The use of ambulatory surgery (AS) for breast pathology (BP) has increased. The objective of this study is to analyse a group of patients treated surgically for breast pathology in order to evaluate its quality and security in a MAS setting in 2017.
MethodsA retrospective review of all patients undergoing breast surgery was conducted within an AS programme from January to December 2017 in Consorcio Hospital General Universitario of Valencia (CHGUV). The number of patients, exclusion reasons, the type of surgical procedures, the evolution of substitution rate (SI), the rate and the causes of conversion to admission, the post-operatory complications, the motives of not including in the ambulatory program and the satisfaction rate of the patients operated with ambulatory surgery have been studied. This has been compared with a 2013 group.
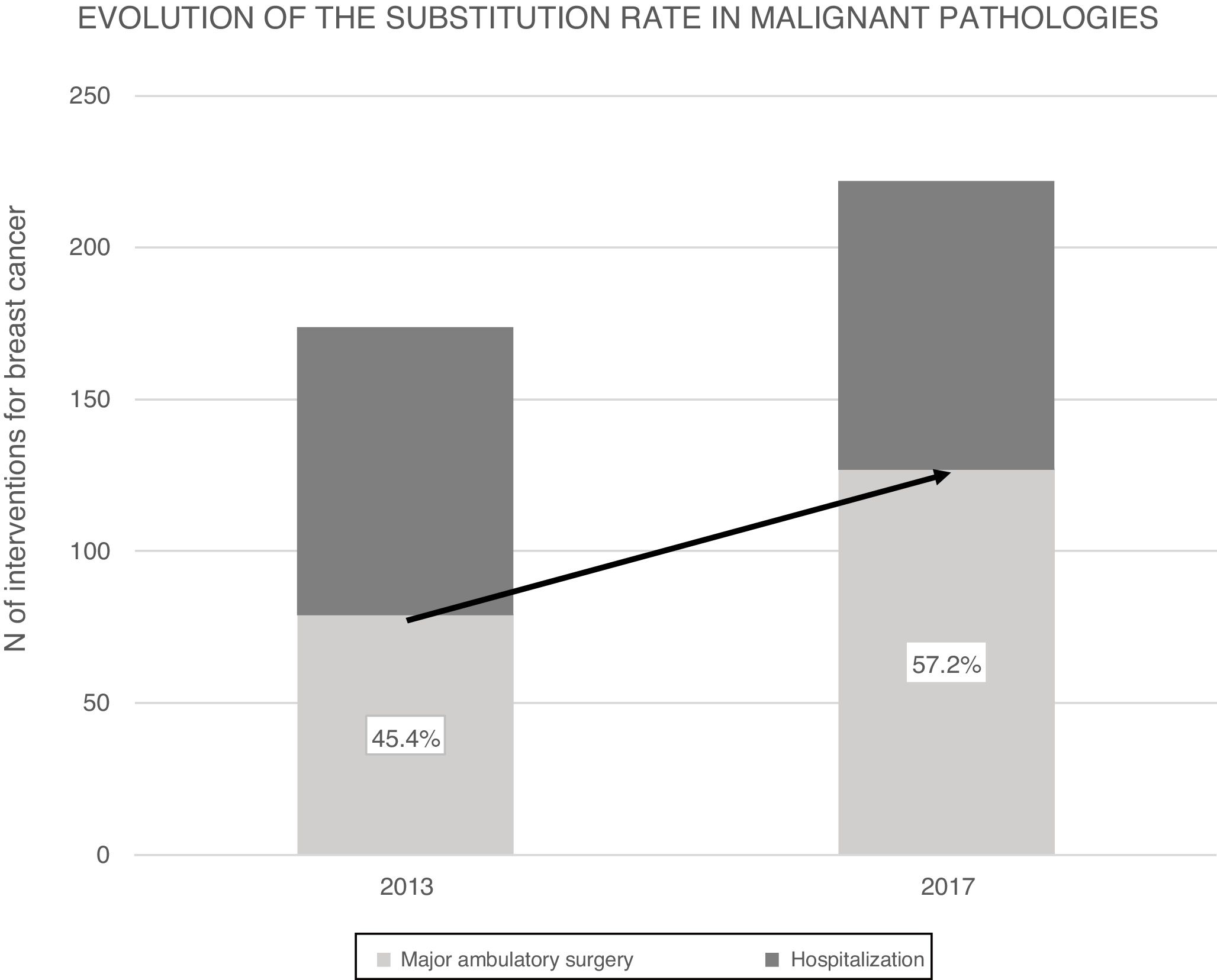
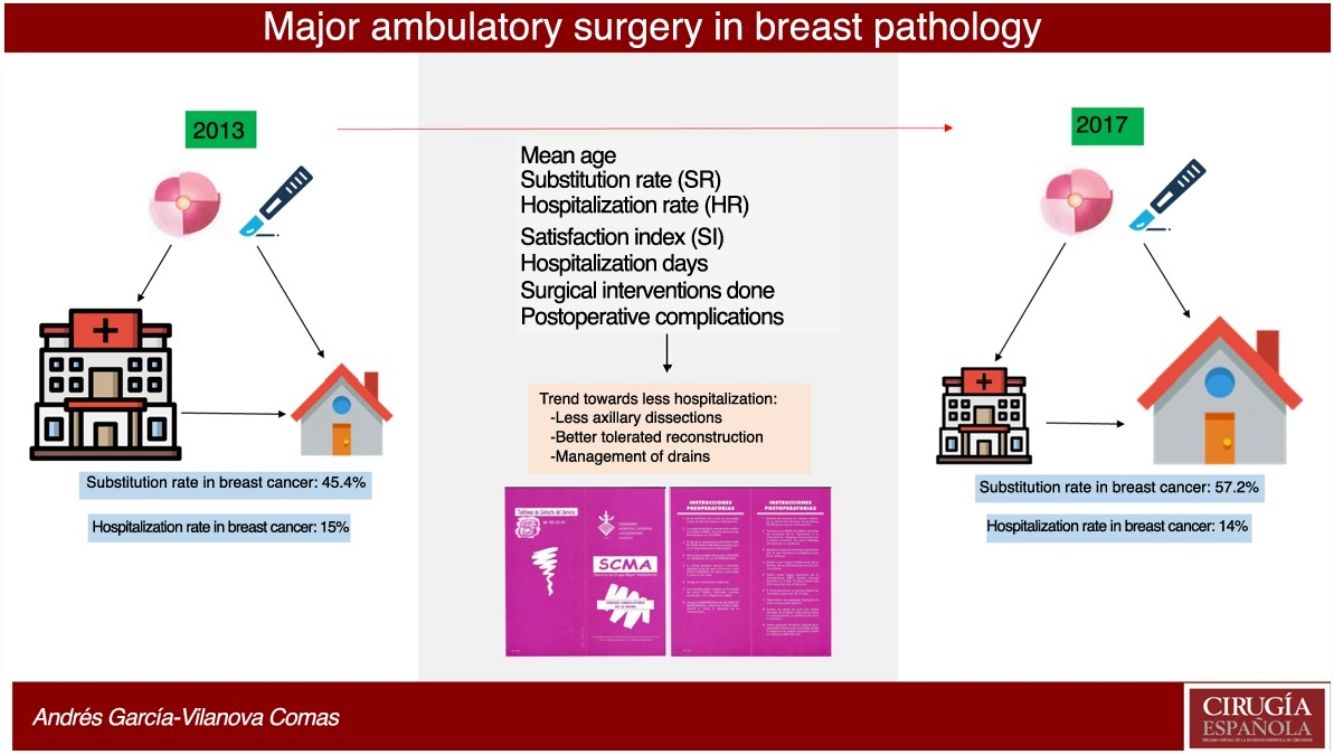
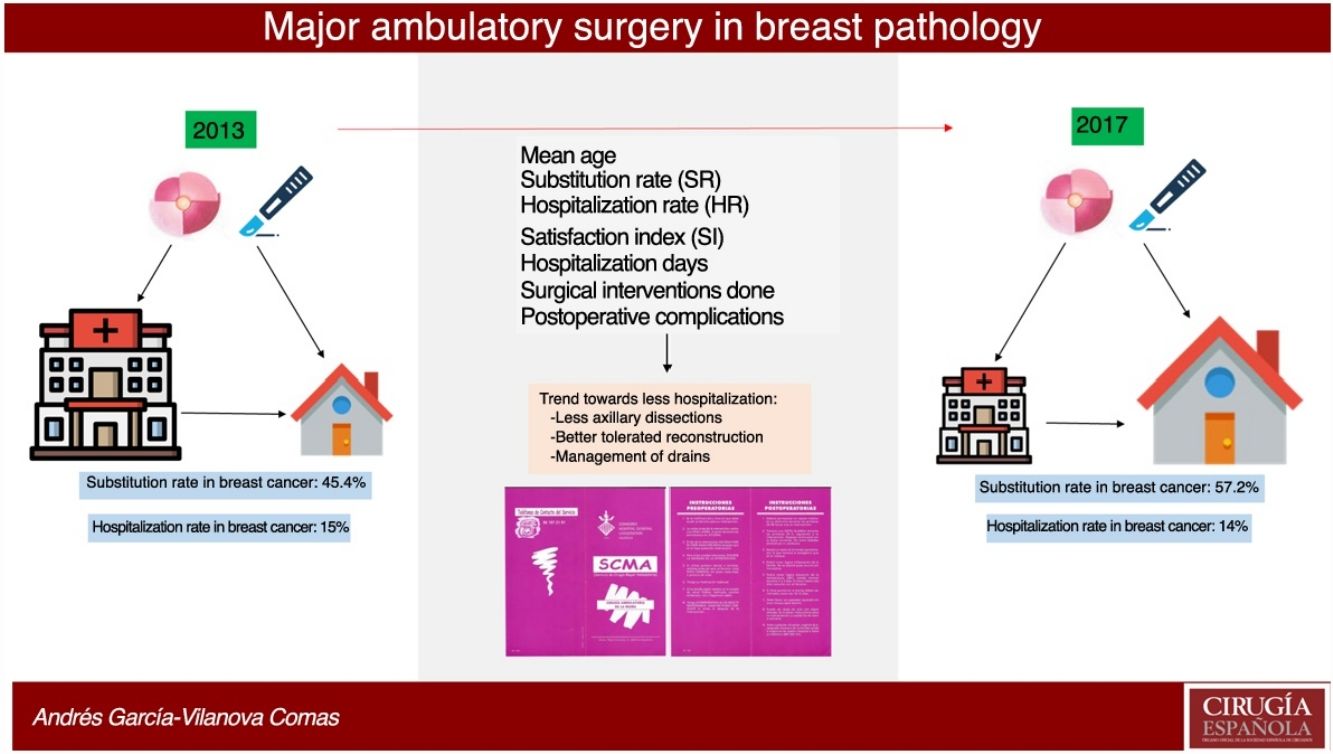
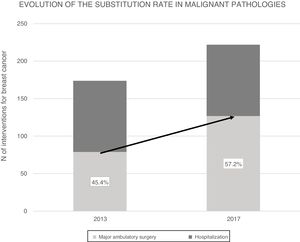
ResultsIn 2017, 396 procedures for BP were performed. 170 operations were carried out for benign and 226 for malignant. The SI for the global mammary pathology is 72.8%. The SI for benign pathology was 93.4%. The SI for malignant pathology was 57.2%, which has increased in the last years from 45.4% in 2013. The index of unexpected admission (TI) of the malignant pathology was 14.1%, while the TI in the benign pathology was 0.6%. Patients hospitalized for malignant pathology presented higher complications (17%) than ambulatory ones (8.5%) and benign (6.5%).
ConclusionsAt the CHGUV, the SR has steadily increased in malignant pathologies. The unexpected hospitalization rate is determined by perioperative sentinel lymph node biopsy results. AS for the treatment of mammary pathology is efficient and safe.
El tratamiento quirúrgico de la patología mamaria (PM) ha evolucionado aumentando su manejo como cirugía mayor ambulatoria (CMA). El objetivo de este estudio es analizar una serie de pacientes intervenidos de patología mamaria en régimen de CMA durante el año 2017 para evaluar su calidad y seguridad.
MétodosSe realiza análisis retrospectivo de los pacientes intervenidos de PM en el Consorcio Hospital General Universitario de Valencia (CHGUV) desde enero hasta diciembre del 2017 incluidas en programa de CMA, estudiando el número de pacientes, motivos de exclusión, el tipo de procedimientos quirúrgicos realizados, el índice de sustitución (IS), la tasa de ingreso (TI) y causas de conversión a ingreso, complicaciones postoperatorias y el índice de satisfacción. Se compara con un grupo control del año 2013.
ResultadosEn 2017 se realizaron 396 intervenciones por PM siendo de patología mamaria benigna (PMB) 170 intervenciones y de patología mamaria maligna (PMM) 226 intervenciones. El IS para la PM global es del 72.8% y para PMB fue 93.4%. El IS para PMM fue 57.2%, que ha progresado en los últimos años desde el 45.4% en 2013. La Tasa de ingreso inesperado (TI) de la PMM fue del 14.1%, mientras que en la PMB fue del 0.6%. La PMM con ingreso presentó más morbilidad (17%) que la PMM sin ingreso (8.5%) y la PMB (6.5%).
ConclusionesEn PMM del CHGUV el IS ha aumentado y la TI depende de la linfadenectomia tras biopsia peroperatoria del ganglio centinela. La CMA para el tratamiento de la patología mamaria es segura y eficiente.
The evolution of major ambulatory surgery (AS) has been significant, especially in the last 2 decades. The quality of care and patient safety have improved, while new indicators have been developed to measure its quality.1
The quality and efficiency of AS are mainly assessed using 2 parameters: the substitution rate (SR), which is the percentage of interventions performed on an outpatient basis out of the total number of operations performed; and the unplanned hospitalization rate (HR), which is the percentage of patients initially scheduled for AS who are admitted to hospital for any reason. The goal is for the SR to be as high as possible and the HR as low as possible.
The AS organizational method for surgical treatment is no longer so new, and patient acceptance levels have reached 80–90%.2 It is considered one of the best systems to reduce waiting lists and improve surgical efficiency. AS reduces surgery-related costs, with an admission rate of 25%–30%, reaching 50% in certain surgical diseases.2,3
Technological developments, new procedures with minimally invasive surgical approaches and short-term anesthesia favor rapid postoperative patient recovery with minimal side effects, and more complex patients and nosological surgical entities have gradually become included in the AS service portfolio.4
The inclusion in the outpatient surgical circuit of patients with benign breast disease is more established than in malignant breast disease, which usually requires more complex breast and axillary surgical interventions.5-7
The objective of this study is to analyze a series of patients treated surgically for benign and malignant disease within the AS setting in 2017, assessing efficiency and safety using AS quality indicators: SR, HR, complications and patient satisfaction index.
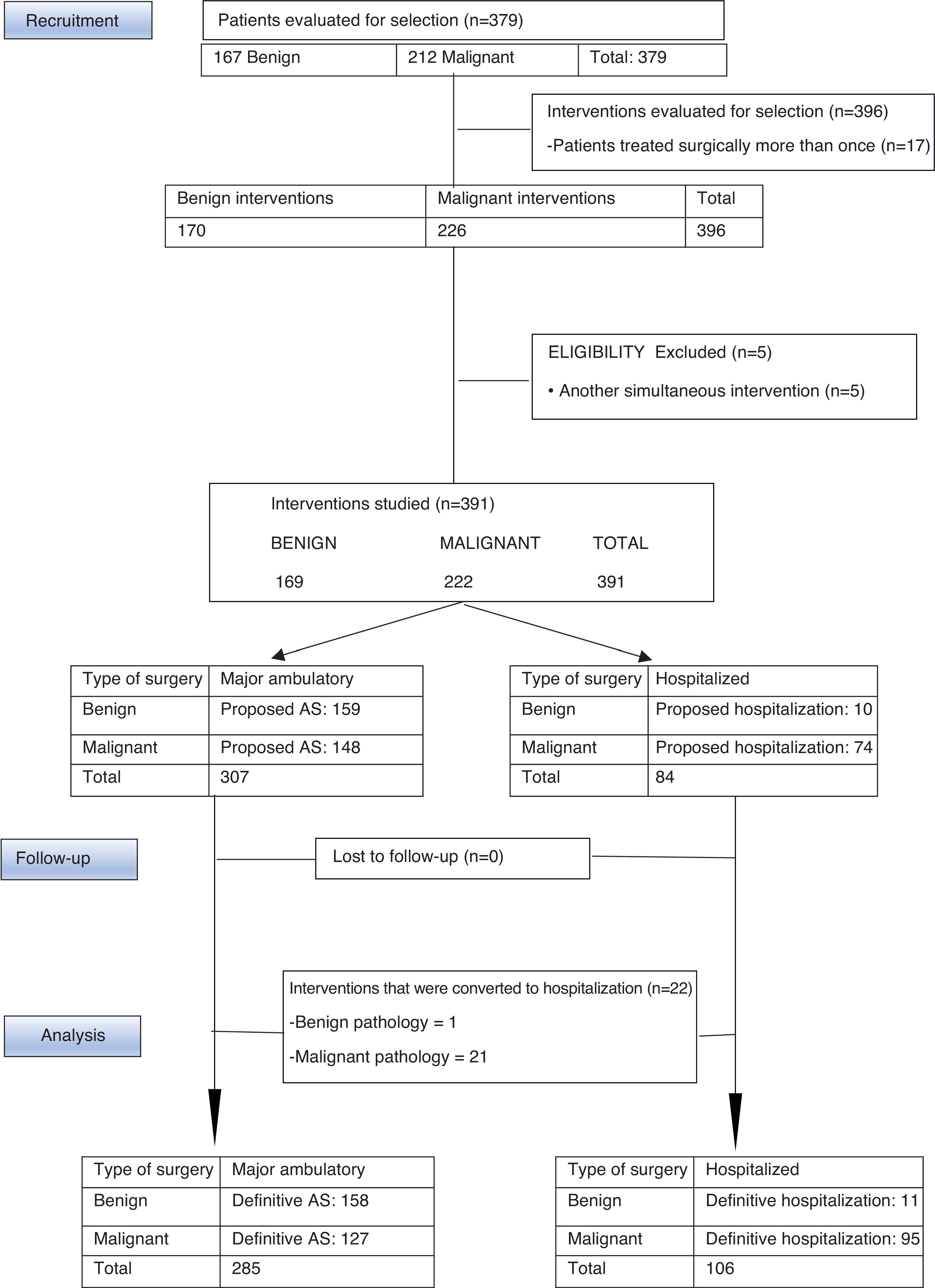
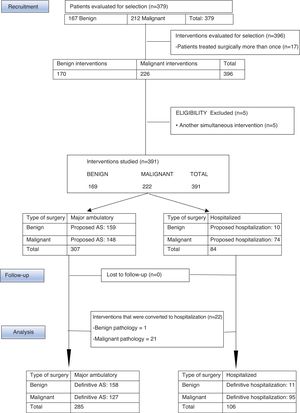
MethodsWe present a retrospective, descriptive, observational study of a series of 378 patients with benign and malignant breast disease, who had undergone surgery at the Consorcio Hospital General Universitario de Valencia (CHGUV) in 2017. We analyzed the AS performed (Fig. 1) and compared the malignant breast disease group with a control group from 2013, the first year in which breast surgery was integrated into the AS circuit. The same inclusion/exclusion criteria and methodology were used, and the surgeries were performed by the same group of surgeons.
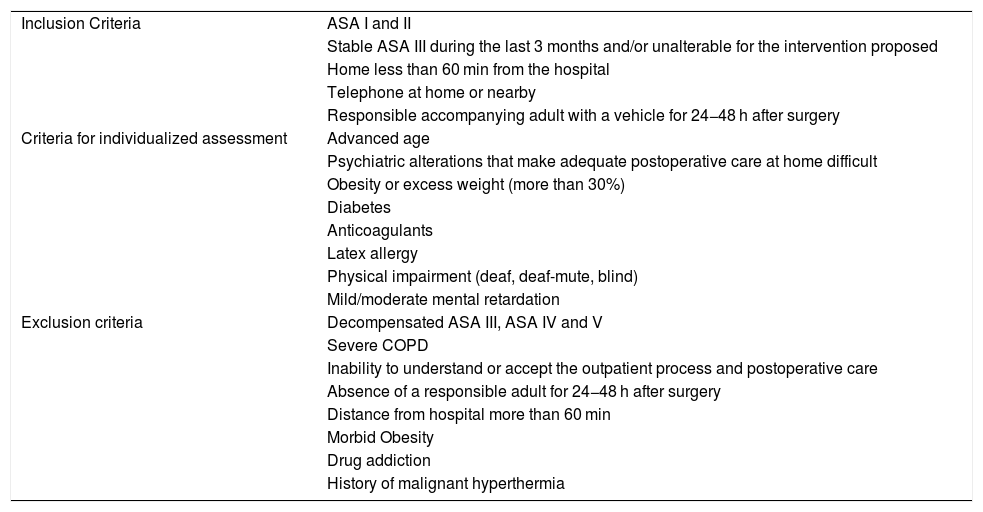
All the patients were evaluated by the Breast Committee, and later in outpatient consultations by members of the General and Digestive Surgery Service of the CHGUV integrated in the Functional Breast Unit (FBU), then evaluated for possible inclusion in the AS program after verifying compliance with the established selection criteria1 (Table 1). Patients were provided the information necessary about the treatment process both verbally and in writing in a specific AS brochure adapted to breast disease, as well as informed surgical, AS and anesthetic consent.
Criteria of Patients With Breast Pathology for AS.
| Inclusion Criteria | ASA I and II |
| Stable ASA III during the last 3 months and/or unalterable for the intervention proposed | |
| Home less than 60 min from the hospital | |
| Telephone at home or nearby | |
| Responsible accompanying adult with a vehicle for 24−48 h after surgery | |
| Criteria for individualized assessment | Advanced age |
| Psychiatric alterations that make adequate postoperative care at home difficult | |
| Obesity or excess weight (more than 30%) | |
| Diabetes | |
| Anticoagulants | |
| Latex allergy | |
| Physical impairment (deaf, deaf-mute, blind) | |
| Mild/moderate mental retardation | |
| Exclusion criteria | Decompensated ASA III, ASA IV and V |
| Severe COPD | |
| Inability to understand or accept the outpatient process and postoperative care | |
| Absence of a responsible adult for 24−48 h after surgery | |
| Distance from hospital more than 60 min | |
| Morbid Obesity | |
| Drug addiction | |
| History of malignant hyperthermia |
Level II and some level III interventions were included in the Davis Classification.8 Exclusion criteria in our AS program were the most aggressive breast interventions that require greater postoperative care, such as, for malignant disease, complete axillary lymphadenectomy (AL) or complete axillary dissection (AD) and mastectomy, with or without reconstruction with prosthesis and augmentation mammoplasties in benign disease. Patients with other simultaneous surgical procedures that could modify the time of discharge were also excluded. All the surgical interventions of the 2013 and 2017 groups were performed by the same surgical team, consisting of 3 general surgeons and a plastic surgeon, integrated in the FBU.
In the sentinel node biopsy technique, a defined protocol was followed,9 following consensus indications10 and assessing the total tumor burden using the one-step nucleic acid amplification (OSNA) technique. The detection of more than 15,000 perioperative copies led to the extension of surgery with AL and subsequent hospitalization of patients initially scheduled as surgery without hospitalization (conversion to hospitalization).11 In the 2013 group using the OSNA technique, the decision to extend surgery with AL included micrometastases and macrometastasis, while in 2017 the total tumor burden of 15,000 copies was used.
General anesthesia was used, preferably using a laryngeal mask, with short-acting opioids, induced with propofol and midazolam12 and maintained with propofol and remifentanil perfusion.13 The wound edges were infiltrated with a long-lasting local anesthetic (levobupivacaine) at the end of the operation.14
Patients were assessed with a modified Aldrete recovery scale, which is included in the manual of the major ambulatory surgery unit. Standards and recommendations of the Ministry of Health and Consumer Affairs1,2 were used to decide on the progressive transfer to the postoperative recovery unit I and II of the AS service or to the day hospital. Patients were subsequently discharged after checking recovery parameters1:
-
Conscious and oriented.
-
Autonomous ambulation without feeling of instability in the last hour.
-
Stable vital signs.
-
Tolerance to liquid intake.
-
Good pain control.
-
Preserved diuresis.
-
Absence of bleeding, nausea and vomiting.
-
Presence of a responsible adult with vehicle for home transport.
The day after surgery, the nursing team of the AS service telephoned each and every one of the patients for home follow-up, assessing the intervention, general condition, whether prescribed medications were being taken, the need to go to the emergency room, presence of fever, nausea or vomiting, wound bleeding, difficult urination or defecation, cognitive state and presence/intensity of pain.
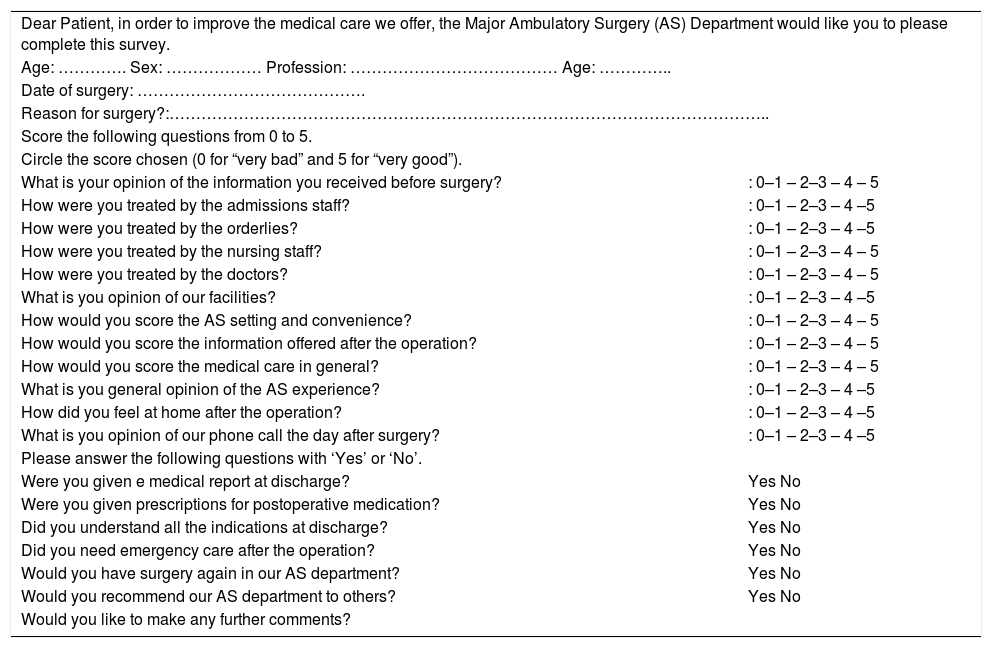
In subsequent visits in outpatient consultations 4 weeks later, satisfaction surveys were administered with specific questions that were answered on a Likert scale (Table 2), and from them the satisfaction index of the patients treated within the program was measured.15
Satisfaction Survey.
| Dear Patient, in order to improve the medical care we offer, the Major Ambulatory Surgery (AS) Department would like you to please complete this survey. | |
| Age: …………. Sex: ……………… Profession: ………………………………… Age: ………….. | |
| Date of surgery: ……………………………………. | |
| Reason for surgery?:………………………………………………………………………………………………….. | |
| Score the following questions from 0 to 5. | |
| Circle the score chosen (0 for “very bad” and 5 for “very good”). | |
| What is your opinion of the information you received before surgery? | : 0–1 – 2–3 – 4 – 5 |
| How were you treated by the admissions staff? | : 0–1 – 2–3 – 4 –5 |
| How were you treated by the orderlies? | : 0–1 – 2–3 – 4 –5 |
| How were you treated by the nursing staff? | : 0–1 – 2–3 – 4 – 5 |
| How were you treated by the doctors? | : 0–1 – 2–3 – 4 – 5 |
| What is you opinion of our facilities? | : 0–1 – 2–3 – 4 –5 |
| How would you score the AS setting and convenience? | : 0–1 – 2–3 – 4 – 5 |
| How would you score the information offered after the operation? | : 0–1 – 2–3 – 4 – 5 |
| How would you score the medical care in general? | : 0–1 – 2–3 – 4 – 5 |
| What is you general opinion of the AS experience? | : 0–1 – 2–3 – 4 –5 |
| How did you feel at home after the operation? | : 0–1 – 2–3 – 4 –5 |
| What is you opinion of our phone call the day after surgery? | : 0–1 – 2–3 – 4 –5 |
| Please answer the following questions with ‘Yes’ or ‘No’. | |
| Were you given e medical report at discharge? | Yes No |
| Were you given prescriptions for postoperative medication? | Yes No |
| Did you understand all the indications at discharge? | Yes No |
| Did you need emergency care after the operation? | Yes No |
| Would you have surgery again in our AS department? | Yes No |
| Would you recommend our AS department to others? | Yes No |
| Would you like to make any further comments? | |
The variables studied included: mean age, days of hospitalization, pathology type and the intervention performed, postoperative complications. Also, indicators of AS quality included: SR, HR and the satisfaction index of the patients undergoing surgery.16 The SR and HR of the breast cancer patient group from 2017 were compared with the 2013 control group.
Statistical AnalysisThe data were included in the Excel 2013 software package and its statistical expansion for analysis, performing a descriptive study of the sample, which included age and type of surgery performed in patient samples from 2017 and 2013. The Kolmogorov–Smirnov test was used to verify the normal distribution of variables, which were homogeneous in terms of age and type of intervention performed. Qualitative variables were expressed as absolute value and percentage. The Chi-squared test was used for the comparison of qualitative variables. A P value <.05 was considered statistically significant.
The Chi-squared proportions analysis was used to analyze the correlation between quadrantectomies, mastectomies, complete axillary dissection and the performance of AS for malignant breast disease in the year 2017 compared to 2013.
ResultsBetween January and December 2017, the UPM of the CHGUV carried out 396 interventions for breast pathologies in 379 patients (167 benign, requiring 170 interventions; and 212 malignant, requiring 226). Patients with simultaneous surgeries other than breast surgery were excluded to avoid confusion due to possible complications associated with the other surgery. Therefore, 5 patients were excluded from the study (4 with malignant disease and one with benign disease). After this exclusion, 374 patients were finally evaluated and 391 interventions were performed, 72.8% of which were performed using AS (285 interventions: 158 for benign disease [55.4%] and 127 for malignant disease [44.7%]) and 27.11% surgery with hospitalization (106 operations: 11 for benign disease [10.3%] and 95 for malignant disease [89.6%]) (Fig. 1).
The mean age of the patients with malignant lesions was 61.06 ± 2 years, while the mean age of patients with benign lesions was 42.74 ± 2 years.
Out of the 169 interventions performed for benign disease in 2017, 156 patients were selected for AS and 159 operations were performed, 158 of which (155 patients) were able to be completed as AS, and one required hospitalization due to persistent postoperative pain. Benign interventions scheduled for hospitalization were related with the need for plastic surgery: replacement of prostheses, removal of prostheses and breast augmentation. Initially, 10 patients were proposed for hospitalization for a total of 10 operations. Therefore, the HR was 0.6% and the SR was 93.4%.
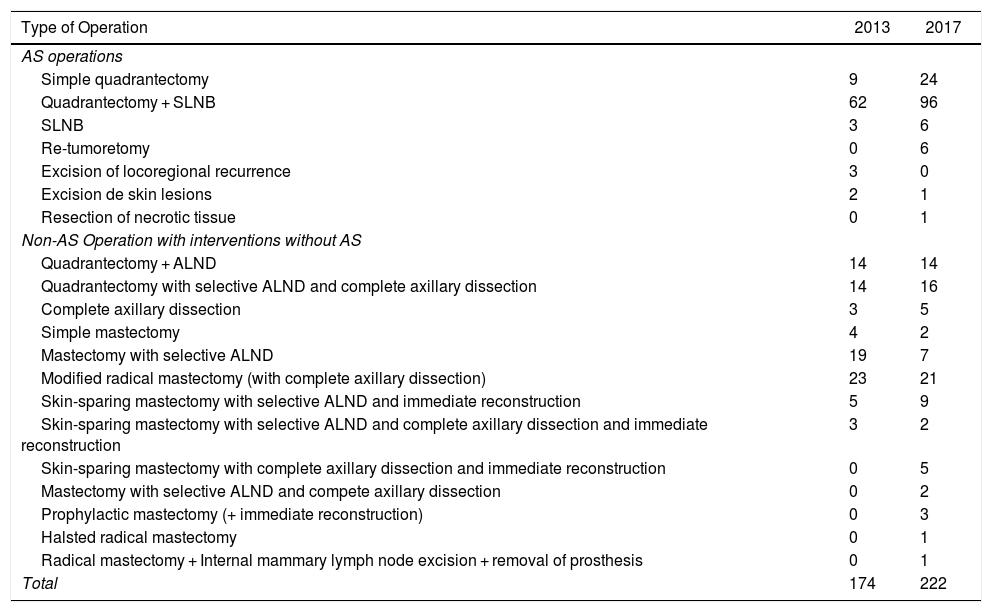
In cases of malignant pathologies in 2013, 174 operations were carried out. In 2017, this figure increased to 222, and the following procedures were selected for AS: quadrantectomy with or without selective sentinel lymph node biopsy (SLNB), SLNB, re-tumorectomy, excision of locoregional recurrence, excision of skin lesions and the resection of necrotic tissue. Basic oncoplastic techniques were used in quadrantectomies, including local mammary gland mobilization, round-block pattern, single-axis superior pedicle vertical mammoplasty, and inferior rotation mammoplasties. No “symmetrization” of the contralateral breast was performed. The remaining procedures were considered surgery requiring hospitalization (Table 3). The following variables were compared between the data from 2013 and 2017: the proportion of interventions initially assigned to AS, those that were actually performed as AS and the percentage of quadrantectomies; the Chi-squared test was used and found a statistically significant difference. In contrast, when the proportion of mastectomies and AD was compared, although these were higher in 2013 compared to 2017, the difference was not statistically significant (Table 3).
Comparison of Interventions for Malignant Breast Pathology Between 2013 and 2017.
| Type of Operation | 2013 | 2017 |
|---|---|---|
| AS operations | ||
| Simple quadrantectomy | 9 | 24 |
| Quadrantectomy + SLNB | 62 | 96 |
| SLNB | 3 | 6 |
| Re-tumoretomy | 0 | 6 |
| Excision of locoregional recurrence | 3 | 0 |
| Excision de skin lesions | 2 | 1 |
| Resection of necrotic tissue | 0 | 1 |
| Non-AS Operation with interventions without AS | ||
| Quadrantectomy + ALND | 14 | 14 |
| Quadrantectomy with selective ALND and complete axillary dissection | 14 | 16 |
| Complete axillary dissection | 3 | 5 |
| Simple mastectomy | 4 | 2 |
| Mastectomy with selective ALND | 19 | 7 |
| Modified radical mastectomy (with complete axillary dissection) | 23 | 21 |
| Skin-sparing mastectomy with selective ALND and immediate reconstruction | 5 | 9 |
| Skin-sparing mastectomy with selective ALND and complete axillary dissection and immediate reconstruction | 3 | 2 |
| Skin-sparing mastectomy with complete axillary dissection and immediate reconstruction | 0 | 5 |
| Mastectomy with selective ALND and compete axillary dissection | 0 | 2 |
| Prophylactic mastectomy (+ immediate reconstruction) | 0 | 3 |
| Halsted radical mastectomy | 0 | 1 |
| Radical mastectomy + Internal mammary lymph node excision + removal of prosthesis | 0 | 1 |
| Total | 174 | 222 |
| Year | 2013 | 2017 | Chi-squared | P |
|---|---|---|---|---|
| Total quadrantectomies | 107 (61%) | 164 (73%) | 6.92 | < .01 |
| Total mastectomies | 54 (31%) | 53 (24%) | 2.54 | > .05 |
| Complete axillary dissection | 60 (34%) | 65 (29%) | 1.22 | > .05 |
| Initial indication of AS | 93(53%) | 148 (66%) | 7.15 | < .01 |
| AS conducted | 79 (45%) | 127 (57%) | 5.45 | < .05 |
During 2017, 144 patients with malignant disease were selected for AS and underwent 148 operations; 123 patients (127 interventions) were able to be completed as AS, while 21 had to be admitted: 16 because initially a quadrantectomy was performed with extemporaneous SLNB, using the OSNA technique, which was compatible with macrometastasis and required completing the intervention with AL; 4 because they were operated on in the month of August, when the day hospital was closed; and one patient was admitted for concomitant Wolff–Parkinson–White syndrome. The SR for malignant disease was 57.2%.
In 2013, 174 operations were performed on patients with malignancies, 93 of which were selected for AS. In the end, 79 interventions were able to be completed as AS, while 14 required hospitalization: 12 because initially a quadrantectomy was performed with extemporaneous SLNB, using the OSNA technique, which was compatible with micrometastases or macrometastasis and required AL; one because the sentinel node could not be detected and the intervention was completed with AL; and one 89-year-old patient was admitted due to comorbidities. The SR for malignant breast disease in 2013 was 45.4%.
Over the course of 4 years (2013–2017) the SR for malignant breast disease in AS has increased 11.8%, going from 45.4% in 2013 to 57.2% in 2017 (Fig. 2). The AS procedures for both years were compared by the Chi-squared test, and a statistically significant association was revealed (Table 3).
The SR for global breast pathologies, both benign and malignant, was 72.8% in 2017.
For the treatment of malignant presentations in 2017, 64 patients were proposed for initial hospitalization for a total of 74 operations performed. These were added to the patients proposed for AS who were converted to hospitalization, in this case 21 patients who underwent a total of 21 interventions. Thus, in 2017, a total of 95 interventions were performed on 85 patients out of the 222 interventions for malignant breast disease (44.81%). In 2013, 95 interventions were completed with hospitalized patients out of the 174 interventions for malignancies (54.59%).
In 2017, 21 patients with malignant presentations were converted to surgery with hospitalization, representing a 14% unexpected hospitalization rate (HR), compared to 14 patients in 2013, representing a HR of 15%. In benign presentations, only one intervention initially selected for AS was converted to surgery with hospitalization.
The average number of hospitalization days after surgery for malignant disease (1.33 days) was similar to the number of days for benign disease (1.1 days).
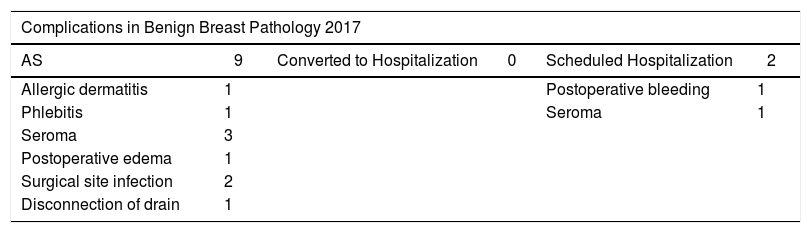
In 169 procedures performed for benign disease, we have only detected 11 complications (6.51%). Seromas were the most frequent, followed by surgical site infection (Table 4).
Complications by Benign and Malignant Breast Pathologies in 2017.
| Complications in Benign Breast Pathology 2017 | |||||
|---|---|---|---|---|---|
| AS | 9 | Converted to Hospitalization | 0 | Scheduled Hospitalization | 2 |
| Allergic dermatitis | 1 | Postoperative bleeding | 1 | ||
| Phlebitis | 1 | Seroma | 1 | ||
| Seroma | 3 | ||||
| Postoperative edema | 1 | ||||
| Surgical site infection | 2 | ||||
| Disconnection of drain | 1 | ||||
| Total complications | 11 | 6.51% | ||||
|---|---|---|---|---|---|---|
| Complications in malignant breast pathology 2017 | ||||||
| During hospitalization | N | Emergencies without hospitalization | N | Emergencies with hospitalization | N | |
| Hospitalization | •→Nausea and vomiting •→Severe emphysematous COPD | 2 | •→Drainage without complete dissection | 1 | •→Necrotic tissue: | 1 |
| •→Bleeding | 1 | •→Plugged drain | 1 | •→reoperation•→Skin necrosis and exposure of prosthetic material: reoperation | 1 | |
| •→Pain + dyspnea | 1 | |||||
| •→Anemic syndrome + transfusion | 1 | |||||
| •→Pain | 1 | |||||
| •→Postoperative bleeding + Anemic syndrome | 3 | |||||
| •→Hypotension + dizziness | 1 | |||||
| •→Hematoma (no operation) | 1 | |||||
| 1 | ||||||
| AS | No hospitalization | 0 | No complications | 0 | No complications | 0 |
| AS converted to hospitalization | •→Hematoma (evacuation) | 1 | •→Hematoma | 1 | No complications | 0 |
| •→Poor function of drain | 1 | |||||
| Total | 8.56% | 19 | ||||
| Scheduled hospitalization | 16 | |||||
| AS | 0 | |||||
| AS converted to hospitalization | 3 | |||||
The interventions performed on an outpatient basis for malignant disease did not generate complications, but we found 3 complications in patients who had been converted to hospitalization: 2 hematomas and one drainage-related.
The operations for malignant pathologies scheduled for hospitalization presented the highest morbidity: 16 complications (17%), most frequently pain, nausea and vomiting. In this group, we found the only 2 readmissions due to necrosis.
In the AS setting, malignant disease generated a higher percentage of complications (19 out of 222 interventions: 8.5%), while benign disease only generated 11 out of 196 operations: 6.51%.
The satisfaction index determined through surveys showed that 94.29% of the patients rated both the care received and the AS service with the highest score (5 out of 5).
DiscussionAmbulatory surgical treatment for malignant breast pathologies has gradually increased, and some authors considered it the new standard therapy for conservative treatments and breast cancer surgery.17–19 The reported incidences of complications and readmissions are equal to or less than those of surgery with hospitalization.17,20,21 However, in certain more complex surgical procedures, including mastectomies, complete axillary dissection or breast reconstructions, the proposal of AS is more debatable by being scheduled as 23-h surgery (overnight stay) or with short-stay hospitalization.22,23
Even though we did not consider age an exclusion criterion in the selection of our patients, the mean age of patients with malignant disease was 61.06 ± 2 yrs, while the mean age in patients with benign disease was 47.74 ± 2 yrs. As in other studies,21,24 it is necessary to consider that the patients treated surgically for malignant breast disease tend to be older, and in some cases the indication for surgical treatment in an AS program may be more difficult due to the comorbidities they present.
In this study, the SR in the breast malignancy group increased by 11.8% in 4 years, which is an indication of the increase in AS treatment for breast cancer in the CHGUV. This is not due to the fact that the indications for AS have been extended by including more complex surgical techniques or patients with more comorbidity. Instead, it is due to the increase in the number of patients requiring simpler surgical procedures (simple quadrantectomies or with SLNB). These cases have a lower complication rate and faster recovery than radical or reconstruction surgery, with less need for traditional hospital admission. It may be related to an increase in patients referred from screening units, after neoadjuvant treatments and for oncoplastic surgery.
The SR detected in our series is similar to those reported by other authors in Spain.24,25
The HR in malignant breast pathologies in 2017 was 14%, a figure similar to those of other Spanish studies24,25 and systematic reviews,20,26 which are between 10% and 11.4%. The most frequent cause of unexpected admission was the extension of surgery with complete AD due to positivity of the sentinel node in the intraoperative pathology study, which coincides with other authors.24 In contrast, in the study by Medina,12 with an HR of 16.5%, the most frequent causes of admission were nausea, vomiting, hematomas, postoperative pain and wound complications.
The average number of days of hospital stay of patients treated surgically for benign disease on a scheduled basis and with admission was 1.1 days, and the average for hospitalized patients treated for malignant disease was 1.3 days. There was a predominance of admission with overnight stay (less than 24 h) in cases of complete AD or mastectomies without immediate reconstruction. Patients undergoing mastectomies with immediate reconstruction had the longest stays. The proportion of patients with quadrantectomies converted to admission due to sentinel lymph node positivity increased from 18% in 2013 to 14% in 2017, and the HR in 2017 would have been even lower if the day hospital had not closed in August of that year.
Future lines of work to increase the SR and reduce the HR in breast pathology surgery at our hospital are aimed at reducing the number of more complex techniques (mastectomies and AD), facilitating adequate patient education in the postoperative care of drain tubes, and the inclusion in the AS program of certain selected patients who require interventions with AD and mastectomies, with and without reconstruction.
It does not seem very likely that the number of mastectomies will decrease significantly in coming years,27 but the tendency of current research is to reduce the indications for AD, because adjuvant axillary radiotherapy can match the benefits of axillary surgery with lower patient morbidity in cases with breast-conserving surgery, as suggested by the results of the ACOSOG-Z011,28 AMAROS29 and OTOASOR30 clinical trials. Likewise, the SOUND31 and BOOG 2013-1832 clinical trials have analyzed the avoidance of performing SLNB to only perform quadrantectomy with radiotherapy and observation. In patients currently undergoing mastectomy with AD, the tendency is also to reduce these dissections.32–34
In patients who have received neoadjuvant therapy in whom the axilla was previously clinically negative, it is under investigation whether it is always necessary to add AD in any sentinel node involvement. In cases that have received neoadjuvant therapy and the axilla was clinically positive prior to neoadjuvant therapy, if certain conditions are met, SLNB is also performed and AD is not performed if the lymph node is no longer affected, according to the ACOSOG Z1071,35 SENTINA36 and SN FNAC37 studies. This current trend towards a progressive decrease in the number of complete axillary dissections should continue to improve AS indicators.
In our series, malignant breast disease operations generated a higher percentage of complications (8.56%) than interventions for benign disease (6.51%), although these also included reconstructions in patients who were considered free of neoplastic disease. The malignant cases treated in AS of this study presented a higher rate of complications among hospitalized patients than in those who were discharged on the same day of the intervention, as indicated by other authors.38
Our rate of infectious complications in benign pathology interventions was 1.26%, which was lower than the rates reported by the Community of Madrid group (which was 3.89%), the Clinical Indicators group for continuous improvement of quality (INCLIMECC) (2.28%) and the National Healthcare Safety Network (NHSN) (2.26%).39–41
Patient opinion and satisfaction surveys inform us of their opinions and encourage patients to participate in planning the AS, thereby increasing their commitment to participate in treatment.42 The 94.29% satisfaction rate of our patients is similar to reports of other authors, also higher than 90%.12
The literature supports that the AS is feasible, safe and beneficial for patients with breast cancer21,24,25,43,44 and improves the efficiency of the healthcare system with savings in hospital stays.45 Therefore, more services should be progressively incorporated into this system.
In conclusion, this study shows an increase in the substitution rate in malignant breast disease from 2013 to 2017, which is related to the decrease in the use of more complex surgical techniques. There was also a reduction in the hospitalization rate, which was associated with the amount of unexpected axillary lymphadenectomies. The rate of complications was lower in the AS group than in the surgery with hospitalization group, and the satisfaction rate surpassed 90%.
It is recommended to improve the quality of the studies, because the majority are not uniform and they are series with few cases. There is also no clear consensus on the pre-evaluation criteria or the quality indicators, making it difficult to make good comparisons between AS units/services at different hospitals (benchmarking).46
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Garcia-Vilanova Comas A, Nadal Gisbert J, Santofimia Chordá R, Fuster Diana C, de Andrés Gómez A, Medrano González J, et al. Cirugía mayor ambulatoria en patología mamaria. Cir Esp. 2020;98:26–35.