We analyze major liver resections performed in 10 years, with the objective of evaluating perioperative results. As secondary objectives, factors related to major complications and comparative analyses of 2 5-year periods are evaluated.
MethodsRetrospective analysis of patients undergoing major hepatic resection (3 or more segments) from January 2005 to December 2014, from pre, intra and postoperative data. The Clavien classification is used for postoperative morbidity.
ResultsA total of 416 major hepatectomies were performed. Transfusions were performed in 38 patients (9.1%). A Pringle maneuver was used in 47.7% of the cases. Half of the patients had no complications, and only 96 patients (23%) had a major complication. Bile leakage was the most frequent complication (n=72, 17.3% of patients), especially due to malignant disease and biliary reconstruction, high risk ASA (III–IV) and prolonged surgical time. Thirteen patients met criteria for liver failure, of which 7 died (5 associated a bacterial infection). The mean hospital stay was 12.5 days, with an 11.8% readmission rate. When comparing 2 5-year periods, at present more complex patients are operated on, with a lower incidence of transfusions and complications (ns).
ConclusionsLiver surgery has increased significantly in recent years. Surgical management of the liver now allows safe and effective surgery, with a very low complication rate. The limit of resectability depends on the residual hepatic volume.
Se analizan las resecciones hepáticas mayores realizadas en 10 años, con el objetivo principal de evaluar los resultados perioperatorios. Como objetivos secundarios, se evalúan los factores relacionados con las complicaciones mayores y el análisis comparativo de 2 periodos de 5 años.
MétodosAnálisis retrospectivo de pacientes intervenidos mediante una resección hepática mayor (3 o más segmentos) desde enero de 2005 hasta diciembre de 2014, de los datos pre-, intra- y postoperatorios. Se utiliza la clasificación de Clavien para el análisis de la morbilidad postoperatoria.
ResultadosSe realizaron 416 hepatectomías mayores, con necesidad de transfusión en 38 pacientes (9,1%) y maniobra de Pringle en el 47,7% de los casos. La mitad de los pacientes no presentaron ninguna complicación y únicamente 96 pacientes (23%) presentaron una complicación mayor. La fuga biliar fue la complicación más frecuente (n=72; 17,3% de los pacientes), sobre todo, por enfermedad maligna y derivación biliar, con ASA elevado (III-IV) y tiempo quirúrgico prolongado. Trece pacientes cumplían criterios de insuficiencia hepática, de los cuales 7 murieron (5 asociaban sobreinfección bacteriana). La estancia hospitalaria media fue de 12,5 días, con una tasa de reingreso del 11,8%. Al comparar 2 periodos de 5 años, se operan pacientes más complejos, con menor incidencia de transfusiones y de complicaciones (ns).
ConclusionesLa cirugía hepática ha aumentado de forma significativa en los últimos años. El manejo quirúrgico del hígado permite en la actualidad ofrecer una cirugía segura y eficaz, con un índice de complicaciones muy bajo. El límite de la resecabilidad viene marcado por el volumen hepático residual.
Liver resection surgery has shown the ability to substantially modify the prognosis of patients who were previously considered non-candidates for treatment with curative intent, such as patients with liver metastases1 or primary hepatic tumors,2 and the technique has progressed remarkably since its introduction. The positive results and universal dissemination have led to an increase in its indications, which in turn has resulted in more highly complex procedures being performed. Consequently, more and more patients with greater volumes of liver disease are being evaluated for resection, and they are ultimately treated with more extensive liver resections.3,4
Generally speaking, the larger the quantity of liver parenchyma resected, more specific postoperative hepatic complications may appear. The morbidity and mortality associated with these complications define the limits of liver resection surgery.5,6 Unlike minor liver surgery, which can be performed in most hospitals with minimal infrastructure, major liver surgery (resection of 3 or more segments) leads to more intraoperative and postoperative complications (hemorrhage, postoperative liver failure, bile leak, etc.) and requires different postoperative management, with a dedicated intensive care unit, and specific additional maneuvers. The result is that these surgeries are normally performed at hospitals with extensive experience in this type of surgical procedures. The experience obtained after these resections has become a key factor to achieve better results in terms of complications and survival in patients operated on at reference hospitals. This has resulted in patients being sent for treatment at tertiary hospitals with high patient volumes and regional policies for the referral of patients requiring complex liver surgery.7
In this present article, we present a descriptive study of consecutive major liver resections performed at our hospital over the last 10 years. Our main objective was to evaluate the results in the immediate intra- and postoperative periods in terms of morbidity and mortality. As secondary objectives, we analyzed the learning curve factor for the technique according to the evolution of the results over time and, finally, we evaluated the factors that could determine the appearance of major complications in the postoperative period.
MethodsThis is a retrospective study of prospectively collected data. Included in the study were patients with major liver resections in a 10-year period from January 1, 2005 to December 31, 2014. In this timeframe, a total of 827 liver resections were performed at our hospital, 416 of which were major hepatectomies, representing 50.3% of the total.
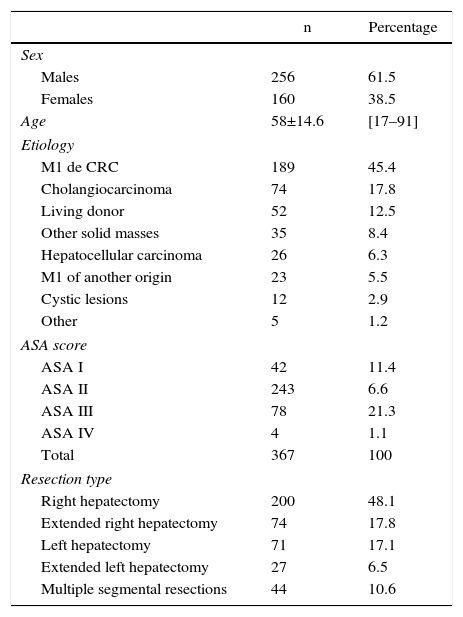
Patient demographic data are shown in Table 1. Most of the patients were male, with a male:female ratio of 61.5%:38.5%, and a mean age of 58±14.6 years (range 17–91). Regarding the assessment of anesthetic risk, the majority of patients (66.2%) presented an anesthetic risk assessment (ASA) score of II.
Demographic Data.
| n | Percentage | |
|---|---|---|
| Sex | ||
| Males | 256 | 61.5 |
| Females | 160 | 38.5 |
| Age | 58±14.6 | [17–91] |
| Etiology | ||
| M1 de CRC | 189 | 45.4 |
| Cholangiocarcinoma | 74 | 17.8 |
| Living donor | 52 | 12.5 |
| Other solid masses | 35 | 8.4 |
| Hepatocellular carcinoma | 26 | 6.3 |
| M1 of another origin | 23 | 5.5 |
| Cystic lesions | 12 | 2.9 |
| Other | 5 | 1.2 |
| ASA score | ||
| ASA I | 42 | 11.4 |
| ASA II | 243 | 6.6 |
| ASA III | 78 | 21.3 |
| ASA IV | 4 | 1.1 |
| Total | 367 | 100 |
| Resection type | ||
| Right hepatectomy | 200 | 48.1 |
| Extended right hepatectomy | 74 | 17.8 |
| Left hepatectomy | 71 | 17.1 |
| Extended left hepatectomy | 27 | 6.5 |
| Multiple segmental resections | 44 | 10.6 |
Out of the 416 procedures, 323 (77.3%) were performed due to malignant diseases and 95 (22.7%) benign diseases. The causes for major hepatectomy are shown in Table 1: the majority (189; 45.4%) was for colorectal liver metastases (CLM). The second most frequent etiology was cholangiocarcinoma, with 74 cases (17.8%), and the third most frequent was split-liver donation for living donor liver transplantation, with 52 cases (12.5%).
Preoperative DataAlmost one-quarter of all patients (24.5%) received chemotherapy before the liver resection procedure, which in all cases was for CLM or metastases of another origin.
Despite the fact that these surgeries were major hepatic resections, only 88 patients (21.2%) underwent volumetry studies, especially in the second period (2010–2014), which also coincided with a higher number of highly complex cases. In those patients whose estimated liver remnant volume was less than 20% of the total in normal livers or less than 30% of the total in pathological livers, or when there was a ratio to body weight lower than 0.6% in normal livers or less than 0.8% in pathological livers (undergoing chemotherapy or in cholestatic liver disease, typical of cholangiocarcinoma), previous portal embolization was performed in order to delay the surgery and achieve hypertrophy of the estimated liver remnant. This procedure was done in 20 patients and was performed by the Angioradiology Department8 in all cases with a percutaneous transhepatic approach.
Most of the patients were treated for the first time for liver disease, although 37 (8.9%) had a previous liver resection.
Surgical ProcedureIn all cases, the surgeries were performed by a surgical team of four surgeons.
In most patients (95%), the surgical procedure used was laparotomy. The preferred laparotomy type was the Makuuchi “J” or right subcostal with epigastric prolongation, depending on each surgeon. A total of 21 patients underwent laparoscopy. The laparoscopic approach in major liver resections was initiated in 2009, after having utilized this technique in minor resections (bi-segmentectomies II–III and anterior segments) in previous years.
In the case of laparoscopic major hepatectomies, we placed the patient in dorsal hyperextension position with open legs and in slight left lateral decubitus for better exposure of the liver. Pneumoperitoneum was created in the midline area 5–10cm above the navel with a Veress needle or Hasson trocar if the patient had a previous midline laparotomy. The rest of the trocars were placed, leaving a separation of 7cm among them, following the right subcostal line (3 additional 12mm trocars), and one in the left flank that was used at the beginning for the intraoperative ultrasound and afterwards to separate the liver toward the left. Before the transection, a band was placed around the hilum for the Pringle maneuver.
Out of the 21 patients who underwent a laparoscopic approach, right hepatectomy was done in 13 and left hepatectomy in 8. The etiologies were CLM (n=14), hepatocellular carcinoma of the liver (n=3), peripheral cholangiocarcinoma (n=2) and hepatic adenomas (n=2).
The approach was intentionally hybrid (initiated by laparoscopy and conversion to complete the procedure) in 3 cases and in one case with assistance. Conversion to open surgery was required in 7 cases (due to adhesions, technical difficulties to evaluate the lesions, intraoperative findings of an implant in the diaphragm, or hemorrhage). In 10 cases, major liver resection was completely laparoscopic.
In all cases, the livers were systematically examined using intraoperative ultrasound (Aloka© Ltd. Model SSD-α5). Both in open as well as laparoscopic surgery, the same methodology was used. The examination began by identifying the portal bifurcation and the end section of the middle hepatic vein. We followed the left portal vein, its ascending branch for segment IV and the branches for segments III and II. Now in segment II, we followed the left hepatic vein and its union with the middle hepatic vein to empty into the vena cava. We followed the right hepatic vein to examine the segments of the right liver lobe. The main objective of this intraoperative examination was not so much the assessment of the known lesions, but to identify previously unknown lesions.
The first step that we carried out was vascular control of the structures of the hepatic hilum, with dissection of the vessels (arterial and portal) of the area to be resected, dissection of arteries and ligation of the portal vein. In most cases, the subsequent vascular control involved drainage of the hepatic veins, with dissection of the space between the right and middle veins, which is later used for the hanging-maneuver. After completing the piggy-back or hepatocaval dissection, we placed a band that reached the hilum above the vena cava that we used to expose the liver. At this time, liver resection was done, utilizing periods of vascular occlusion of the portal pedicle9 (Pringle maneuver) for 15min, with 5-min rest periods.
For liver transection, in all patients compact ultrasonic surgical aspirator (CUSA®10) dissection was used. For coagulation, we initially used a bipolar clamp from the Tissuelink® system11 from 2005 until November 2011, after which the Aquamantys® system was used.12
In 47 patients (11.3%), perfusion of somatostatin was established at a dose of 21mL/h at the standard concentration as estimated for a small remnant according to the patient's weight. After a bolus of 250g, we proceeded with a perfusion of 250μg/h for 5 days, with the patient fasting. Somatostatin has an effect on splanchnic irrigation with a final result of decreased portal flow. We initiated intraoperative perfusion of somatostatin when macroscopically the remnant was considered very small, with the aim to reduce the portal flow. In these remnants, the triggering factor for the future development of small-for-size syndrome is endothelial damage (shear-stress) caused by the excess portal blood flow in the hepatic sinusoid.13,14 In most cases, (n=44) the perfusion of somatostatin was initiated during surgery, and in 3 cases during the immediate postoperative period.
Postoperative DataPostoperative Liver FailureAfter surgery, orotracheal intubation was withdrawn in the operating room and the patient was transferred either to the conventional hospital ward or the Intensive Care Unit (ICU). The need for postoperative hospitalization in the ICU in these patients is determined by the risk for developing liver failure. This postoperative risk was assessed by 3 factors.
- -
Extension of the liver resection and small-sized hepatic remnant: 100% of the patients that required intraoperative somatostatin required ICU.
- -
Quality of the liver parenchyma: there are basically 2 patient groups: patients with cholestatic liver, especially cholangiocarcinoma; and patients who have received neoadjuvant chemotherapy that is potentially toxic for liver remnant function (with the development of a sinusoidal obstruction syndrome or “blue liver” in the case of oxaliplatin and steatohepatitis or “yellow liver” in the case of irinotecan).15
- -
Intraoperative hemorrhage: directly related with postoperative morbidity and mortality.
The appearance of postoperative liver failure was assessed clinically (hepatic encephalopathy and ascites) and analytically. The analytical parameters were measured before the intervention and on postoperative days 1, 3, 5, 7, 15 and 30, with special emphasis on bilirubin and prothrombin time, using the 50–50 morbidity criteria (3rd to 8th days), 50–50 score mortality (5th day),16 and peak serum bilirubin greater than 7mg/dL during post-op.17 In addition, we analyzed the evolution of these 2 parameters during the first week after surgery. When there was no trend toward normalization, this analytical alteration correlated with the appearance of postoperative complications.
DefinitionsMajor liver resection is defined as the resection of 3 liver segments or more (standard right and left hepatectomies, extended right and left hepatectomies, a standard liver resection of 3 or more segments). For nomenclature, we used the Brisbane classification18 from 2000 about the terminology in the different types of liver resection.
The presence of ascites in the postoperative period was determined by discharge of ascitic liquid through the abdominal drains of at least 500cc/day for at least three consecutive days.
The presence or absence of biliary leak was confirmed by the macroscopic appearance of the drained liquid or after percutaneous aspiration. In cases of doubt, a biochemical analysis was always done and a concentration of bilirubin in the ascitic liquid at least three times higher than in serum was used for confirmation.19 When the macroscopic appearance of an aspirated collection or drainage sample was not bilious and, furthermore, the biochemical analysis showed no alterations in bilirubin level, we defined the event as an intraabdominal collection.
Statistical AnalysisContinuous variables are expressed as median±standard deviation. The categorical variables were analyzed with the Chi-squared or Fisher's F tests, and the Student's t was used for the difference between the continuous variables. Results with a P<.05 were considered statistically significant. A univariate statistical analysis was used to evaluate the predictive factors. The variables that obtained statistical significance (P≤.05) in the univariate analysis were included in the logistic regression model to identify the associated risk factors. All the statistical tests were completed with SPSS Statistics 20 for Windows (SPSS Inc., Chicago, IL, USA).
ResultsIntraoperative DataTable 1 shows the surgical procedures performed. Almost half (48.1%) were standard right hepatectomies.
In 19 cases (4.6%), an associated intraoperative ablative treatment was used (radiofrequencies, microwaves or alcoholization), mostly in the second period (2010–2014).
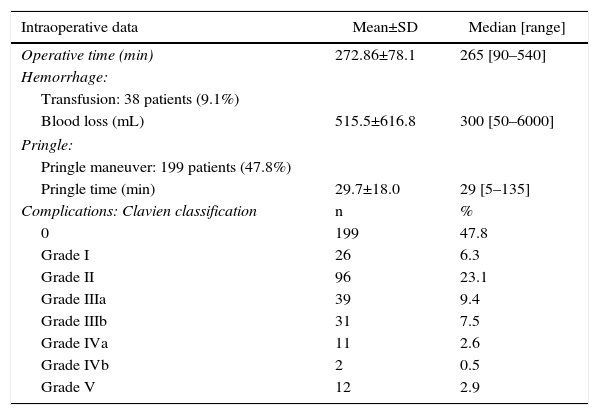
Intraoperative data are shown in Table 2. Mean operative time was 272.86min. The Pringle maneuver was used in 199 patients (47.8%), with a mean time of 29.7min. A total of 38 patients (9.1%) received blood transfusions. In these patients, the mean number of transfused blood units was 2.32 (median: 2 units; range: 1–8). Mean blood loss per procedure was 515.5mL. Portal reconstruction was performed in 29 patients (7.0%), in the context of patients with Klatskin tumors (n=28) in order to carry out a no-touch technique,20 and one case in a patient with liver metastasis of colorectal cancer.
Main Intraoperative Data and Postoperative Complications According to the Clavien–Dindo Classification.
| Intraoperative data | Mean±SD | Median [range] |
|---|---|---|
| Operative time (min) | 272.86±78.1 | 265 [90–540] |
| Hemorrhage: | ||
| Transfusion: 38 patients (9.1%) | ||
| Blood loss (mL) | 515.5±616.8 | 300 [50–6000] |
| Pringle: | ||
| Pringle maneuver: 199 patients (47.8%) | ||
| Pringle time (min) | 29.7±18.0 | 29 [5–135] |
| Complications: Clavien classification | n | % |
| 0 | 199 | 47.8 |
| Grade I | 26 | 6.3 |
| Grade II | 96 | 23.1 |
| Grade IIIa | 39 | 9.4 |
| Grade IIIb | 31 | 7.5 |
| Grade IVa | 11 | 2.6 |
| Grade IVb | 2 | 0.5 |
| Grade V | 12 | 2.9 |
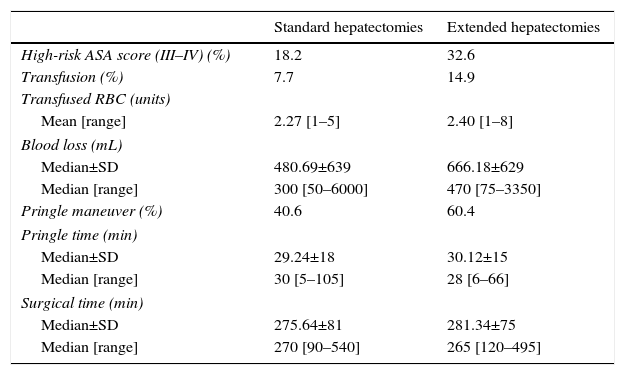
In Table 3, we analyzed parameters associated with surgical complexity according to the type of procedure used. We compared standard hepatectomies (right and left) and extended hepatectomies (extended right and left).
Comparison of Intraoperative Data Between Standard and Extended Hepatectomies.
| Standard hepatectomies | Extended hepatectomies | |
|---|---|---|
| High-risk ASA score (III–IV) (%) | 18.2 | 32.6 |
| Transfusion (%) | 7.7 | 14.9 |
| Transfused RBC (units) | ||
| Mean [range] | 2.27 [1–5] | 2.40 [1–8] |
| Blood loss (mL) | ||
| Median±SD | 480.69±639 | 666.18±629 |
| Median [range] | 300 [50–6000] | 470 [75–3350] |
| Pringle maneuver (%) | 40.6 | 60.4 |
| Pringle time (min) | ||
| Median±SD | 29.24±18 | 30.12±15 |
| Median [range] | 30 [5–105] | 28 [6–66] |
| Surgical time (min) | ||
| Median±SD | 275.64±81 | 281.34±75 |
| Median [range] | 270 [90–540] | 265 [120–495] |
In the group of extended major resections, the patients presented a higher anesthetic risk. When we compared the standard hepatectomies with the extended major hepatectomies, in the latter we observed greater intraoperative blood loss, which entailed a higher perioperative rate of blood transfusion and a greater use of the Pringle maneuver. The Pringle times and total surgical times were similar.
Postoperative DataPostoperative ComplicationsAccording to the Clavien classification21 of the 416 major resections, 199 patients (47.8%) presented no complications. There were minor complications (Clavien–Dindo grades I and II) in 122 patients (29.3%) and major complications (Clavien III–IV–V) in 95 patients (22.8%), mostly type III. Mortality was 2.9% (12 patients).
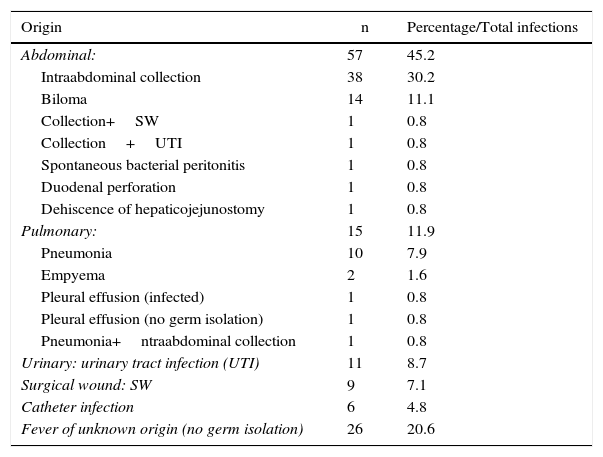
Table 4 reports the infectious complications that appeared in 126 patients (30.3%). There was a predominance of abdominal infections (infected intraabdominal and biliary collections), followed by urinary tract infections, surgical wound infections and pneumonias. In 26 patients, it was not possible to identify the origin of the fever.
Infectious Complications After Major Resections.
| Origin | n | Percentage/Total infections |
|---|---|---|
| Abdominal: | 57 | 45.2 |
| Intraabdominal collection | 38 | 30.2 |
| Biloma | 14 | 11.1 |
| Collection+SW | 1 | 0.8 |
| Collection+UTI | 1 | 0.8 |
| Spontaneous bacterial peritonitis | 1 | 0.8 |
| Duodenal perforation | 1 | 0.8 |
| Dehiscence of hepaticojejunostomy | 1 | 0.8 |
| Pulmonary: | 15 | 11.9 |
| Pneumonia | 10 | 7.9 |
| Empyema | 2 | 1.6 |
| Pleural effusion (infected) | 1 | 0.8 |
| Pleural effusion (no germ isolation) | 1 | 0.8 |
| Pneumonia+ntraabdominal collection | 1 | 0.8 |
| Urinary: urinary tract infection (UTI) | 11 | 8.7 |
| Surgical wound: SW | 9 | 7.1 |
| Catheter infection | 6 | 4.8 |
| Fever of unknown origin (no germ isolation) | 26 | 20.6 |
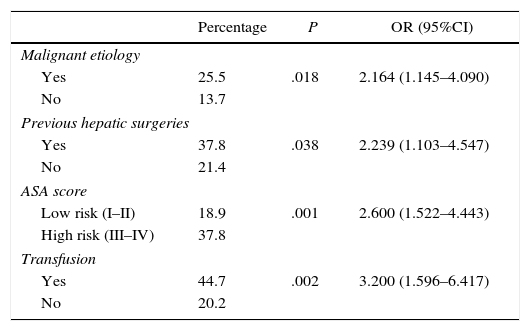
The univariate study of the different factors that seemed to influence the appearance of major postoperative complications (Clavien III–V) is shown in Table 5.
Factors Associated With the Appearance of Major Postoperative Complications (Clavien III–V).
| Percentage | P | OR (95%CI) | |
|---|---|---|---|
| Malignant etiology | |||
| Yes | 25.5 | .018 | 2.164 (1.145–4.090) |
| No | 13.7 | ||
| Previous hepatic surgeries | |||
| Yes | 37.8 | .038 | 2.239 (1.103–4.547) |
| No | 21.4 | ||
| ASA score | |||
| Low risk (I–II) | 18.9 | .001 | 2.600 (1.522–4.443) |
| High risk (III–IV) | 37.8 | ||
| Transfusion | |||
| Yes | 44.7 | .002 | 3.200 (1.596–6.417) |
| No | 20.2 | ||
The characteristic complications of major hepatectomies include bile leaks, and this complication appeared in 72 patients (17.3%).
Out of the 416 patients in the entire series, 69 (16.6%) underwent biliary bypass. Out of these 69, 36 cases (52.2%) presented postoperative bile leak.
In the 72 patients that presented bile leak, we have analyzed the results by dividing the cases into 2 groups: patients with biliary tract reconstruction (n=36) and patients without biliary reconstruction (n=36).
As for the location of the bile leak, out of the 36 patients who did not present biliary bypass, in 33 cases (91.7%) the leak was located in the transection margin. As for the 36 patients who did present biliary bypass, in 14 (38.9%) it was located in the hepaticojejunostomy, and in 10 cases (27.8%) the leak was present in the transection margin (in the remaining cases of patients with biliary bypass diversion, the location of the leak was not determined).
The treatment of bile leaks varied in terms of the location of the leak, in the resection margin or in a hepaticojejunostomy. The majority of patients with leaks in the resection margin were treated conservatively, or drainage was assisted with ultrasound. As for the patients with bile leak in the hepaticojejunostomy, in half of the cases we decided on an initial re-operation.
The bile leaks appeared in all the different types of hepatectomy performed. These were more frequent in patients with malignant etiology (60 vs 12) and more prevalent in patients with cholangiocarcinoma (30 cases; 41.7%).
By completing a univariate study to predict bile leak, statistically significant differences were found in diagnosis, ASA and parameters related with the surgical difficulty: perioperative transfusion, blood loss and surgical time.
As for the etiology, the patients with cholangiocarcinoma presented bile leak in 40.5% of cases, with a P<.001 when compared to patients of other diagnoses. The patients with high-risk ASA scores (III–IV) presented higher risk for the incidence of bile leak (26.8%) than those with low-risk ASA scores (I–II) (14%), with a P=.004. There were also statistically significant differences for the appearance of a bile leak in the post-op among patients who received perioperative transfusion compared to those with no transfusion (342 vs 14.8%; P=.015), those with long surgical times (298 vs 267min; P=.003) and in those with more blood loss (480 vs 660mL; P=.039).
Incidence of Liver FailureDuring post-op, 27 patients had ascites and 21 patients presented a certain degree of encephalopathy.
Out of the 416 patients treated, 13 patients met the 50–50 criteria on day 5. Of these, 7 died (prediction 53.8%). The second criterion that we used was post-op peak bilirubin ≥7mg/dL (Mullen score). A total of 38 patients met this criterion in the postoperative period and 9 of them died (23.7%).
MortalityThe mortality rate was 2.88% (12 patients).
Immediate mortality: Two patients died during the immediate postoperative period, one after 72h due to massive bleeding, and the other 48h later as a result of cardiorespiratory arrest.
Post-resection liver failure: 7 patients died as a consequence of developing post-hepatectomy liver failure, which appeared in all cases during the first 7 days. In 5, the presence of hepatic encephalopathy was associated (grades 2–4), and 3 cases presented ascites. All except for one patent died as a consequence of multiple organ failure associated with the appearance of an infectious complication. The infectious focus was abdominal in 5 patients and pulmonary in one case. In the case of pneumonia, no germs were isolated. In the other cases, the germ causing the infection included a gram-negative bacillus or an enterococcus (which were usually associated; 3 out of 5, 60%).
In the 3 remaining patients, the cause of death was massive pulmonary embolism in one case, thrombosis of the hepatic artery in another case, and hospital-acquired pneumonia in the third case.
All the deceased patients presented malignant baseline disease, cholangiocarcinoma in 8 patients (66.7%) and CLM in 4 (33.3%). The mean age was 67.33 (median: 69 yrs). Two patients had undergone previous liver resection. The 8 patients with cholangiocarcinoma who died represented 10.8% of the patients treated with this pathology.
Postoperative Hospital StayMean postoperative stay was 12.50 days (median: 9 days).
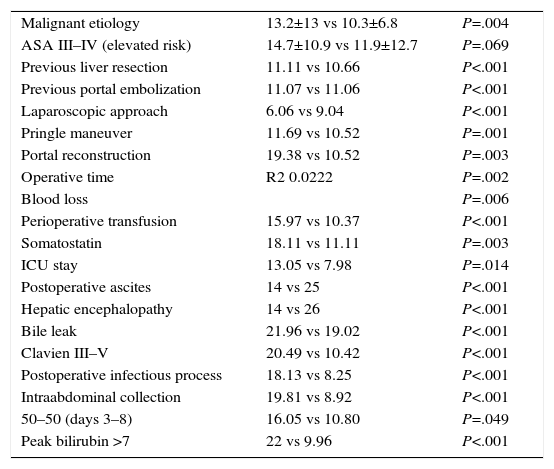
Predictive factors for postoperative hospitalization were analyzed with a univariate analysis. The statistically significant variables (P<.05) for prolonged postoperative hospital stay are shown in Table 6. The preoperative and intraoperative parameters associated with prolonged postoperative hospitalization were related with greater surgical difficultly. The postoperative parameters associated with longer hospitalization were derived from the appearance of postoperative complications.
Predictive Factors for Postoperative Hospital Stay, in Days.
| Malignant etiology | 13.2±13 vs 10.3±6.8 | P=.004 |
| ASA III–IV (elevated risk) | 14.7±10.9 vs 11.9±12.7 | P=.069 |
| Previous liver resection | 11.11 vs 10.66 | P<.001 |
| Previous portal embolization | 11.07 vs 11.06 | P<.001 |
| Laparoscopic approach | 6.06 vs 9.04 | P<.001 |
| Pringle maneuver | 11.69 vs 10.52 | P=.001 |
| Portal reconstruction | 19.38 vs 10.52 | P=.003 |
| Operative time | R2 0.0222 | P=.002 |
| Blood loss | P=.006 | |
| Perioperative transfusion | 15.97 vs 10.37 | P<.001 |
| Somatostatin | 18.11 vs 11.11 | P=.003 |
| ICU stay | 13.05 vs 7.98 | P=.014 |
| Postoperative ascites | 14 vs 25 | P<.001 |
| Hepatic encephalopathy | 14 vs 26 | P<.001 |
| Bile leak | 21.96 vs 19.02 | P<.001 |
| Clavien III–V | 20.49 vs 10.42 | P<.001 |
| Postoperative infectious process | 18.13 vs 8.25 | P<.001 |
| Intraabdominal collection | 19.81 vs 8.92 | P<.001 |
| 50–50 (days 3–8) | 16.05 vs 10.80 | P=.049 |
| Peak bilirubin >7 | 22 vs 9.96 | P<.001 |
Within 30 days of discharge, 47 patients (11.3%) were re-admitted to hospital. The most frequent causes were biloma (12 cases) (most of which were already identified), intraabdominal collection (9 cases) and fever of unknown origin (9 cases). Five patients were re-operated on: one due to evisceration, one intestinal fistula, 2 in the context of septic shock due to an over infected collection, and one patient due to hemoperitoneum on the 3rd day after discharge, secondary to hemorrhage from the left phrenic vein (10 days after hepatic surgery). The remaining causes for re-hospitalization were: an isolated case (n=1) of pleural effusion, surgical wound infection, general malaise, urinary infection, deep vein thrombosis, etc.
Analysis by PeriodsThe 10-year period was divided into two 5-year periods: 2005–2009, and 2010–2014. Out of the 416 major liver resections, in the first period (2005–2009) 183 procedures were performed (44%), and 233 procedures (56%) in the second period (2010–2014).
A comparison study of the 2 periods, showed no statistically significant differences in the variables compared, except in 4 sections. The first 3 refer to the complexity of the patient, which was greater in the second period because there was a higher proportion of a malignant disease, greater utilization of volumetry and older patient age. In spite of the greater complexity of these cases, mean blood loss was lower in the second period: 618mL vs 445mL (P=.02).
Without presenting statistically significant differences, in the second period 8.7% of the patients received transfusions compared to 14.5% in the first period. In addition, in the second period 52.8% of the patients presented no complications, versus 41.5% in the first period.
DiscussionHepatobiliary and pancreatic surgery has demonstrated a logarithmic progression over the last 20 years. The incorporation of hepatic and pancreatic transplantation has led to a very relevant increase in hepatic surgery activities. Normally, this highly complex surgery is associated with elevated morbidity, with a 30-day incidence of major complications that reaches 50% and mortality 6%.22–30
In spite of everything, there have been no global studies about what this surgery currently represents. Therefore, our group decided to review data from the last 10 years in the context of our surgical activity, which includes living-donor liver transplantation, demonstrating that our team is highly experienced in these surgical techniques.
Recently, Clavien published the multinational experience in right hepatectomy in living donors with more than 5000 procedures from 12 hospitals, including ours. They established a global perspective of what should be the “ideal” rate of complications associated with this complex surgery, although it was quite specific. This is known as benchmarking, which provides for comparative evaluation of surgical results.
This study established an incidence of complications of 12% (3.8% major complications). However, this is in the ideal context of a healthy subject and with the highest probability of a positive outcome. Even so, the complication rate is not low.
Procedure CharacteristicsThe main characteristics of the current surgery are associated with a very safe technique. The preoperative evaluation by imaging studies associated with systematic use of measures to provide for bloodless liver transection has resulted in a notable reduction in bleeding during and after surgery. With data from our experience, we have analyzed blood loss and the transfusion requirements related with the magnitude of the procedure (extended hepatectomies). 90.9% of the patients required no transfusions, while only 9.1% required transfusions, compared to 49% of the patients receiving transfusion in a series published in the later 1990s31 or 12.4% of the patients receiving transfusions in a more recent publication.32 It should be mentioned that these series include major and minor resections. In the multi-center series of major hepatectomies by Vauthey in 200717 in non-cirrhotic patients, 16.1% of the patients required perioperative transfusion. In the “ideal” cases of Rössler with major resections,33 2% of the patients received transfusions.
The Pringle maneuver is a safety mechanism that is very frequently used. Initially, it involves two factors: one positive, as it can have a certain preconditioning effect9; and one negative, related with the later appearance of ischemic injury to the bile duct. In this series, the Pringle maneuver was done in 47.7% of the patients, usually related with the extended liver resection. The periods used by our group are widely used in the literature (15min of vascular occlusion, followed by 5min without clamping). In the publication by Zimmitti32 in 2013, the Pringle maneuver was carried out in 58.2% of the resections (major as well as minor).
Until now, the series published of liver resections have encompassed both major and minor hepatectomies.31,32,34,35 No one has focused on analyzing major hepatectomies, except in the publication by Mullen from 2007 to define the peak serum bilirubin >7 as a predictive factor of liver failure,17 and the recent multicenter study by Clavien.33
Postoperative CourseOverall ComplicationsIn our series, we selected only major liver resections and ruled out the minor resections due to the low impact on postoperative morbidity and mortality.
Complications appeared in 52.3% of the patients; 23% of the cases were major complications. These major complications were more frequent in malignant etiologies, patients with previous resections, high-risk ASA scores and those who required intraoperative transfusion.
Until the Clavien–Dindo classification21 was published in 2004 and subsequently used, reliable comparisons between series of postoperative complications were not possible. Thus, in the period prior to its utilization, there was great variability in the description of postoperative morbidity, from the 22% described in the series by Belghiti in 200031 to the 74.9% of the series by Jarnagin in 200234 and, afterwards, the 39% of global complications in the series by Imamura in 2003.35 In the series from 2007 by Vauthey about major resections,17 complications were reported according to the Clavien classification in 42.8% of the patients, 16.8% of which were major complications. Meanwhile, in 2013, Zimmitti32 presented complications in 36.7% of patients (14.1% major complications).
Blood loss is an independent risk factor for major postoperative complications and perioperative mortality.34,36 The literature reports that independent factors that predict intraoperative bleeding of more than 1500mL include37: INR <70%, non-peripheral tumor, hepatic vein involvement, BMI≥23.0kg/m2, major resections, operative time and tumor size.38
Bile LeakToday, bleeding has passed to a second plane as a problem in major hepatic surgery, while bile leaks continue to be the Aquiles heel in this type of procedures, especially in extended liver resections.
In our series, there is an incidence of bile leaks of 17.3%. Predictive factors for bile leak can be divided into 2 different groups. On the one hand, procedures with greater surgical complexity (patients with high ASA risk, greater blood loss, blood loss requiring perioperative transfusions, longer surgical time, and patients in whom somatostatin perfusion was used as the liver remnant was considered very small in size). The other group refers to the etiology, with a greater incidence of bile leaks in cholangiocarcinoma (P<.001) due to having created a biliary diversion. We found no differences in terms of bile leaks comparing the 2 periods. The presence of this complication causes longer hospitalization and, likewise, a higher rate of associated complications.
The incidence of bile leak in the literature remains constant, with differences between series of 4.8%–7.6% (mean: 5.5%).39–45 Even the incidence of bile leak increases by comparing different periods, due to a greater complexity of the procedures32: 5.9 vs 3.7%; P=.011).
Liver Failure and MortalityThe main risk after major liver resection is the development of hepatic insufficiency and liver failure, which is related with the volume and quality of the liver remnant.
In our series, we have found a correlation between the scores used (50–50 criterion16 and peak serum bilirubin>7mg/dL17) and the associated morbidity and mortality, with 13 and 38 patients, respectively, and a predicted mortality of 53.8 and 23.7%. In the article by Belghiti, which included 775 major (60%) and minor liver resections, the patients who represented a positive 50–50 criterion on the 5th day had a predictive mortality factor of 59%. The publication by Mullen included 1059 patients with major liver resection, with a mortality of 4.7% after 90 days (3.2% after 30 days) and a powerful correlation with morbidity and mortality based on a serum bilirubin >7mg/dL. These scores always have to do with an insufficient liver remnant, so an initial intraoperative evaluation is required; at the same time, somatostatin perfusion can be initiated and maintained for 5 days.
Liver failure after resection is the main cause of death after liver resection, with incidences in the literature that vary from 1.2 to 32%.
In our series, the appearance of infection in a patient who had developed post-resection liver failure was a determining factor in postoperative mortality.
The incidence of mortality was 2.88%. Compared to the series of hepatectomies, which include both major and minor resections, the reported incidences of mortality were 4.4,31 3.134 and 2.3%.32
Analysis by PeriodsIn our comparative study between the 2 periods, we observed a greater proportion of malignant disease, utilization of volumetry and older patients. In spite of having a higher number of patients in the second period and greater surgical complexity, there was a statistically significant lower rate of blood loss, without differences in terms of bile leaks or postoperative morbidity and mortality.
Similarly, in 2014 Nanashima et al. published a study with an analysis in 3 periods with 544 patients who underwent liver resection.46 They observed an increase in age of the patients operated on, as well as steatosis and jaundice of the liver. The surgical procedures were more complex in the latter period, but there was less blood loss and a lower rate of blood transfusions. This was accompanied by a lower rate of postoperative complications, including bile leaks, which resulted in shorter hospitalization.
In the series by Zimmitti in 2013, about 2628 liver resections performed between 1997 and 2011 were divided into 2 periods with the same number of cases.32 They found that in the second period there was a higher incidence of complex resections (previous hepatic re-operations, two-stage resections, extended hepatectomies and previous portal embolization; P<.05) and also an increase in bile leaks (5.9 vs 3.7%; P=.011).
In conclusion, major hepatic surgery currently offers extraordinarily good results, with very low mortality and complication rates. The referral of patients with the disease is an aspect that may have had an influence from a healthcare standpoint, as it contributes to patients receiving treatment at hospitals with greater experience.
Authorship/Collaborations- -
David Calatayud: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
- -
Santiago Sánchez Cabús: data collection, critical review and approval of the final version.
- -
Jaime Sampson: data collection, critical review and approval of the final version.
- -
Aridai Resendiz: data collection, critical review and approval of the final version.
- -
Víctor Molina: critical review and approval of the final version.
- -
Constantino Fondevila: critical review and approval of the final version.
- -
Josep Fuster: critical review and approval of the final version.
- -
Juan Carlos García-Valdecasas: study design, critical review and approval of the final version.
The authors have no conflict of interests to declare.
Please cite this article as: Calatayud D, Sánchez Cabús S, Sampson J, Resendiz A, Molina V, Fondevila C, et al. Resección hepática mayor: una cirugía segura y eficaz. Cir Esp. 2017;95:437–446.