Even though evidence is lacking, a low-fat diet has been traditionally recommended after cholecystectomy. The main aim of this study was to assess the potential correlation between postoperative symptoms and type of diet after cholecystectomy.
MethodsSymptoms were prospectively assessed by the GIQLI (Gastrointestinal Quality of Life Index) score at baseline, one month and six months after cholecystectomy in 83 patients operated on at our institution. Patients completed a questionnaire about their diet and were classified into four groups according to the amount of fat intake. Differences in the GIQLI score depending on the type of diet were assessed over time.
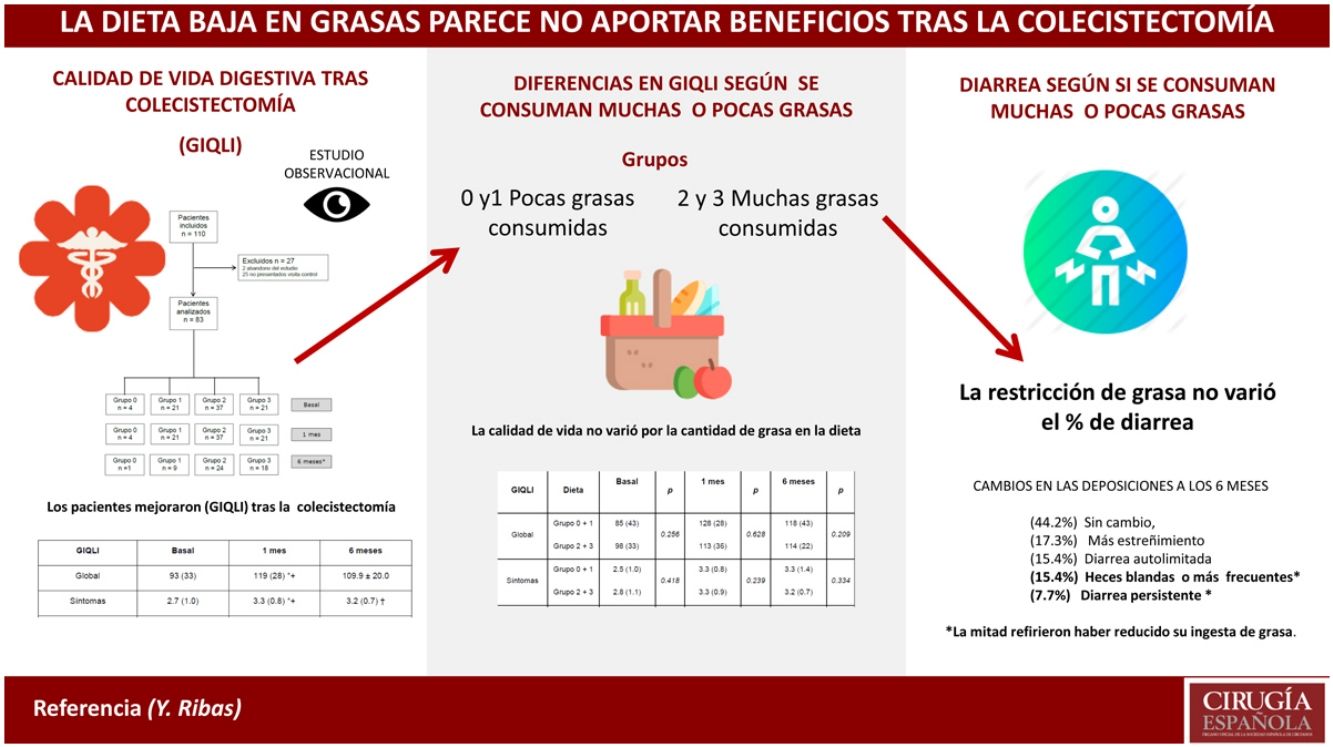
ResultsThe overall GIQLI score and most subdomains significantly increased after surgery compared to baseline, regardless of the intake of dietary fat. Constipation improved after cholecystectomy compared to baseline, whereas diarrhea and bowel urgency got worse. More than 50% of patients experienced a change in their bowel habit after surgery, which persisted six months later in 23% of cases.
ConclusionsA low fat diet does not seem to have an influence on the improvement of symptoms after cholecystectomy. However, a randomized study is ongoing at our institution to confirm the results of this prospective study
A pesar de la falta de evidencia, tradicionalmente se ha recomendado seguir una dieta baja en grasas tras la colecistectomía. El objetivo principal fue analizar la correlación potencial entre los síntomas postoperatorios y el tipo de dieta tras la colecistectomía.
MétodosLos síntomas fueron evaluados de forma prospectiva mediante el cuestionario Gastrointestinal Quality of Life Index (GIQLI) antes de la intervención, al mes y 6 meses después de la colecistectomía en 83 pacientes operados en nuestro centro. Los pacientes completaron un cuestionario sobre su dieta y fueron clasificados en 4 grupos de acuerdo a la cantidad de grasa ingerida. Las diferencias en la puntuación GIQLI dependiendo del tipo de dieta se evaluaron en el tiempo.
ResultadosLa puntuación GIQLI total y varias dimensiones aumentaron significativamente tras la cirugía respecto al valor basal, independientemente de la ingesta de grasa en la dieta. Entre los síntomas evaluados por el GIQLI, la diarrea y la urgencia defecatoria empeoraron mientras que el estreñimiento mejoró. Más del 50% de los pacientes experimentaron cambios en el ritmo deposicional después de la cirugía, que fueron persistentes durante 6 meses en el 23% de los casos.
ConclusionesLa dieta baja en grasas no parece influir en la mejoría de los síntomas tras la colecistectomía. No obstante, los resultados de un estudio aleatorizado que se está realizando en nuestro centro contribuirán a confirmar los resultados de este estudio prospectivo.
Postcholecystectomy syndrome involves a heterogeneous group of gastrointestinal symptoms after cholecystectomy, including biliary and non-biliary disorders.1,2 Certain entities should be ruled out, such as choledocholithiasis, bile duct lesions or bile leaks, which have specific treatments.1
Symptoms can persist or present de novo after surgery and can include abdominal pain, dyspepsia, diarrhea, constipation, abdominal distension, flatulence, acidity or nausea;3,4 their prevalence varies greatly. A recent systematic review4 demonstrated the lack of reliable data because only one of the 38 studies included met all quality criteria. Diarrhea was the most frequent symptom but also showed greater variability. Some symptoms considered secondary to cholecystectomy could be explained by coexisting pathologies like irritable bowel syndrome.
Data showing that most preoperative symptoms improve after surgery, except diarrhea,3 are controversial. While some studies report a frequency of postcholecystectomy diarrhea between 5 and 12%,5–8 others argue that it is uncommon.9–11 A recent study12 showed that cholecystectomy was associated with an increased postoperative risk of diarrhea and abdominal pain, although validated questionnaires were not used.
The low-fat diet has not been uniformly recommended after cholecystectomy,13 and there are no standardized guidelines for nutrition after surgery.14 One study15 found no differences in postoperative symptoms between patients who followed a low-fat diet compared to patients who followed a normal diet; meanwhile, 2 other studies16,17 reported that patients who did not follow a low-fat diet had more postoperative symptoms. As in our institution, there seems to be great variability in dietary recommendations after cholecystectomy, and evaluation of results is controversial.
Our objective was to prospectively assess postcholecystectomy symptoms using a validated questionnaire and to determine the correlation with the type of diet followed.
MethodsStudy DesignFrom November 2015 to March 2016, patients admitted for treatment of symptomatic cholelithiasis or related complications at the Consorci Sanitari de Terrassa (Barcelona) were prospectively included in the study.
ParticipantsPatients included in the study were over the age of 18 and had biliary colic or complications related with cholelithiasis (pancreatitis, cholangitis, cholecystitis).
The exclusion criteria were: refusal to participate in the study, inability to understand information due to mental illness or language barrier, and serious complications that could affect the postoperative course.
EvaluationsData were collected prospectively, including patient demographic data, comorbidities and type of surgery. The evaluation included questions about symptoms and diet before surgery, in person one month after surgery, and then again 6 months after surgery by mailed questionnaires.
To assess the symptoms, all patients completed the validated Spanish version of the Gastrointestinal Quality of Life Index (GIQLI) questionnaire.18,19 The GIQLI includes 36 questions to assess the quality of life specifically in terms of gastrointestinal health. It has been widely used in different digestive disorders, including cholelithiasis. A higher score represents a better quality of life. The survey is comprised of 5 sections: symptoms, physical sphere, emotional sphere, social sphere and effects of treatment.
In addition, all patients completed a questionnaire about the types of food included in their usual diet before surgery, one month post-op and 6 months post-op. To do so, patients chose the types of food from a non-indicative list obtained from the low-fat diet recommendations used at our hospital center (Appendix 1). The diet followed was classified into 4 groups according to the amount of fat ingested: intake of 3 or less fatty foods was considered as a very low fat diet (group 0); from 4 to 7 it was considered as low fat (group 1); from 8 to 10, a normal diet in the amount of fat (group 2), and more than 10 was considered a high-fat diet (group 3).
The final 6-month evaluation included questions about bowel movements and diet, as well as fat intake, tolerance to high-fat foods, changes in diet after surgery, and from whom the dietary recommendations were obtained (Appendix B).
Statistical AnalysisBaseline data are presented as median and interquartile range (IQR) for quantitative variables, and as frequencies for qualitative variables. The Student’s t test for paired samples was applied to analyze the differences in the GIQLI subdimensions between baseline, one month and 6 months after treatment. The Student’s t for independent data was applied to analyze the differences in the GIQLI over time depending on the type of diet. A P value<.05 was considered statistically significant. The data analysis was performed using the SPSS statistical package version 20.
Approval by the Ethics CommitteeThe study was approved by the Ethics Committee of the Consorci Sanitari de Terrassa (approval number 01-15-102-014). All patients provided written informed consent.
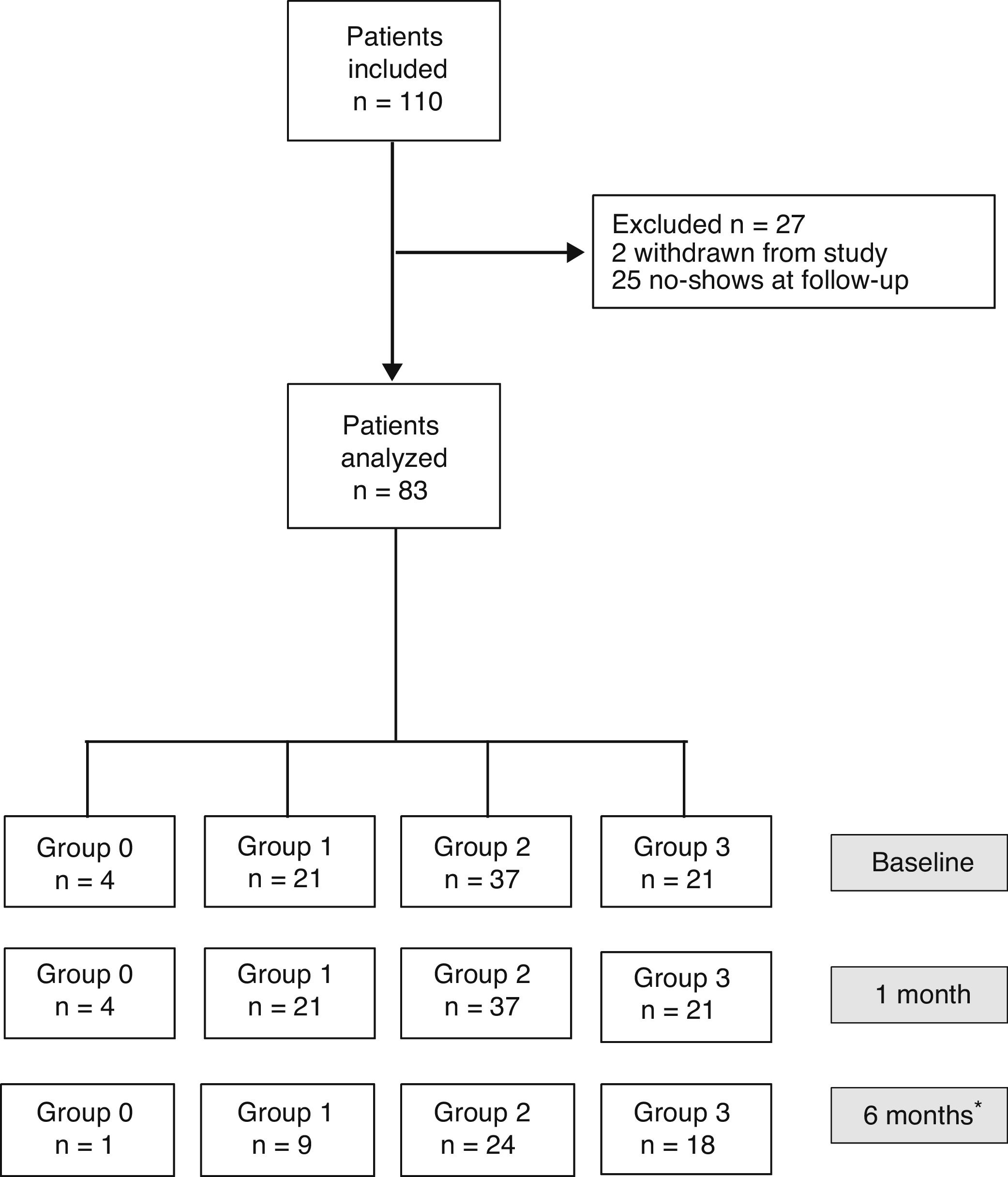
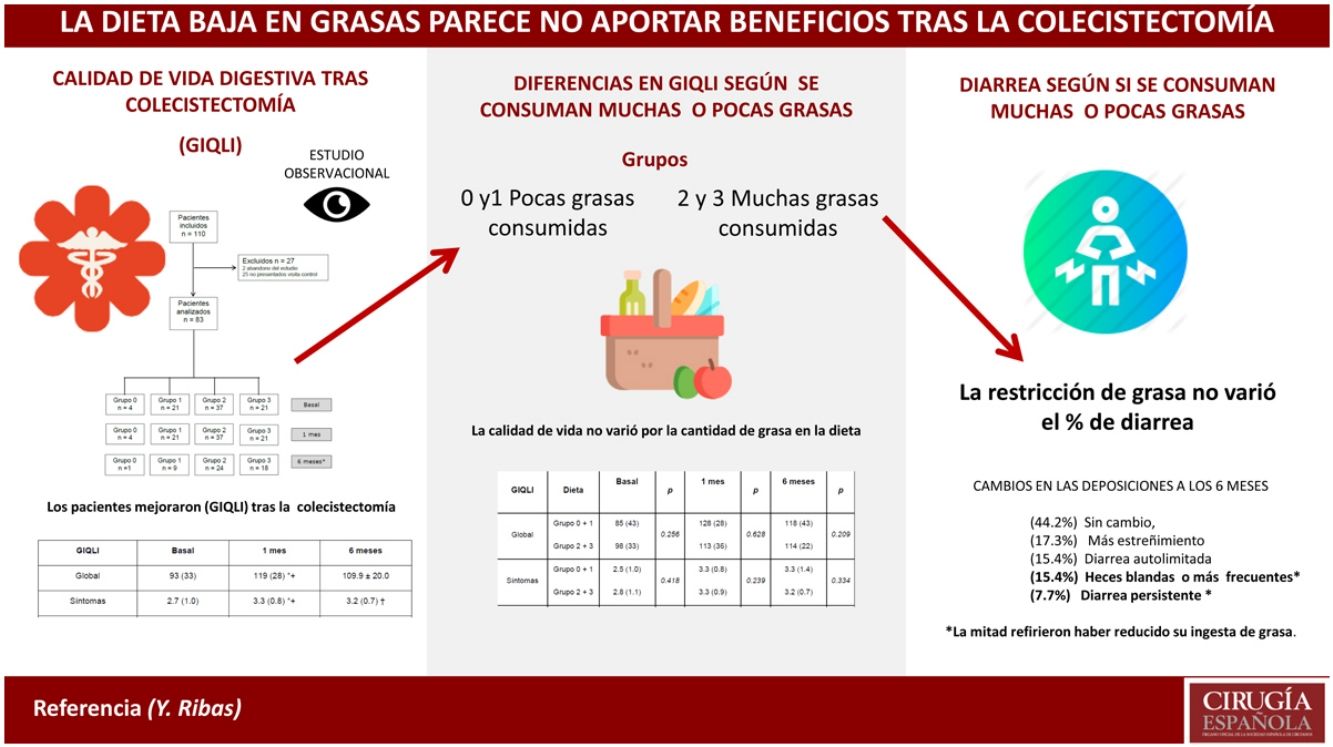
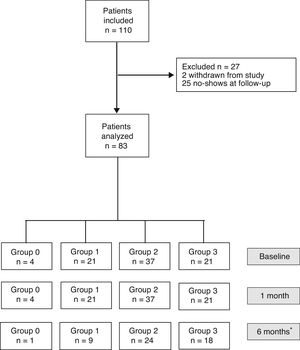
ResultsBaseline DataOut of the 110 patients included in the study, 27 were excluded one month after surgery (2 dropped out of the study, and 25 did not attend the follow-up visit). Thus, the sample was ultimately made up of 83 patients (34 men and 49 women) with a median age of 58 (24) and a body mass index of 27.9 (5.9) (Fig. 1). No patient was excluded due to postoperative complications.
Flow diagram of patients included and classified into groups according to the quantity of fat ingested one month after surgery (Group 0=diet very low in fat; Group 1=low-fat diet; Group 2=diet with normal amount of fat; Group 3=diet rich in fat). *Thirty-one patients did not complete the questionnaire sent 6 months after surgery.
Indications for cholecystectomy were: hepatic colic in 65 cases, acute cholecystitis in 5 cases, 6 interventions after mild acute biliary pancreatitis, and 7 patients underwent endoscopic sphincterotomy due to acute cholangitis or choledocholithiasis. Eleven patients had a gastrointestinal history (2 bariatric surgery, 7 gastroesophageal reflux, one symptomatic colonic diverticular disease and one Roux-en-Y reconstruction due to duodenal trauma). Laparoscopic cholecystectomy was performed in all patients except 4 (2 laparotomies due to multiple abdominal surgeries, 2 conversions due to technical difficulty). Two patients presented with bruises associated with antithrombotic therapy, which did not interfere with food intake or digestion.
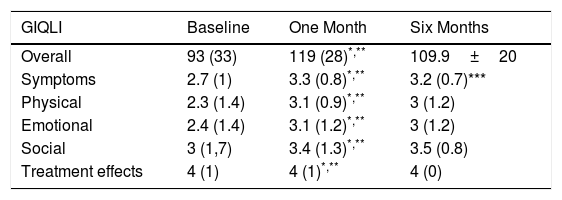
Symptom EvaluationThe overall GIQLI score and its dimensions, except for the treatment effect, increased significantly one month after surgery compared to the baseline score (Table 1).
Overall GIQLI Score and Dimension Scores: Baseline, One Month and 6 Months After Surgery.
| GIQLI | Baseline | One Month | Six Months |
|---|---|---|---|
| Overall | 93 (33) | 119 (28)*,** | 109.9±20 |
| Symptoms | 2.7 (1) | 3.3 (0.8)*,** | 3.2 (0.7)*** |
| Physical | 2.3 (1.4) | 3.1 (0.9)*,** | 3 (1.2) |
| Emotional | 2.4 (1.4) | 3.1 (1.2)*,** | 3 (1.2) |
| Social | 3 (1,7) | 3.4 (1.3)*,** | 3.5 (0.8) |
| Treatment effects | 4 (1) | 4 (1)*,** | 4 (0) |
Data expressed as median and IQR.
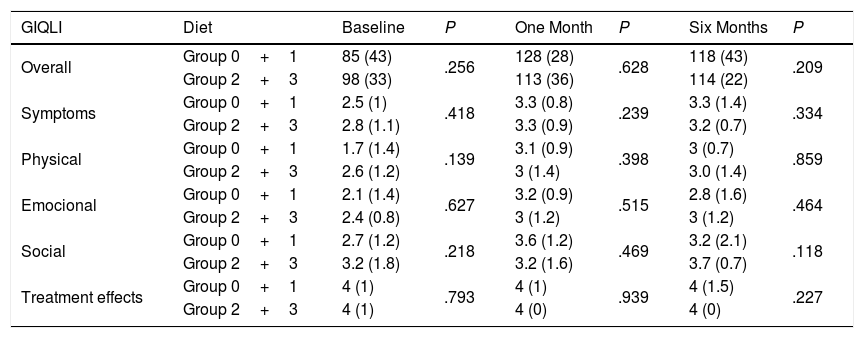
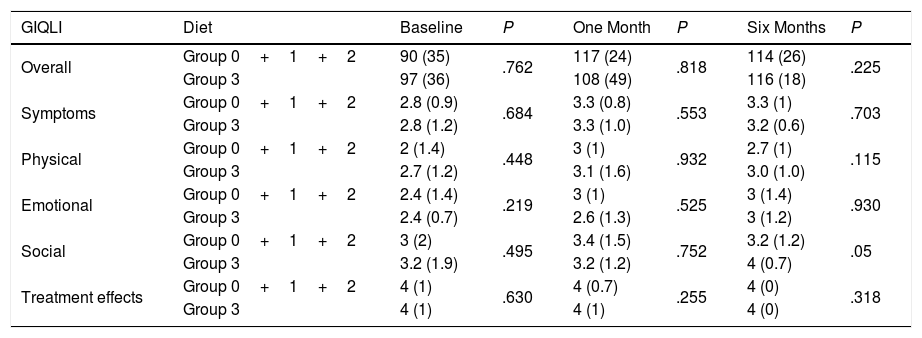
Regarding the impact of the diet, there were no statistically significant differences in the Overall GIQLI score and its dimensions when groups 0 and 1 were compared with groups 2 and 3 (Table 2). When the comparison was made between group 3 (high-fat diet) and the rest of the aggregate groups (0, 1 and 2), no differences were found either (Table 3).
Overall GIQLI Score and Dimension Scores at Baseline (Groups 0 and 1N=25 vs. Groups 2 and 3N=58), One Month (Groups 0 and 1N=25 vs. Groups 2 and 3N=58), and 6 Months (Groups 0 and 1N=10 vs. Groups 2 and 3N=42), After Surgery According to Fat Intake.
| GIQLI | Diet | Baseline | P | One Month | P | Six Months | P |
|---|---|---|---|---|---|---|---|
| Overall | Group 0+1 | 85 (43) | .256 | 128 (28) | .628 | 118 (43) | .209 |
| Group 2+3 | 98 (33) | 113 (36) | 114 (22) | ||||
| Symptoms | Group 0+1 | 2.5 (1) | .418 | 3.3 (0.8) | .239 | 3.3 (1.4) | .334 |
| Group 2+3 | 2.8 (1.1) | 3.3 (0.9) | 3.2 (0.7) | ||||
| Physical | Group 0+1 | 1.7 (1.4) | .139 | 3.1 (0.9) | .398 | 3 (0.7) | .859 |
| Group 2+3 | 2.6 (1.2) | 3 (1.4) | 3.0 (1.4) | ||||
| Emocional | Group 0+1 | 2.1 (1.4) | .627 | 3.2 (0.9) | .515 | 2.8 (1.6) | .464 |
| Group 2+3 | 2.4 (0.8) | 3 (1.2) | 3 (1.2) | ||||
| Social | Group 0+1 | 2.7 (1.2) | .218 | 3.6 (1.2) | .469 | 3.2 (2.1) | .118 |
| Group 2+3 | 3.2 (1.8) | 3.2 (1.6) | 3.7 (0.7) | ||||
| Treatment effects | Group 0+1 | 4 (1) | .793 | 4 (1) | .939 | 4 (1.5) | .227 |
| Group 2+3 | 4 (1) | 4 (0) | 4 (0) |
Data expressed as median and IQR.
Overall GIQLI Score and Dimension Scores at Baseline (Group 0+1+2N=62; Group 3N=21), One Month (Group 0+1+2N=62; Group 3N=21), and 6 Months (Group 0+1+2N=34; Group 3N=18), After Surgery According to Fat Intake.
| GIQLI | Diet | Baseline | P | One Month | P | Six Months | P |
|---|---|---|---|---|---|---|---|
| Overall | Group 0+1+2 | 90 (35) | .762 | 117 (24) | .818 | 114 (26) | .225 |
| Group 3 | 97 (36) | 108 (49) | 116 (18) | ||||
| Symptoms | Group 0+1+2 | 2.8 (0.9) | .684 | 3.3 (0.8) | .553 | 3.3 (1) | .703 |
| Group 3 | 2.8 (1.2) | 3.3 (1.0) | 3.2 (0.6) | ||||
| Physical | Group 0+1+2 | 2 (1.4) | .448 | 3 (1) | .932 | 2.7 (1) | .115 |
| Group 3 | 2.7 (1.2) | 3.1 (1.6) | 3.0 (1.0) | ||||
| Emotional | Group 0+1+2 | 2.4 (1.4) | .219 | 3 (1) | .525 | 3 (1.4) | .930 |
| Group 3 | 2.4 (0.7) | 2.6 (1.3) | 3 (1.2) | ||||
| Social | Group 0+1+2 | 3 (2) | .495 | 3.4 (1.5) | .752 | 3.2 (1.2) | .05 |
| Group 3 | 3.2 (1.9) | 3.2 (1.2) | 4 (0.7) | ||||
| Treatment effects | Group 0+1+2 | 4 (1) | .630 | 4 (0.7) | .255 | 4 (0) | .318 |
| Group 3 | 4 (1) | 4 (1) | 4 (0) |
Data expressed as median and IQR.
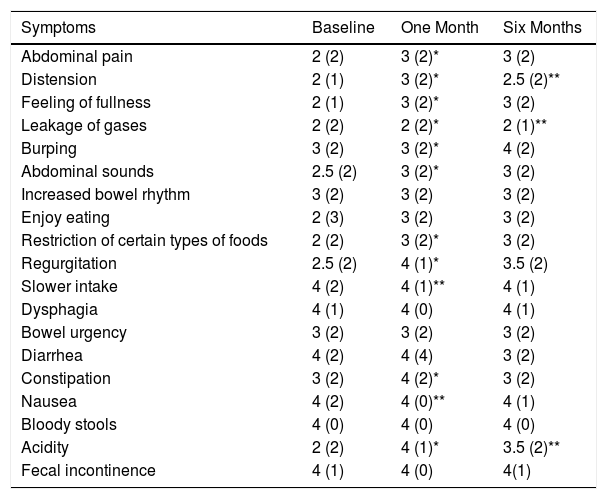
Table 4 compiles the symptoms that make up the symptomatic score, showing that constipation improved one month after surgery and diarrhea had a worse score 6 months afterwards. In parallel, approximately 10% of patients had a lower-than-baseline score after one month in all items except bowel frequency, bowel urgency and diarrhea, in which lower scores were given at 26, 30 and 28%, respectively. In contrast, the constipation item score improved in 42% of cases. There were no differences between the different diet groups.
Symptoms According to the GIQLI Score at Baseline, and One Month/6 Months After Surgery.
| Symptoms | Baseline | One Month | Six Months |
|---|---|---|---|
| Abdominal pain | 2 (2) | 3 (2)* | 3 (2) |
| Distension | 2 (1) | 3 (2)* | 2.5 (2)** |
| Feeling of fullness | 2 (1) | 3 (2)* | 3 (2) |
| Leakage of gases | 2 (2) | 2 (2)* | 2 (1)** |
| Burping | 3 (2) | 3 (2)* | 4 (2) |
| Abdominal sounds | 2.5 (2) | 3 (2)* | 3 (2) |
| Increased bowel rhythm | 3 (2) | 3 (2) | 3 (2) |
| Enjoy eating | 2 (3) | 3 (2) | 3 (2) |
| Restriction of certain types of foods | 2 (2) | 3 (2)* | 3 (2) |
| Regurgitation | 2.5 (2) | 4 (1)* | 3.5 (2) |
| Slower intake | 4 (2) | 4 (1)** | 4 (1) |
| Dysphagia | 4 (1) | 4 (0) | 4 (1) |
| Bowel urgency | 3 (2) | 3 (2) | 3 (2) |
| Diarrhea | 4 (2) | 4 (4) | 3 (2) |
| Constipation | 3 (2) | 4 (2)* | 3 (2) |
| Nausea | 4 (2) | 4 (0)** | 4 (1) |
| Bloody stools | 4 (0) | 4 (0) | 4 (0) |
| Acidity | 2 (2) | 4 (1)* | 3.5 (2)** |
| Fecal incontinence | 4 (1) | 4 (0) | 4(1) |
Data expressed as median and IQR.
After 6 months, 52 patients completed the questionnaire sent by mail, showing a persistent improvement of the overall GIQLI score as well as in the physical, emotional and social dimensions. The symptoms dimension showed a slight decrease, although the improvement over the baseline score remained statistically significant (Table 1).
Among the patients who returned the questionnaire after 6 months, there were no differences in the overall GIQLI score or the dimensions between the first- and sixth-month follow-up when we compared patients with a low-fat diet (groups 0 and 1) and those with a high-fat diet (groups 2 and 3) (Table 2). This lack of differences was maintained when patients with a high-fat diet (group 3) and the other groups (groups 0, 1 and 2) were compared (Table 3).
Final EvaluationSix months after surgery, 26 patients (50%) reported eating less fat, 17 (32.7%) followed their usual diet and 9 (19.3%) had reduced fat intake temporarily. Only 3 patients (5.7%) reported significant intolerance to fatty foods. The proportion of consumers of a high-fat diet (group 3) increased over time (21 out of 83 one month after surgery and 18 out of 52 after 6 months).
Regarding bowel habit, 23 patients (44.2%) had not experienced any change, 9 (17.3%) reported more constipation, 8 (15.4%) self-limited diarrhea (liquid feces), 8 (15.4%) soft stools or more bowel movements per day and 4 (7.7%) had persistent diarrhea. In this group of 12 patients with soft stools and/or persistent diarrhea, only 6 reported having reduced their fat intake.
The source of information for the postoperative diet was provided by healthcare workers in 46 (88.4%), while 14 (26.9%) had obtained information from websites or magazines and 9 (17.3%) from relatives or friends.
DiscussionThe main results of our study were that the overall GIQLI score and scores of most of the dimensions increased significantly one month and 6 months after surgery compared to baseline, regardless of dietary fat intake. More than 50% of patients experienced changes in bowel habit after surgery, and 23% had persistent soft stools or diarrhea 6 months after surgery. The type of diet was not a determining factor, since half of the patients had reduced the fat in their diet, while the other half had begun consuming the amount of fat they considered normal, when asked 6 months after surgery.
The GIQLI score18,19 is a validated questionnaire that has been shown to be useful for assessing post-cholecystectomy symptoms.20,21 A recent study21 that administered the GIQLI after cholecystectomy showed that 90% of patients experienced an overall improvement 24 weeks after surgery. This correlated with the results of our study, in which the overall GIQLI score and the symptoms dimension scores improved after the intervention.
Cholecystectomy improves biliary colic, but there may be previous symptoms and others that appear de novo after the intervention, diarrhea being the most frequent.3,4 However, the prevalence of diarrhea varies greatly among the studies due to the lack of a uniform definition and a baseline evaluation, in addition to the variety of instruments used. According to our final 6-month evaluation, 4 patients (8.7%) had persistent diarrhea and 8 more had soft stools or increased stool frequency. The prevalence of diarrhea is controversial, but data from several studies5,8,10 show that a percentage of patients experience a change in bowel rhythm after cholecystectomy, as shown in more than 50% of patients in our series. Nevertheless, one of these studies10 concluded that there is no real change in bowel rhythm, except for more frequent bowel movements and the perception of less constipation. Fort et al.5 reported an accelerated colonic transit time one month after the intervention that was maintained 4 years after cholecystectomy. For this reason, some authors advise being cautious in the indication of cholecystectomy in women with irritable bowel syndrome.22
However, the main objective of our study was to evaluate the potential differences in symptoms after surgery depending on the amount of fat in the diet. Despite the lack of evidence, a recently published review13 recommended avoiding high-fat foods and the recommendation for dietary fat restriction after cholecystectomy is extended, although the restriction time may vary considerably. In the review of the literature, we have only identified 3 studies15–17 that assess the impact on postoperative symptoms of the diet with fat. In the first study,15 40 patients were randomized to either a postoperative low-fat diet or a normal diet, finding no significant differences between the groups. However, this study has several methodological limitations, as it does not calculate the sample size, justify exclusions, specify the comparability of the groups in the baseline evaluation, or use a validated questionnaire; moreover, few symptoms were evaluated. In the second study,16 a low-fat diet was recommended to 125 consecutive patients after laparoscopic cholecystectomy, and postoperative diarrhea was evaluated. Patients who did not follow the low-fat diet were more likely to have diarrhea, especially one week after surgery, although this correlation was only moderate. Finally, a third study17 evaluated 59 patients 3 months after surgery and indicated that the diet had a potential impact on post-cholecystectomy symptoms. The authors concluded that post-cholecystectomy symptoms were associated with the intake of cholesterol, animal proteins and eggs, and negatively correlated with the intake of vegetables. Yet patients were classified as symptomatic or asymptomatic, with no clear definition and without using validated questionnaires. The last 2 studies16,17 were performed in Asian patients, where functional dyspepsia is frequent23 and, although fats can exacerbate dyspeptic symptoms,24 the causal mechanism is not clear.
Our study has several limitations. First, it is an observational study in which the sample size was not calculated because there were no previous data in the scientific literature. However, the results of the present study have allowed us to calculate the sample size, knowing the statistical power of the randomized prospective study that we are initiating at our hospital. Another limitation is the monitoring of the diet followed since, when a low-fat diet is recommended, there is no guarantee that patients will follow it in the medium term. Although most patients receive advice from healthcare professionals, they also report getting information online or from family and friends who have gone through the same procedure, which contributes to actions that are not sufficiently documented. Finally, a clear limitation of our study is that the term ‘diarrhea’ has different meanings for different patients and, therefore, the prevalence data for diarrhea are less reliable than if the Bristol scale had been determined, as other authors have done previously.10
In conclusion, a low-fat diet does not seem to influence the improvement of symptoms after cholecystectomy. At our medical center, a randomized study comparing the effect of a normal diet with a low-fat diet is underway, the results of which may contribute towards confirming the results of the present observational study.
FundingThis research project has received no specific funding from public, commercial or non-profit organizations.
Conflict of InterestsNone.
Please cite this article as: Ribas Blasco Y, Pérez Muñante M, Gómez-Fernández L, Jovell-Fernández E, Oms Bernad LM. Dieta baja en grasas tras colecistectomía: ¿se debería recomendar de forma sistemática? Cir Esp. 2020;98:36–42.