To analyze whether clinical and analytical parameters differ according to histopathology in cases of acute appendicitis (AA).
MethodsThis is a retrospective, observational study including patients (>14 years of age) admitted for suspicion of AA from 1 April 2014 to 31 July 2016. Histopathology was divided into complicated (including perforated and gangrenous AA) and uncomplicated appendicitis (phlegmonous). Sex, age, temperature of patients on admission to the Emergency Department, symptom duration, preoperative white blood cell (WBC) count, neutrophil percentage, mean platelet volume (MPV), platelet distribution width (PDW), C-reactive protein (CRP) and hospital stay were compared in the two groups.
ResultsThree hundred and thirty-five patients were analyzed, and 284 were included. Appendicitis was uncomplicated in 194 (68.3%) and complicated in 90 (31.7%). Age, symptom duration, neutrophil percentage, CRP and hospital stay were higher in the complicated AA group (P < .05). The mean differences between uncomplicated and complicated AA were: age 13.2 years (95% CI: 8.2–18.2), symptom duration 14.1 h (95% CI: 6.3–21.9), neutrophil percentage 5.0% (95% CI: 3.2–6.8), CRP 73.6 mg/l (95% CI: 50.0–97.2) and hospital stay 2.2 days (95% CI: 1.4–3.0), with p < 0.05 for all these variables. A model based on the preoperative parameters (age, symptom duration, neutrophil percentage and CRP) was calculated to predict the likelihood of complicated AA. The receiver operating characteristic (ROC) of the model had an area under the curve of 0.80 (95% CI 0.75−0.85).
ConclusionThis model is able to diagnose complicated AA without the need for imaging techniques, although it must be validated with prospective analysis.
Los parámetros clínicos y analíticos de la apendicitis aguda (AA) son la base diagnóstica. Se analiza la diferencia de sus valores según la histología para distinguir las AA simples de las complicadas.
MétodosAnálisis observacional retrospectivo que incluye pacientes (>14 años) que ingresan con diagnóstico de AA desde el 1 abril 2014 al 31 julio 2016. Histopatológicamente se dividen en AA complicada (perforada y/o gangrenada) y AA no complicada (flemonosa). Entre los 2 grupos se compara sexo, edad, temperatura al ingreso, duración de sintomatología, recuento leucocitario preoperatorio (WBC), porcentaje de neutrófilos, volumen plaquetario medio (VPM), índice de distribución de plaquetas (PDW), proteína C-reactiva (PCR) y estancia hospitalaria.
ResultadosSe analizan 335 pacientes y se incluyen 284, de los cuales 194 (68,3%) tienen AA no complicada (AAnc) y 90 (31,7%) AA complicada (AAc). La edad, la duración de sintomatología, el porcentaje de neutrófilos, la PCR y la estancia hospitalaria son mayores en la AAc (p < 0,05). Las diferencias de las medias entre AAnc y AAc son: edad 13,2 años (IC 95%: 8,2-18,2), duración de sintomatología 14,1 h (IC 95%: 6,3-21,9), porcentaje de neutrófilos 5,0% (IC 95%: 3,2-6,8), PCR 73,6 mg/l (IC 95%: 50,0-97,2) y estancia hospitalaria 2,2 días (IC 95%: 1,4-3,0), con p < 0,05. Un modelo basado en parámetros preoperatorios (edad, duración de sintomatología, porcentaje de neutrófilos y PCR) se calcula para predecir la posibilidad de AAc. El área bajo la curva del modelo es 0,80 (IC 95%: 0,75-0,85).
ConclusionesEl modelo predice la posibilidad de desarrollar AAc, pero debe validarse de manera prospectiva.
Acute appendicitis (AA) is the most common abdominal surgical emergency.1 In many cases, the anamnesis, examination and laboratory parameters are sufficient for an early diagnosis.2,3 Its relevance was shown by the recent publication of the RIFT analysis, which analyzed different scoring scales used in patients with suspected AA.4 There is great interest in an early diagnosis because any delay in surgery increases morbidity and mortality5 and is also associated with increased perforation and complication rates.1,6 Imaging techniques have improved the diagnostic process,7 but ultrasound has shown low sensitivity,4 and computed tomography (CT) scan increases the risk of radiation8 and does not differentiate between uncomplicated and complicated AA.9 Thus, quick and easy-to-apply methods are required to predict the probability of complications in AA. Certain clinical and analytical data, such as age,10 duration of symptoms,10 and increased inflammatory markers,11,12 have been used to predict perforated AA. Other analytical parameters have been analyzed for the diagnosis of AA, such as platelet distribution width (PDW) and mean platelet volume (MPV).13,14 MPV is a marker of platelet function and activation, and its levels have been shown to be lower in AA.15
While individually these elements are poor predictors of AA complications, when used together they may have a high discriminatory power. The objective of this analysis is to develop a predictive model based on clinical and analytical parameters to differentiate between uncomplicated and complicated AA (gangrenous and/or perforated) in adults, without using imaging tests to guide decisions for the surgical approach, duration of antibiotic therapy and hospital stay prediction. The application of conservative treatment of AA is also assessed.
MethodsPatientsWe conducted a retrospective observational analysis of appendices whose histology showed inflammation (phlegmonous, gangrenous, perforated) in patients (>14 years) admitted by the emergency department of a level 2 hospital in Spain. The participants or their legal guardians signed the informed consent for the surgical procedure. Patients under 14 years of age were excluded, since the analytical parameters have different ranges of normal at our hospital.
The analysis was approved by the hospital’s Clinical Research Ethics Committee.
We retrospectively analyzed 335 patients whose appendix was received by the anatomic pathology department between April 1, 2014 and July 31, 2016.
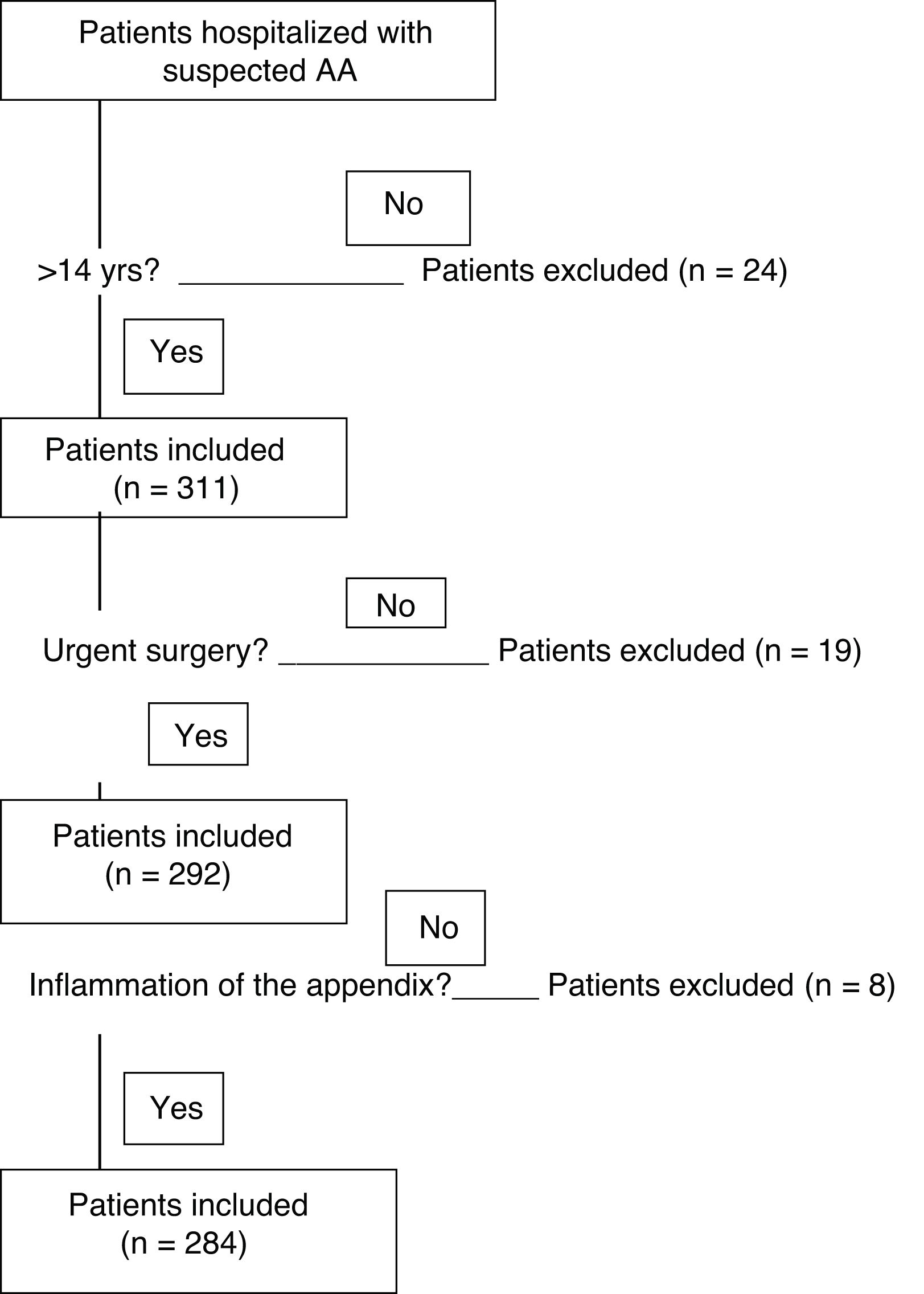
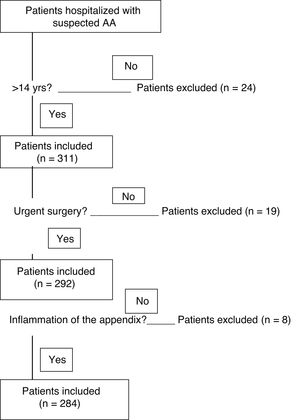
Twenty-four patients under 14 years of age were excluded. Nineteen appendices extracted in elective surgery were not included, and another 8 appendices were excluded because their histology did not show inflammation (Fig. 1). The 284 patients included in the study were divided into 2 groups according to the histopathological findings for the infiltration of inflammatory cells in the layers of the wall of the appendix and the associated defects. The groups are the uncomplicated AA (phlegmonous) group and the complicated AA (perforated and/or gangrenous) group.
Age, sex, temperature upon admission to the emergency department, duration of symptoms (from the onset of pain until surgery), preoperative leukocyte count (white blood cell [WBC]), percentage of neutrophils, MPV, PDW, C-reactive protein (CRP) and hospital stay.
Laboratory testsVenous samples were obtained and processed in EDTA tubes. They were automatically analyzed in internationally certified devices approved by a clinical analysis practitioner. The reference intervals in the hospital are: 3.7–9.5 × 103/μL WBC, 40%–74% neutrophils, 7.2–11.1 fL MPV, 10%–18% PDW, and 0.0–8.0 mg/L CRP.
Statistical analysisThe statistical analysis was carried out using SPSS® software for Windows 21.0 (IBM Corp., Armonk, NY, USA). Percentages and medians with interquartile range (IQR) were used to describe the main results. The Student’s t test and the χ2 test were used to compare quantitative and categorical variables.
Logistic regression was used to find the odds ratio (OR) of each variable in the univariate and multivariate analysis to predict AAc (dependent variable).
In addition, sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated with 2 cutoff points, 0.1 and 0.2. The receiver operating characteristic (ROC) curve was obtained for each variable and for the predictive model found according to the multivariate analysis. The results are shown with a 95% confidence interval (95%CI), and a P < .05 was considered statistically significant.
ResultsThe 284 patients were divided into 2 groups according to histopathological findings: 194 (68.3%) were uncomplicated AA (phlegmonous AA) and 90 (31.7%) were complicated AA (gangrenous and/or perforated AA).
In terms of sex, there were no statistically significant differences between the groups (P = .92): there were 81 women (41.8%) in the uncomplicated AA group and 37 (41.1%) in the complicated AA group. The characteristics of the patients are shown in Table 1. Mean age, duration of symptoms, percentage of neutrophils, CRP, and hospital stay were higher in the complicated AA group, while there were no statistically significant differences between the groups in terms of body temperature at admission, WBC or the currently analyzed parameters, like MPV and PDW (Table 1).
Comparison of uncomplicated and complicated AA groups.
| Variables (mean) | Mean uncomplicated AA (n = 194) | Mean complicated AA (n = 90) | Difference of means | SD | 95%CI of the difference | P value* |
|---|---|---|---|---|---|---|
| Age (yrs) | 35.0 | 48.2 | 13.2 | 2.5 | 8.2–18.2 | <0.001 |
| Symptoms duration (h) | 26.9 | 41.0 | 14.1 | 3.9 | 6.3–21.9 | <0.001 |
| Percentage neutrophils (%) | 80.0 | 85.0 | 5.0 | 0.9 | 3.2–6.8 | <0.001 |
| CRP (mg/L) | 37.1 | 110.7 | 73.6 | 11.8 | 50.0–97.2 | <0.001 |
| Hospital stay (days) | 2.2 | 4.4 | 2.2 | 0.4 | 1.4–3.0 | <0.001 |
| Temperature (°C) | 36.5 | 36.6 | 0.1 | 0.1 | −0.1 to 0.3 | 0.52 |
| WBC (cells/μL) | 14 210.1 | 14 548.8 | 338.7 | 589.1 | −839.5 to 1.516.9 | 0.57 |
| PDW (%) | 16.5 | 16.6 | 0.1 | 0.1 | −0.1 to 0.3 | 0.23 |
| MPV (fL) | 8.5 | 8.5 | 0.0 | 0.1 | −0.2 to 0.2 | 0.94 |
AA: acute appendicitis; SD: standard deviation; 95%CI: 95% confidence interval; CRP: C-reactive protein; PDW: platelet distribution width; MPV: mean platelet volume; WBC: white blood cell.
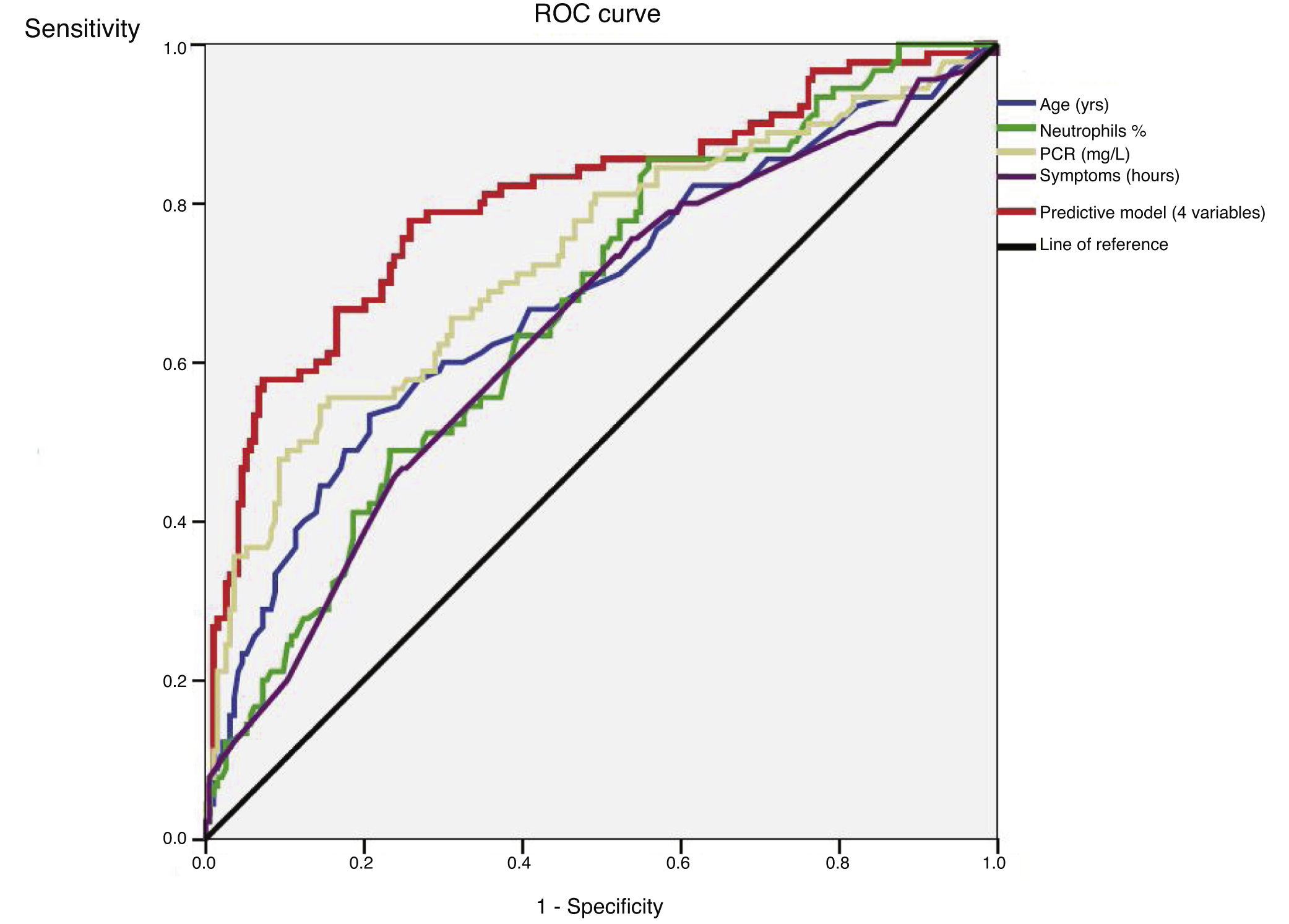
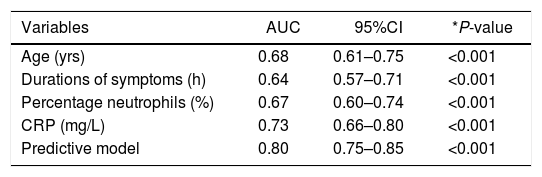
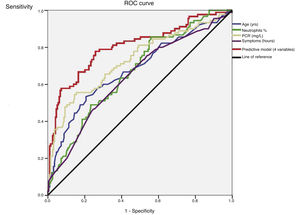
An ROC curve was developed to define the predictive values of the preoperative parameters and biomarkers for the diagnosis of complicated AA (Fig. 2). The areas under the curve were 0.68 (95%CI: 0.61−0.75) for age, 0.64 (95%CI: 0.57−0.71) for the duration of symptoms, 0.67 (95% CI: 0.60−0.74) for the percentage of neutrophils and 0.73 (95%CI: 0.66−0.80) for CRP.
After the multivariate logistic regression model based on the statistically significant preoperative values shown in Table 2 (age, duration of symptoms, WBC and percentage of neutrophils), Nagelkerke’s R2 has a value of 0.36. The ROC curve with 95%CI shown in Fig. 2 for the adjusted regression model based on these parameters has an area under the curve of 0.80 (95%CI: 0.75−0.85) (Table 3). The predictive probability (P) of complicated AA was calculated using the following formula:
Variables included in the formula for the predictive clinical model.
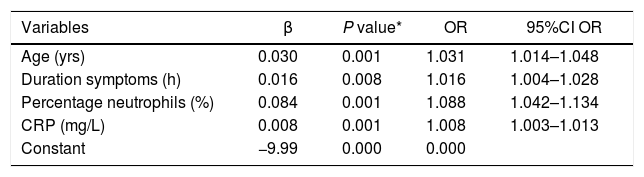
| Variables | β | P value* | OR | 95%CI OR |
|---|---|---|---|---|
| Age (yrs) | 0.030 | 0.001 | 1.031 | 1.014–1.048 |
| Duration symptoms (h) | 0.016 | 0.008 | 1.016 | 1.004–1.028 |
| Percentage neutrophils (%) | 0.084 | 0.001 | 1.088 | 1.042–1.134 |
| CRP (mg/L) | 0.008 | 0.001 | 1.008 | 1.003–1.013 |
| Constant | −9.99 | 0.000 | 0.000 |
95%CI OR: 95% confidence Interval OR; OR: odds ratio; CRP: C-reactive protein.
These are the parameters included in the model calculated for complicated AA. Per year of age, the possibility of complicated AA increases 3.1%. For every hour of symptoms, the possibility increases 1.6%. For every 1% of neutrophils, risk increases 8.8%; and for every 1 mg/L of CRP, it increases 0.8%.
ROC curve of statistically significant variables and the predictive model.
| Variables | AUC | 95%CI | *P-value |
|---|---|---|---|
| Age (yrs) | 0.68 | 0.61–0.75 | <0.001 |
| Durations of symptoms (h) | 0.64 | 0.57–0.71 | <0.001 |
| Percentage neutrophils (%) | 0.67 | 0.60–0.74 | <0.001 |
| CRP (mg/L) | 0.73 | 0.66–0.80 | <0.001 |
| Predictive model | 0.80 | 0.75–0.85 | <0.001 |
AUC: area under the curve; 95%CI: 95% confidence interval; CRP: C-reactive protein; ROC: receiver operating characteristic.
Using this formula, 2 models are proposed according to 2 different cutoff values.
In the first model, with a cut-off value of 0.1, the sensitivity and specificity were 96.7% and 22.8%, respectively, while the positive and negative predictive values were 36.9% and 93.6%, respectively. The positive likelihood ratio was 1.26 and the negative was 0.146.
In the second model, with a cut-off value of 0.2, the sensitivity, specificity, positive and negative predictive values were 80.3%, 52.8%, 45.2% and 87.2%, respectively, and the positive and negative likelihood ratios were 1.70 and 0.374, respectively.
DiscussionAA is the most frequent urgent abdominal surgery, and the delay in its diagnosis may lead to perforation and peritonitis. In the literature, most of the analyses focus on predicting appendicitis,4,6,7,12 and only a few articles have analyzed the differences between simple and complicated AA.11,16,17
The analysis published by the RIFT Study Group4 compares the different scoring scales used in the diagnosis of AA. The proposed algorithm recommends the use of the appendicitis inflammatory response (AIR) score in males and the adult appendicitis score (AAS) in women, based on clinical and analytical parameters. Hence, the use of radiological techniques is reserved for cases in which diagnostic efficacy is to be obtained, that is, patients who have obtained a score with a lower risk for AA, but who cannot be observed. In the United Kingdom, however, the use of CT is lower compared to the other European countries analyzed (Spain, Italy, Portugal, Ireland), but they have a higher rate of negative appendectomies (20%) compared to the total from all the other countries (6.2%). Thus, these scales allow patients at higher risk for AA to be identified, but, at the same time, a high percentage of negative appendectomies has been registered in the United Kingdom (probably due to the lower rate of CT usage).4
In the analysis presented, the duration of symptoms (from the onset of the pain until urgent surgery) is a parameter that increases the risk of complicated AA, as the possibility of developing complications increases by 1.6% for each hour of symptoms. In the meta-analysis published by Li et al.,18 a shorter duration of symptoms (<24 h) is not associated with developing complicated AA, but the risk is greater if the duration is >24 h. Also, when a subgroup with symptoms <6 h is compared to a group with a duration of 6−12 h, there are statistically significant differences, with longer hospital stays and more surgical wound infection in the 6−12 h subgroup. In this regard, the data are contradictory, as the meta-analysis published by Van Dijkl et al.19 found no correlation between duration of symptoms and complicated AA (although the duration of symptoms was defined from either arrival to the emergency room or from diagnosis, depending on the analyses included). This lack of consensus in the definition of the duration of symptoms generates bias in the results. A prospective analysis would be necessary, including both the duration from the onset of symptoms until surgery as well as the duration from emergency room admission until surgery.20
Regarding radiological techniques, ultrasound of the right iliac fossa (RIF) has a diagnostic sensitivity for acute appendicitis of 38% and 37% in women and men, respectively, with high diagnostic suspicion.4
CT has a high sensitivity for diagnosing acute appendicitis (92% in women and 94% in men),4 but it has a low sensitivity (62%) for diagnosing perforated appendicitis.16,17 In addition, it is associated with radiation exposure in a disease that usually affects young patients, with the corresponding risk of developing cancer (risk of 1:1250 for a dose of radiation equivalent to that used in CT).8 Furthermore, there is also the risk of developing contrast-induced nephropathy (11%) and allergic reactions, while it is also a technique that is not available at all hospitals.
In this analysis, a scoring system was developed to distinguish between simple and complicated appendicitis, based on patient characteristics and analytical parameters that are routinely collected in clinical practice.
The study included patients with AA, but patients with abscess were excluded (who were not treated surgically), given that it is a different entity.
The selected cut-off points were chosen to show different diagnostic options of the same proposed model. In a diagnostic test, high sensitivity is required to diagnose true positives, and thus the model with the 0.1 cut-off point was considered more applicable, despite the lower specificity.
This system identifies a substantial group of patients with a high probability of complicated AA. In these patients, the use of longer duration antibiotic therapy21,22 and the surgical approach should be considered, and we should also assume that the hospital stay will be longer.23–26 Furthermore, although urgent appendectomy is the current treatment for complicated AA, different analyses, including randomized trials,27–31 have evaluated conservative treatment with antibiotic therapy. This is not currently accepted systematically, however, because 39% of patients with complicated AA treated conservatively require appendectomy either during hospitalization or afterwards due to recurrence.30,31 Although more evidence is required to administer antibiotic treatment in uncomplicated AA, the search for clinical-analytical parameters that are able to identify patients who are candidates for this treatment should be initiated as soon as the scientific evidence allows.
Several parameters have been analyzed in the literature as possible predictors of AA complication. The risk of perforation is higher in the elderly10 and in the presence of abdominal pain for a longer duration.11 Mean age and duration of symptoms are higher in complicated AA, and the difference is statistically significant.
Regarding the analytical results, many studies have shown that CRP levels increase proportionally with the severity of the inflammatory response in AA.6,11,32–36 Higher neutrophil counts have in turn been observed in this pathology.13 In this analysis, CRP and percentage of neutrophils have statistically significant differences between simple AA and complicated AA.
Some thrombocyte markers, including MPV and PDW, have been associated with thrombocyte activation, thrombosis, and the pathophysiology of diseases related with inflammation, such as AA.37 In our analysis, neither MPV nor PDW were statistically significant predictors for the diagnosis of complicated AA.
Thus, it is known that individual elements of clinical findings and analytical results are poor predictors of complicated AA, but, in combination, they have a high discriminatory power. Laboratory tests showing inflammatory response and clinical descriptors of abdominal pain are powerful diagnostic parameters and should be included in the diagnostic evaluation.
Older age, longer duration of symptoms, and higher percentage of neutrophils and CRP are predictive factors for gangrenous and/or perforated AA. This analysis shows a predictive model capable of indicating an increased risk of complicated AA (gangrenous and/or perforated AA).
Thus, with the use of this model, imaging techniques are not required for the diagnosis of complicated AA. Although there is some limitation because imaging studies must be performed if there is suspicion of certain diseases also included in the differential diagnosis (such as abscess or neoplasm), it is true that they do not have high sensitivity to differentiate between complicated AA and uncomplicated AA. The clinical and analytical parameters presented in this analysis provide diagnostic orientation.
For the above reasons, we use patient age, duration of the symptoms, and two analytical parameters (neutrophil count and CRP) to calculate the probability of developing complicated AA.
In terms of clinical applicability, the model provides a better therapeutic approach, including the choice of laparotomy or laparoscopy and the use of longer antibiotic therapy.21,22 It is also capable of predicting longer hospital stays.
The limitations of this analysis are based on the retrospective methodology, which means that there is a notable bias in the duration of the symptoms. Prospective assessment is necessary for its internal validation and subsequent external validation as well.
FundingThis study has received no specific funding from public, commercial or non-profit organizations.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank the Statistics Department at the Hospital de Guadalajara for their contribution to the analysis.
Please cite this article as: García-Amador C, Arteaga Peralta V, de la Plaza Llamas R, Torralba M, Medina Velasco A, Ramia JM. Valoración de parámetros clínicos y analíticos preoperatorios en apendicitis aguda complicada. Score para predecir apendicitis complicada. Cir Esp. 2021;99:282–288.