Endocrine pancreatic segregation is regulated by the autonomic nervous system. The parasympathetic system stimulates insulin production by the beta cells and inhibits the adrenergic discharge by the sympathetic nervous system. The aim of this study was to evaluate the effect of percutaneous neurostimulation (PENS) of dermatome T7, generating a somato-autonomic reflex, whose efferent pathway is the vagal branches that specifically stimulate the pancreas. The effect of this treatment on glycemia, insulin secretion and insulin resistance was investigated.
MethodsA prospective randomised clinical trial was performed. Patients with BMI≥30kg/m2 and diagnosis of diabetes mellitus treated with metformin were included. Patients were divided into 2 groups: patients undergoing PENS of dermatome T7 (12 sessions of 30min weekly) associated with a 1200kcal/day diet (Group 1) and patients following only a 1200kcal/day diet (Group 2). All the patients underwent a blood sample extraction before the treatment and 7 days after finishing it.
Results60 patients were included: 30 in each group. After finishing the treatment, in Group 1 a significant decrease in glycemia (mean decrease of 62.1mg/dl; P=.024) and HOMA (mean decrease 1.37; P=.014) was observed. In Group 2, no significant differences between pre and post-treatment values were observed.
ConclusionPENS of dermatome T7 associated with a 1200kcal/day diet achieves a greater reduction in glycemia and insulin resistance than with diet exclusively after 3 months of treatment.
La secreción endocrina pancreática está regulada por el sistema nervioso autónomo. El sistema parasimpático estimula la producción de insulina por las células beta e inhibe la liberación adrenérgica por el sistema nervioso simpático. El objetivo del presente estudio es evaluar el efecto de la neuroestimulación percutánea (PENS) del dermatoma T7, creándose un reflejo somato-autonómico, cuya vía eferente serán las ramas del nervio vago que estimulan específicamente el páncreas. Se analizará el efecto de este tratamiento sobre la glucemia, la secreción de insulina y la resistencia a la acción de la insulina.
MétodosRealizamos un estudio prospectivo aleatorizado. Se incluyeron pacientes con IMC≥30kg/m2 y diagnóstico de diabetes mellitus en tratamiento con metformina. Los pacientes fueron aleatorizados en 2 grupos: pacientes sometidos a PENS del dermatoma T7 (12 sesiones de 30min semanales) asociado a dieta de 1.200kcal/día (grupo 1) y pacientes que seguían una dieta de 1.200kcal/día exclusivamente (grupo 2). A todos los pacientes se les realizó una analítica sanguínea en ayunas antes de empezar el tratamiento y a los 7 días de finalizarlo.
ResultadosSe incluyeron 60 pacientes: 30 pacientes en cada grupo. Al finalizar el tratamiento en el grupo 1 se observa un descenso significativo en la glucemia (descenso medio de 62,1mg/dl; p=0,024) y en el HOMA (descenso medio 1,37; p=0,014). En el grupo 2 no se observan diferencias significativas en los valores pre y postratamiento.
ConclusiónLa PENS del dermatoma T7 asociada a dieta de 1.200kcal/día produce una mayor reducción de la glucemia y de la resistencia insulínica que la obtenida solo mediante dieta tras 3 meses de tratamiento.
It has been widely proven that obesity is a risk factor for the development of type-2 diabetes mellitus (DM2). In recent decades, the prevalence of diabetes mellitus has increased, matching the increase in obesity. Some authors have estimated that the risk of developing DM2 increases by 4.5% with each kg gained over the normal limit. This means that in patients with severe obesity or morbid obesity, the probability of developing DM2 during their lifetime is 100%.1–3 Resistance to the effect of insulin has become the major determinant for the development of DM2, which appears when the pancreatic beta cell is unable to produce more insulin to defeat peripheral insulin resistance.4
It has been shown that weight loss leads to an improvement in blood sugar level profile. Weight loss secondary to bariatric surgery has proven to increase the secretion of insulin in the pancreas, as well as reduce peripheral resistance to the action of insulin, both mechanisms contributing to improvement and even disappearance of DM2 in morbidly obese patients.4,5
The secretion of the endocrine pancreas is regulated by the autonomous nervous system. The parasympathetic system stimulates the production of insulin by beta cells. The administration of exogenous acetylcholine also increases the release of insulin. On the other hand, parasympathetic activation inhibits adrenergic release of the sympathetic nervous system.6
The purpose of this study is to evaluate the effect of percutaneous electrical nerve stimulation (PENS) of dermatome T7 and the subsequent creation of a somato-autonomic reflex, with sensory nerve endings of dermatome T7 as afferent paths and the vagal nerve branches as efferent paths which will specifically stimulate the pancreas. The effect of this treatment on blood sugar levels, the secretion of insulin and the resistance to the action of insulin were analysed.
MethodsWe carried out a prospective randomised study at the Obesity Unit of Hospital General Universitario de Elche and Neurostimulation Units for the Treatment of Obesity at Clínica Garcilaso (Madrid) and Clínica Maisonnave (Alicante) from January 2013 to January 2014. The calculation of the sample size was based on a reduction of insulin resistance according to the formula Homeostasis model assessment (HOMA) of 0.5 in the control group and double (1) in the experimental group. The calculation in the control group was based on historical data obtained at Hospital General Universitario de Elche in obese and type-2 diabetic patients after 12 weeks of dietary follow-up based on a 1200kcal/day diet. With 80% statistical power and significance level P<.05, it was calculated that it was necessary to include 30 patients in each study arm. The inclusion criteria were patients with BMI≥30kg/m2 and diagnosis of non-insulin-dependent DM treated with metformin. The exclusion criteria were patients with other endocrine disorders causing their obesity or DM, patients with insulin-dependent DM or patients treated with oral antidiabetic drugs other than metformin.
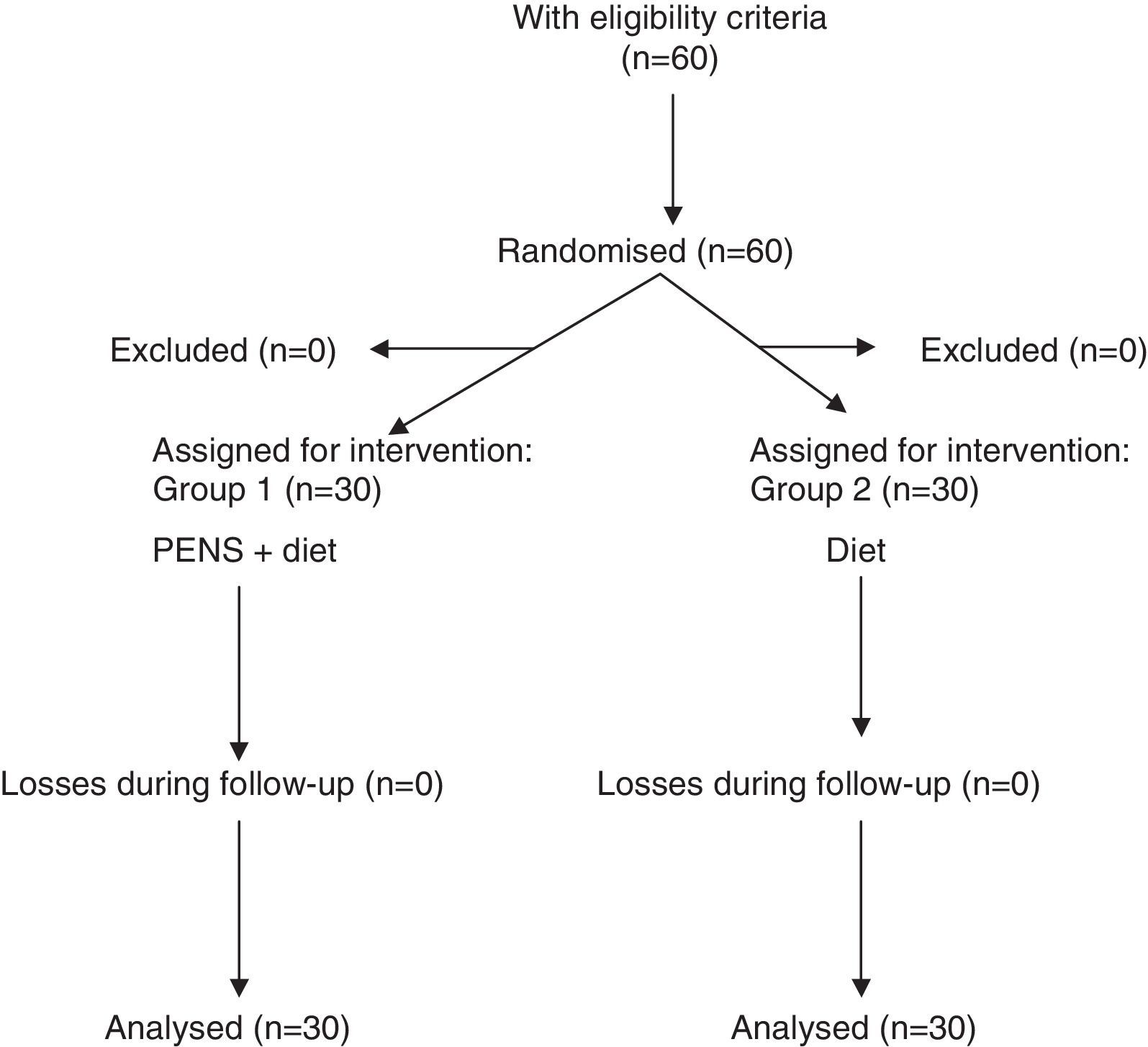
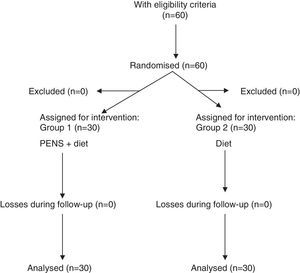
Patients were randomised into 2 groups through a randomising model taken from the Internet: patients subject to percutaneous electrical nerve stimulation of dermatome T7 (PENS) associated with a 1200kcal/day diet (Group 1) and patients who followed a 1200kcal/day diet exclusively (Group 2) (Fig. 1).
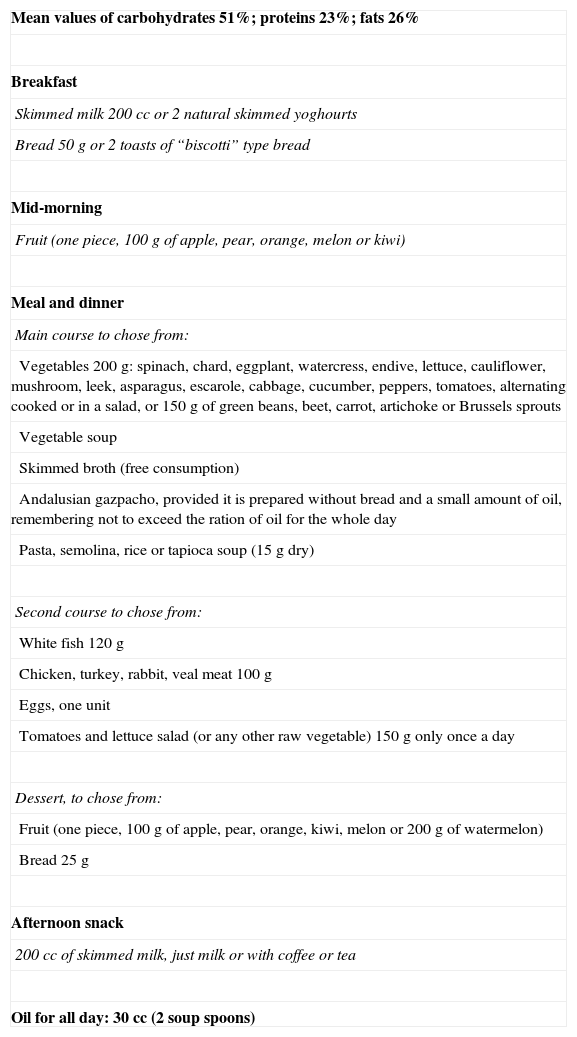
All patients were under treatment with metformin 850mg every 12h. The diet prescribed of the 1200kcal/day is shown in Table 1. Patients in both groups were told of the importance of weight loss with the diet to improve their diabetes and avoid the development of the associated complications. Patients subject to PENS of dermatome T7 were told that this therapy was intended to reduce appetite and help to keep up with the diet, as in a previous study of our group, where dermatome T6 was stimulated.7
Diet 1200kcal/day.
| Mean values of carbohydrates 51%; proteins 23%; fats 26% |
| Breakfast |
| Skimmed milk 200 cc or 2 natural skimmed yoghourts |
| Bread 50g or 2 toasts of “biscotti” type bread |
| Mid-morning |
| Fruit (one piece, 100g of apple, pear, orange, melon or kiwi) |
| Meal and dinner |
| Main course to chose from: |
| Vegetables 200g: spinach, chard, eggplant, watercress, endive, lettuce, cauliflower, mushroom, leek, asparagus, escarole, cabbage, cucumber, peppers, tomatoes, alternating cooked or in a salad, or 150g of green beans, beet, carrot, artichoke or Brussels sprouts |
| Vegetable soup |
| Skimmed broth (free consumption) |
| Andalusian gazpacho, provided it is prepared without bread and a small amount of oil, remembering not to exceed the ration of oil for the whole day |
| Pasta, semolina, rice or tapioca soup (15g dry) |
| Second course to chose from: |
| White fish 120g |
| Chicken, turkey, rabbit, veal meat 100g |
| Eggs, one unit |
| Tomatoes and lettuce salad (or any other raw vegetable) 150g only once a day |
| Dessert, to chose from: |
| Fruit (one piece, 100g of apple, pear, orange, kiwi, melon or 200g of watermelon) |
| Bread 25g |
| Afternoon snack |
| 200 cc of skimmed milk, just milk or with coffee or tea |
| Oil for all day: 30 cc (2 soup spoons) |
All patients were subject to a fasting blood test prior to commencing treatment and another one 7 days after completion (the interval between both blood tests was 13 weeks). ClinicalTrials.gov ID: NCT02122874.
Methodology of Percutaneous Electrical Nerve Stimulation of Dermatome T7The PENS was carried out by surgeons at the centres participating in the study. The Urgent PC 200 Neuromodulation System® device was used (Uroplasty, Minnetonka, MN, EE USA). Patients were subject to weekly sessions of 30min for 12 consecutive weeks. The patient was laid in supine decubitus position and, with no need for local anaesthesia, a needle was inserted in the upper left quadrant of the abdomen in the mid-clavicular line, 4cm below the rib cage border (to match dermatome T7). Although either side could have been chosen for stimulation, the left arm was chosen due to the position of the furniture used for the appointments, which required placing the nerve stimulator on a side table next to the stretcher. The needle was introduced perpendicular to the abdominal wall plane with a depth of 0.5–1cm. Correct placement was confirmed when the patient noticed an “electric tingling” sensation at least 5cm lateral to the point where the needle was inserted, within the territory of dermatome T7. The PENS was applied at a frequency of 20Hz and maximum amplitude (0–20mA) before causing pain.
VariablesIn the beginning and at the end of the treatment, weight, BMI, weight loss, blood sugar levels, glycosylated haemoglobin, insulin and HOMA were assessed. Patients subject to PENS of dermatome T7 were asked to quantify their perception of appetite before and after treatment using a visual analogue scale (VAS) with scores 0 (no appetite) to 10 (uncontrollable appetite).
StatisticsAll statistical analyses were performed using the SPSS 17.0 program (SPSS Inc., Chicago, IL). Quantitative variables with formal distribution were defined using mean and standard deviation; in non-Gaussian variables, median and range were used. Qualitative variables were defined by the number of cases and percentage. Comparison between variables was carried out using Student's t test and Pearson's correlation for quantitative variables with Gaussian distribution, and Mann–Whitney, Friedman and Spearman tests for non-Gaussian variables. P values <.05 were considered significant.
Results60 patients were included in the study: 30 patients in each group. The sample was 80% women and 20% men with a mean age of 48.9±13.8 years (interval 29–72 years). There were no significant differences in age and gender between both groups.
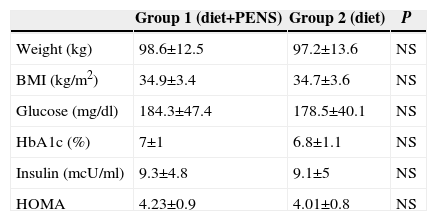
The measurements for pre-operative weight, BMI, glucose, glycosylated haemoglobin, insulin and HOMA in both groups are shown in Table 2. No statistically significant differences were found between groups.
Anthropometric and Analytical Measurements Before Commencing Treatment in Both Groups.
| Group 1 (diet+PENS) | Group 2 (diet) | P | |
|---|---|---|---|
| Weight (kg) | 98.6±12.5 | 97.2±13.6 | NS |
| BMI (kg/m2) | 34.9±3.4 | 34.7±3.6 | NS |
| Glucose (mg/dl) | 184.3±47.4 | 178.5±40.1 | NS |
| HbA1c (%) | 7±1 | 6.8±1.1 | NS |
| Insulin (mcU/ml) | 9.3±4.8 | 9.1±5 | NS |
| HOMA | 4.23±0.9 | 4.01±0.8 | NS |
HbA1c: Glycohemoglobin; HOMA is calculated according to the formula glucose×insulin/405; BMI: Body Mass Index.
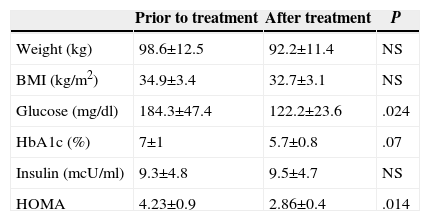
Upon finishing the treatment in Group 1, a significant decrease in blood sugar levels was observed (mean loss of 62.1mg/dl; 95% CI: 41.6–82.6; P=.024) and in HOMA (mean loss 1.37; 95% CI: 0.63–2.11; P=.014). A tendency towards reduction was observed in the values for glycosylated haemoglobin, without reaching statistical significance (P=.014) (Table 3).
Prior to Treatment and After Treatment Values in Group 1.
| Prior to treatment | After treatment | P | |
|---|---|---|---|
| Weight (kg) | 98.6±12.5 | 92.2±11.4 | NS |
| BMI (kg/m2) | 34.9±3.4 | 32.7±3.1 | NS |
| Glucose (mg/dl) | 184.3±47.4 | 122.2±23.6 | .024 |
| HbA1c (%) | 7±1 | 5.7±0.8 | .07 |
| Insulin (mcU/ml) | 9.3±4.8 | 9.5±4.7 | NS |
| HOMA | 4.23±0.9 | 2.86±0.4 | .014 |
HbA1c: Glycohemoglobin; HOMA is calculated according to the formula glucose×insulin/405; BMI: Body Mass Index.
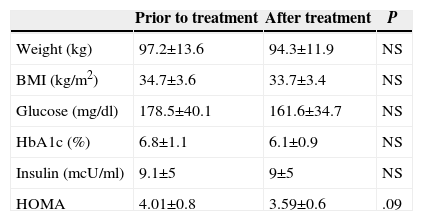
No significant differences were observed in Group 2 prior to treatment or after the treatment values in any of the parameters analysed (Table 4). Only a tendency towards a decrease in the values for HOMA was observed (P=.09).
Prior to Treatment and After Treatment Values in Group 2.
| Prior to treatment | After treatment | P | |
|---|---|---|---|
| Weight (kg) | 97.2±13.6 | 94.3±11.9 | NS |
| BMI (kg/m2) | 34.7±3.6 | 33.7±3.4 | NS |
| Glucose (mg/dl) | 178.5±40.1 | 161.6±34.7 | NS |
| HbA1c (%) | 6.8±1.1 | 6.1±0.9 | NS |
| Insulin (mcU/ml) | 9.1±5 | 9±5 | NS |
| HOMA | 4.01±0.8 | 3.59±0.6 | .09 |
HbA1c: Glycohemoglobin; HOMA is calculated according to the formula glucose×insulin/405; BMI: Body Mass Index.
A higher tendency for weight loss was observed in Group 1 (6.4±1.4kg against 2.5±0.8kg; P=.08).
In Group 2, a direct correlation between the weight loss and decrease in HOMA was observed (Pearson 0.345; P=.043). In Group 1, this correlation could not be determined (P=.258).
Quantification of Appetite in Group 1The median for appetite before treatment was 6 (range 4–10) and the median after treatment was 4 (range 3–7) (P=.215).
DiscussionThe effect of PENS has been widely shown in the stimulation of the posterior tibial nerve for treatment of anal incontinence, by creating a somato-somatic reflex.8,9 Recently, our group has published the first study on the use of PENS of dermatome T6 to reduce appetite and, as a result, obtain a significant weight loss. In this case, a somato-autonomic reflex is created, with the vagus nerves that connect specifically to the stomach as an efferent pathway. As a consequence of this parasympathetic stimulation, the stomach slows down its emptying process and increases the feeling of fullness, which translates into a reduction in appetite.7 This study is based on the same grounds as in this last work mentioned, by also creating a somato-autonomic reflex, with sensory nerve endings to dermatome T7 as afferent pathway and parasympathetic branches of the vagus nerve which stimulate the pancreatic gland as efferent pathway. Despite a tendency towards a higher weight loss in the group subject to PENS of dermatome T7, this does not attain the results in our previous study with PENS of dermatome T6. When evaluating the quantification of the perception of appetite, the loss after finishing treatment is not statistically significant. However, the tendency to greater weight loss and the small reduction in appetite after PENS of dermatome T7 may possibly be due to mild collateral stimulation of nerve terminations of dermatome T6, due to its proximity to dermatome T7.
It has been widely shown that weight loss improves blood sugar levels, mainly due to a reduction in resistance to the action of insulin in peripheral tissues. This usually determines a decrease in hyperinsulinism.10,11 In patients in Group 2 of our study (subject to dietary treatment only) no significant decrease of blood sugar levels were observed, but there was a trend for a decrease in HOMA values after dietary treatment (P=.09), with practically unaltered insulin values (9.1mcU/ml pre-treatment and 9mcU/ml post-treatment). In this case, no significant decrease in the levels of insulin was observed, although these are within the normal range both before and after treatment (normal range 5–20mcU/ml). Therefore, since there was no hyperinsulinism, there was no significant reduction in pancreatic release of insulin.
In the group of patients subject to PENS of dermatome T7, associated with diet, a significant reduction of blood sugar levels (P=.024) and HOMA values (P=.014) was obtained, along with a tendency to a fall in glycosylated haemoglobin levels. Here we saw a non-significant increase in insulin levels (9.3mcU/ml before the treatment and 9.5mcU/ml after completion of the treatment). Therefore, we could say that pancreatic stimulation through PENS of dermatome T7 does not produce a significant increase of pancreatic secretion of insulin. It would be logical to think, then, that the highest decrease in blood sugar levels is due to the reduction of insulin resistance as a consequence of the weight loss. In the group of patients subject only to diet, the weight loss shows a direct correlation with the decreased HOMA. However, in patients subject to PENS in addition to the diet, this direct correlation is lost, and therefore the high weight loss in this group of patients is not fully accounted for by the greater decrease in insulin resistance.
Parasympathetic stimulation of the pancreatic gland, in addition to its own effects on the gland, produces an inhibition of the sympathetic nervous system, which implies a decrease in the release of counter-regulatory hormones (adrenaline, growth hormone and cortisol). These hormones produce an increase in the synthesis of endogenous glucose and suppress their use on peripheral tissues, mainly in the muscle, conditioning the resistance to the action of insulin.12 Therefore, inhibition of the sympathetic nervous system through parasympathetic stimulation of the pancreas may account for, at least in part, the decrease in insulin resistance after PENS of dermatome T7. In our opinion, this may be the main physio-pathological mechanism justifying the effects of neuromodulation on the homeostasis of blood sugar levels.
As we mentioned above, during the PENS of dermatome T7, the reflex of dermatome T6 is possibly also partially activated. The delay in gastric emptying this produces inhibits the release of ghrelin by the gastric fundus.13,14 The inhibition of ghrelin has been shown to increase pancreatic secretion of insulin and reduce resistance to its peripheral action, especially in the adipose tissue, which is why this action is more surprising in obese patients.15 Therefore, a certain degree of inhibition of ghrelin can also favour the decrease of HOMA. In our previous study on the PENS of dermatome T6, we did not find a significant decrease of blood sugar levels after the treatment and we did not analyse the HOMA. However, most patients included in that previous study were not diabetic, and therefore no conclusive data can be extracted from that analysis.7 New studies would be necessary on the PENS of dermatome T6 in diabetic patients and its effect on blood sugar levels and insulin resistance.
The main limitations of this study are that it is an unblinded clinical trial and the potential duration of the results, given that only the results after the end of the treatment are included. New studies will be carried out to evaluate the duration of the benefit obtained over time on glucose homeostasis, as well as analysing the effect of neurostimulation of dermatome T6 on hormone levels (adrenaline, growth hormone, cortisol, ghrelin, etc.).
ConclusionAfter 3 months of treatment, percutaneous electrical nerve stimulation of dermatome T7 associated with a 1200kcal/day diet produces a higher reduction of blood sugar levels and insulin resistance than that obtained only through diet.
Conflict of InterestThe authors declare that there are no conflicts of interest.
Please cite this article as: Ruiz-Tovar J, Llavero C, Ortega I, Diez M, Zubiaga L, Calpena R. La neuroestimulación eléctrica percutánea del dermatoma T7 mejora el perfil glucémico en pacientes obesos y diabéticos tipo 2. Cir Esp. 2015;93:460–466.