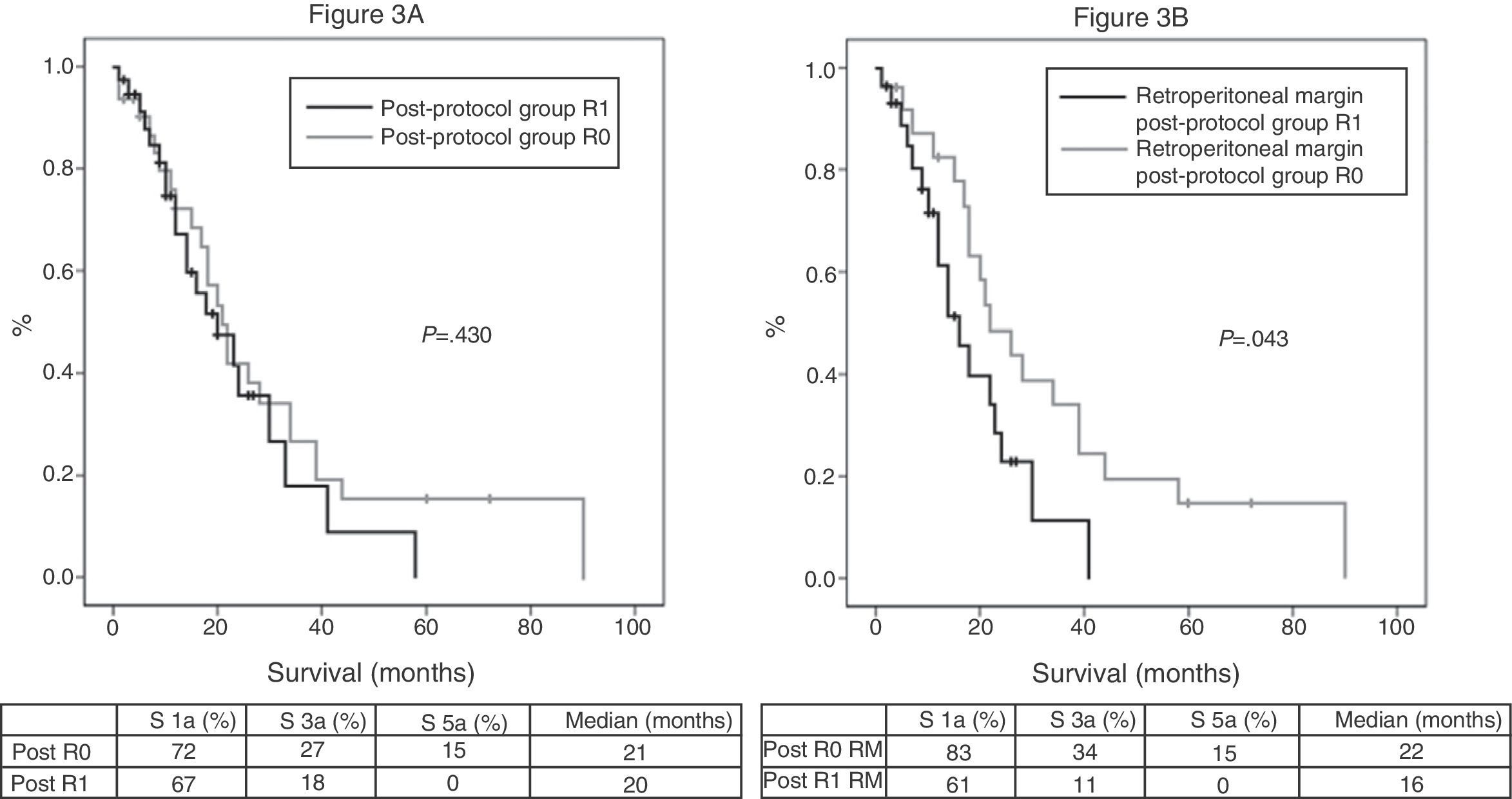
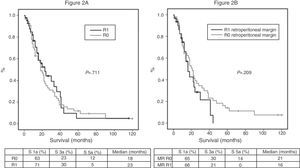
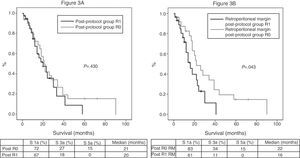
Of the 100 patients in the case series, 10 patients have been excluded for the survival analysis: 3 due to postoperative mortality, and 7 that were considered stage IV with microscopic peritoneal or liver implants detected in the final pathology. Accordingly, in this phase of the study, 29 patients form the pre-protocol group, and 61 form the post-protocol group. Overall survival in the case series at 1, 3 and 5 years was 73%, 29%, and 11% respectively, with a median of 20 months. When analyzing the entire case series, including pre-protocol and post-protocol patients, there are no significant differences between the survival rates of R0 patients compared to R1 patients (Fig. 2A). Furthermore, no differences were found in survival between the R0 and R1 patients when analyzing the retroperitoneal margin of cases with pancreatic head tumors (including both pre-protocol and post-protocol groups together) (Fig. 2B), or when analyzing the differences between R0 and R1 patients only in the post-protocol group (Fig. 3A). However, when evaluating the retroperitoneal margin in patients with pancreatic head tumors studied with a standardized protocol, a clear statistically significant difference is indeed seen in survival rates (Fig. 3B), more favorable in cases with R0 retroperitoneal margin.
(A) Overall survival curves of the case series, analyzed according to the R0/R1 margin. Hazard ratio: 0.916 (95% CI 0.56–1.47). (B) Survival curves for patients with pancreatic head tumors in the entire case series, analyzed according to the involvement of the retroperitoneal margin (RM). Hazard ratio: 1.414 (95% CI 0.81–2.45).
(A) Survival curves of patients in the post-protocol group (Post), analyzed according to the presence of R0 and R1 margin. Hazard ratio: 1.275 (95% CI 0.69–2.35). (B) Survival curves of patients with tumors of the pancreatic head of the post-protocol group (Post), analyzed according to the involvement of the retroperitoneal margin (RM). Hazard ratio: 2.044 (95% CI 1.00–4.16).
The wide variability in rates of involvement of surgical resection margins in pancreatic cancer probably reflects the lack of standardized protocols. In cancer surgery, obtaining tumor-free resection margins has been linked to good surgical technique, and generally, low levels of R1 resections are considered a reflection of high quality and the surgery's radical nature. However, a series of studies2,6 question this relationship in pancreatic cancer. When standardized protocols have been implemented to analyze surgical specimens, it has been demonstrated that many of the specimens rated as R0 were actually R1.2 Although the first reference to this problem dates back to 19934 with a 51% R1 rate, 2 more recent papers have actually started raising flags. The first clearly shows the lack of standardized protocols, and identifies an 85% R1 rate in pancreatic cancer.6 The second, which has greater impact due to the title chosen for publication, Most pancreatic resections are R1 resections is from the Esposito and Büchler group; it changes from a 14% R1 rate, when specimens are not analyzed according to a protocol, to 76% when analyzed with standardized histopathological protocol.2 The clinical significance of this observation is crucial, because when we examine randomized trials that have influenced resected pancreatic cancer treatment, such as the ESPAC study, that observed low levels of R1 involvement (19%)11,12 and participation of multiple hospitals (61 sites in 11 countries) it suggests that, in reality, a standardized analysis of the specimens has not been performed; therefore, non-oncologic resection rates have been underestimated.
Our case series confirms, in line with the works of Verbecke6 and Esposito,2 that a standardized analysis protocol for resection margins increases the rate of R1 resections, which when lacking this analysis, would be considered as R0. There are several distinguishing aspects in our study with respect to other case series. First, we included only pancreatic cancer.13 Although all tumors in the periampullary and pancreatic areas should be analyzed with this standardized histopathological protocol, the circumferential resection margin would be rarely reached in duodenal or papilla tumors. This choice is preferred because it is a more homogeneous group that allows minimizing potential biases when analyzing survival and its relation to resection margins. Second, the definition used for R1. For American authors, according to the UICC/American Joint Commission on Cancer System,14,15 the tumor must reach the transection edge to be considered R1, whereas for most European authors, following the criteria of the Royal College of Pathologists16–18 which we have adopted in our protocol, R1 occurs when the distance between tumor and resection margin is equal to or less than 1mm. However, a study of the prognostic relationship between the distance from the tumor to the resection margin and survival, concludes that the minimum distance to establish a more favorable prognosis must be greater than 1.5mm.19
Our experience with the resection margins analysis in a standardized manner begins between 2003 and 2004, when we question the veracity of our high rate of R0 “oncology” resections, with 10% survival rates at 5 years, similar to most studies in the literature, and failing to find any difference in survival rates among patients with R0 and R1 resections.20 The introduction of a standardized protocol to analyze surgical specimens has shown that our R0 resection rate was wrong. This means that detailed analysis of surgical specimens may invert oncologic resection rates, changing from a majority of R0 resections to a majority of R1 resections. And, of course, as they were performed by the same surgeons and without changes in the surgical technique, i.e., these results cannot be attributed to the surgeon factor.
For pancreatic cancer, even after the recent case series where surgical specimens have been analyzed with a standardized protocol, the real impact of the R1 factor on long-term survival remains unclear. While in some case series it does significantly affect survival,13,18,21,22 in others, this impact is not observed.2,3,5,8 In our study, microscopic invasion of the circumferential margin is particularly important, because the R1 resection has an adverse effect on survival. This relevance comes at the expense of the retroperitoneal posterior margin in patients with pancreatic head tumors, when specimens are analyzed with the standardized protocol, since they are not significantly different before or after application of the protocol in this margin for corporocaudal resections or for the transection margin; for corporocaudal resections, because the posterior involvement is readily observable by the pathologist with or without a standardized protocol, and for the transection margin, because when a section of this margin is analyzed intraoperatively, in the event it turns out to be positive, it expands; thus, it is rarely positive in the final analysis. In addition, the relevance is revealed only when analyzing patients with pancreatic head tumors (Fig. 3B) and not when analyzing all patients in the standard protocol (Fig. 3A), since statistical significance was lost when the latter group was included, i.e., patients with body–tail resections and total pancreatectomies.
Although in our investigation, we obtained significant results with standardized analysis of the surgical specimens in pancreatic cancer, we must acknowledge a number of limitations. It is a retrospective analysis of a single institution, which includes not only CPD but also corporocaudal resections and total pancreatectomies. Furthermore, we have not studied the pattern of recurrence or cause of death, nor whether patients received postoperative chemotherapy or not.
In conclusion, in this study, we observed that the rate of R0 oncology resections changes from 78%, when the resected specimens are analyzed without a standardized protocol, to 47% when the protocol is applied. In patients with pancreatic head cancer, the microscopic invasion of the retroperitoneal posterior margin significantly affects survival. These findings, consistent with those of other well established surgical groups,2,17,18,21 justify the need for further studies to reduce the R1 rate. The use of more effective neoadjuvant therapies,23 or the initial approach of the superior mesenteric artery,24 a technical modification seemingly able to obtain a few more millimeters of retroperitoneal tissue, could be useful strategies to extend the resection margin, and consequently, improve our results.
Presentations at ConferencesPreliminary versions of this research were presented at the following conferences:
- -
SIXTEENTH National Surgery Meeting, San Sebastián, October 2007.
- -
TENTH Biliopancreatic Spanish Club Meeting, Santander, September 2007.
- -
2nd International Conference Advances in Surgery. Discussions from the cutting edge, Barcelona, December 2010.
The authors declare having no conflict of interest.
Please cite this article as: Sabater L, Gómez-Mateo MC, López-Sebastián J, Muñoz-Forner E, Morera-Ocón F, Cervantes A, et al. Implicaciones pronósticas del estudio estandarizado de los márgenes de resección en el cáncer de páncreas. Cir Esp. 2014;92:532–538.