Oesophageal reconstruction by gastroplasty with cervical anastomosis has a higher incidence of dehiscence. The aim of the study is to analyze the incidence of anastomotic leakage in patients undergoing gastroplasty with cervical anastomosis following angiographic ischemic conditioning of the gastric conduit.
MethodsProspective analysis of patients who underwent gastric conditioning two weeks prior to oesophageal reconstruction, from January 2001 to January 2014. The conditioning was performed by angiographic embolization of the left and right gastric artery, and splenic artery.
The main variable analyzed was the incidence of anastomotic leakage in patients undergoing gastroplasty with cervical anastomosis. Secondary variables analyzed were the result of the conditioning, complications arising from that procedure and in the postoperative period, and mean length of postconditioning and postoperative hospital stay.
ResultsGastric conditioning was indicated in 97 patients, with neoplasia being the most frequent etiology motivating the oesophageal reconstruction (76%). 96 procedures were successfully carried out, arterial embolization was complete in 80 (83%). The morbidity rate was 13%, with no mortality. Postoperative morbidity was 45%; the most frequent complications associated with the surgery were respiratory problems. Six (7%) patients experienced cervical fistula, and all received conservative treatment. The rate of postoperative mortality was 7%.
ConclusionsIn our series the incidence of anastomotic leakage in patients undergoing gastroplasty with cervical anastomosis following angiographic ischemic conditioning is 7%.
Angiographic ischemic conditioning is a procedure with acceptable morbidity.
La reconstrucción esofágica mediante gastroplastia con anastomosis cervical es una técnica una mayor dehiscencia anastomótica remarcable. El objetivo de este estudio es analizar la incidencia de dehiscencia anastomótica en pacientes sometidos a gastroplastia con anastomosis cervical tras acondicionamiento isquémico angiográfico del tubo gástrico.
MétodosAnálisis prospectivo de pacientes que se sometieron a acondicionamiento gástrico 2 semanas antes de la reconstrucción esofágica, desde enero de 2001 hasta enero de 2014.
El acondicionamiento se realizó mediante embolización angiográfica de las arterias gástricas izquierda y derecha, y la arteria esplénica.
La variable principal analizada fue la incidencia de dehiscencia anastomótica en pacientes sometidos a gastroplastia con anastomosis cervical.
Las variables secundarias analizadas fueron el éxito del acondicionamiento, las complicaciones tras este procedimiento y postoperatorias, y la duración media de la estancia hospitalaria postacondicionamiento.
ResultadosEl acondicionamiento gástrico se indicó en 97 pacientes, siendo la neoplasia la etiología más frecuente que motivó la reconstrucción esofágica (76%). Se realizaron 96 procedimientos con éxito, la embolización arterial fue completa en 80 (83%). La morbilidad fue del 13%, sin mortalidad. La morbilidad postoperatoria fue del 45%; las complicaciones más frecuentes asociadas a la cirugía fueron los respiratorios. Seis (7%) pacientes presentaron fístula cervical y todos tratados de forma conservadora. La mortalidad postoperatoria fue del 7%.
ConclusionesEn nuestra serie, la incidencia de dehiscencia anastomótica en pacientes sometidos a gastroplastia con anastomosis cervical tras acondicionamiento isquémico angiográfico es del 7%. El acondicionamiento isquémico angiográfico es un procedimiento con una morbilidad aceptable.
Oesophageal reconstruction via gastroplasty with thoracic or cervical anastomosis has a higher incidence of dehiscence (wound rupture) than other types of anastomoses of the gastrointestinal tract, due to the considerable risk of ischemia associated with gastroplasties.1–4 Ischemic conditioning of the gastric conduit is a therapy preceding the creation of the gastric tube that aims to prevent this eventuality.
In 1996 and 1998, Akiyama et al. were the first to publish results reporting their work on arteriographic embolization of the left gastric artery (LGA), right gastric artery (RGA) and splenic artery (SA) as a method of gastric conditioning prior to gastroplasty.5,6 The results obtained showed a smaller reduction in tissue blood flow from baseline (33%) and a lower incidence of anastomotic leakage (2%) compared to the control group, at 67% and 8%, respectively. Later, in 1999, Isomura et al. published their results on a series of 34 patients with cervical or thoracic gastroplasty with prior angiographic conditioning.7 As with Akiyama's group,6 they observed a smaller reduction in tissue blood flow during the construction of the gastric tube, at 27.5% compared to the 68.9% reduction observed in the control group (P<.005), with an anastomotic leakage rate of 2.9%.
Since 2006, some authors have advocated laparoscopic gastric conditioning,8–17 the longest series being that of Schröder 419 patients. Nevertheless this approach has some disadvantages: the need for general anesthesia and two surgical procedures, and a possible lower efficacy compared to the arteriographic one, as a recent meta-analysis18points out, probably influenced by the waiting time from the conditioning to the surgery is less.
Actually there is a debate about the benefit of ischemic conditioning. In a recent publication,19 its general recommendation is questioned, recommending it only in selected cases with high risk of dehiscence (aortic calcification, hypertension, renal failure).
The present study is the series published with the highest number of patients arteriographically embolized, being able to provide information about the success of this technique, its morbidity and incidence of cervical anastomotic leakage.
The aim of the present study is to analyze the incidence of anastomotic leakage in patients undergoing gastroplasty with cervical anastomosis following angiographic ischemic conditioning of the gastric conduit.
Material and MethodsStudy DesignWe conducted a retrospective analysis of prospective database of all patients referred for arteriographic gastric conditioning from January 2001 to January 2014 in the Oesophageal Surgery Unit of the Hospital Universitari de Bellvitge.
Inclusion CriteriaAll patients undergoing gastroplasty with cervical anastomosis, either immediately following oesophageal resection (transhiatal or Mc Keown procedure) or at a later time, with deferred reconstruction.
In oesophageal cancer cases, the embolization was indicated after a reevaluation in an expert committee the staging of the oesophageal cancer. In patients that were performed neoadjuvant treatment, the embolization was indicated once the tumor progression was ruled out.
All patients gave informed consent on the risks and benefits of the procedure.
Exclusion CriteriaUntil 2009 exclusion criteria was the same described by Akiyama5: gastroduodenal ulcer, prior history of pancreatitis, more than 75 years of age, or known vascular abnormality that preclude a angiographic occlusion of the arteries. After January 2010, the only exclusion criteria was the last one.
Study ObjectivesThe main study objective was to analyze the incidence of anastomotic leakage in patients undergoing gastroplasty with cervical anastomosis after ischemic conditioning of the gastric conduit via angiography.
The secondary objectives were to analyze the result of the conditioning, complications arising from that procedure and in the postoperative period, and mean length of postconditioning hospital stay.
VariablesDescriptive data included demographic characteristics, comorbidities (heart disease, pulmonary disease, renal failure), aetiological diagnosis of the oesophage al diseases, and the type of surgical technique used.
We defined the result of the conditioning procedure as: complete technique for the correct embolization of the three arteries (LGA, RGA, and SA), incomplete technique of one or two of the arteries, and failure of the technique in case of no embolized arteries.
Morbidity after embolization included: pancreatitis, abscess, pseudocyst, spleen ischemia (diagnosed by CT or abdominal ultrasound and needs some treatment), liver ischemia (diagnosed by CT or abdominal ultrasound), bleeding, artery dissection and, arterial pseudoaneurysm.
Postoperative mortality: during hospitalization and/or 30 days after surgery.
Major and minor morbidity after surgery procedure was considered, according to the Clavien-Dindo classification.20 These include: pulmonary complications, chylothorax (diagnosed macroscopically or biochemically) wound infection, dysphony, paralytic ileus (that implies some treatment), hemorrhage (hemothorax, haemoperitonuem.
About oesophagogatsric anastomotic leakage, it was considered with the presence of one or more of the following conditions: radiologic confirmation by water-soluble contrast study or thoracoabdominal TC with oral contrast of dehiscence of oesophagogastric anastomosis, Thoracic drain output of oesophagogastric content or methylene blue, confirmation by the surgeon during a reintervention, endoscopic confirmation of anastomotic leakage.
Type IV oesophagogastric anastomotic leakage (plasty ischemia)21 was considered when one or more of the following criteria was present: endoscopic evidence of gastric mucosa ischemia, evidence of low captation of the plasty in a thoracoabdominal CT with endovenous contrast that required a reintervention.
Gastric Conditioning TechniquePatients underwent ischemic conditioning two weeks before surgery. Physicians performed an angiogram of the celiac trunk via a femoral access pre- and post-procedure.
Embolization at the base of the SA helps to maintain blood supply to the spleen through collateral circulation, averting splenic necrosis. Thus, the embolization of this artery was initially performed with 8.89mm coils (0.035 inches) (Cook, IN, USA), aided by proximal splenic artery balloon occlusion (Boston Scientific, MA, USA). Operators usually embolized the artery at a mid-portion of the main trunk using a 5 Fr long sheath and an Amplatzer device (AGA Medical, MN, USA).
Likewise, the LGA was embolized with the 8.89mm coils and/or an Amplatzer device, positioned from the main trunk to the first branch point. Where accessory left gastric branches (which often occur when the LGA originates from the left hepatic artery) were present, they were also catheterised and embolized.
Because professionals from our institution have sometimes had to section the RGA to allow a tension-free mobilization of the gastric tube via the mediastinum, we prefer to also embolize this artery.
For the RGA embolization, physicians must usually insert a microcatheter for its selective catherisation. However, the anterograde approach of the right gastric catheterisation can be challenging. In that case, we attempted retrograde catheterisation via the arcade on the lesser curvature with a microcatheter, inserting a 4 or 5 Fr Simmons or Cobra catheter (Terumo Europe N.V., Leuven, Belgium) into the LGA to serve as a guide. We also placed coils or microcoils proximally in the artery (from the main trunk to the first branch point). Final celiac angiography confirmed both embolization and the lack of gastric blood supply from arteries other than the right gastroepiploic artery.
Surgical TechniqueIn all of the patients with immediate oesophageal reconstruction (transhiatal or Mc Keown procedure) the gastroplasty was through the posterior mediastinum. For the patients with deferred reconstruction, it was through the anterior mediastinum.
The cervical anastomosis was performed manually end-to-side via simple stitches with resorbable sutures (VICRYL™ 3/0. Johnson & Johnson International, Lieje, Belgium) in all cases.
Statistical MethodCategorical outcomes were expressed as percentages. For the analyses on the incidence of dehiscence, we used the Chi-squared test with Fisher's correction. The threshold for statistical significance was set as P<.05.
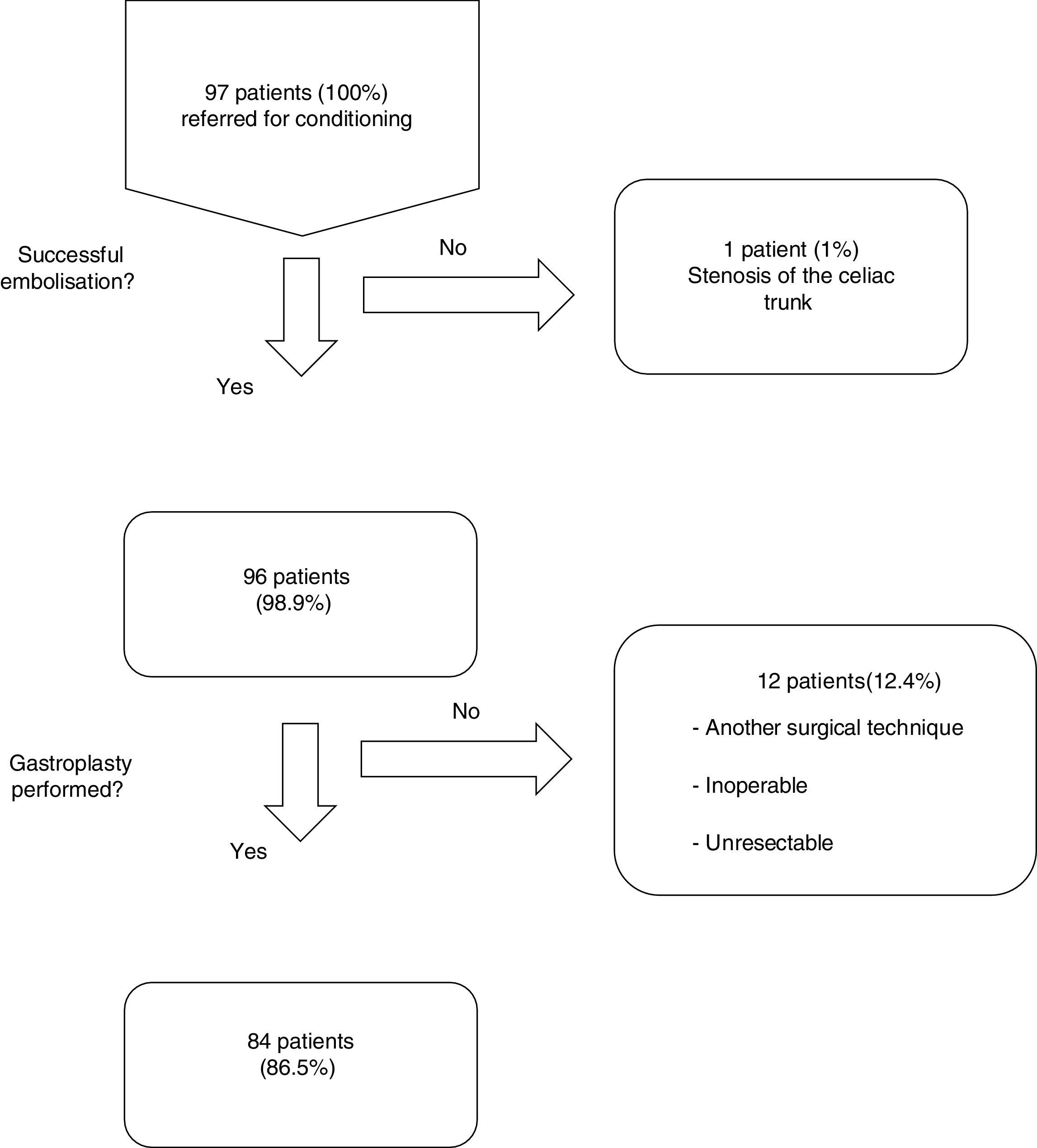
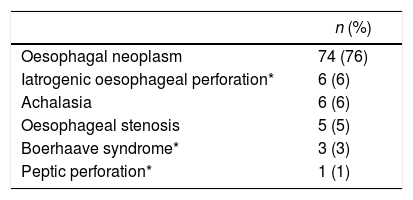
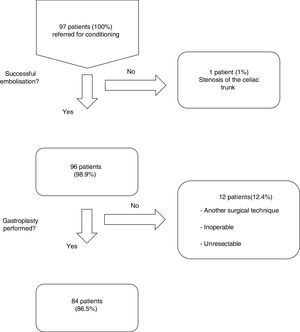
ResultsFrom January 2001 to January 2014, a total of 97 consecutive patients were referred for ischemic conditioning of the gastric conduit prior to the performance of a gastroplasty with cervical anastomosis. 79 (81%) were men and 18 (18%) were women, with a mean age of 59.9±11.4 years. The most frequent aetiological diagnosis was oesophageal neoplasia, which was present in 74 cases (76%), followed by different non-cancer related causes in the remaining 23 patients (Table 1). Of the 97 patients, the complete procedure (cervical gastroplasty with prior gastric conditioning) was only performed in 84 (Fig. 1). Surgeons did not perform the complete gastroplasty in eight cases due to inoperability/unresectability, in one case due to intraoperative surgical conversion to total gastrectomy, and in three cases due to conversion to transthoracic oesophagectomy. The surgical techniques employed in the 84 patients were: 39 three-field oesophagectomy (46%); 34 transhiatal oesophagectomy (41%); and 11 deferred reconstruction (12%).
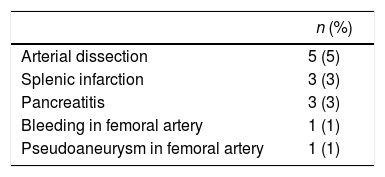
Of the 96 procedures that were successfully performed, arterial embolization was complete in 80 (83%) and incomplete in 16 (17%). The most frequent cause of incomplete embolization was the lack of catheterisation in the right gastric artery, occurring in 9 cases (56%). Morbidity associated with the technique was 13%, and there was no mortality (Table 2). The cases with lesion(s) on the femoral artery were treated at the time of the arteriographic procedure. The cases of pancreatitis and splenic infarction were diagnosed by computed tomography (CT) if patients were suffering abdominal pain, and these conditions were managed conservatively, presenting a favourable evolution. The mean length of hospital stay after the procedure was 1.3±0.6 days. The period of time between the gastric conditioning and the surgery was 20.4±5 days in the cases of neoplastic pathology.
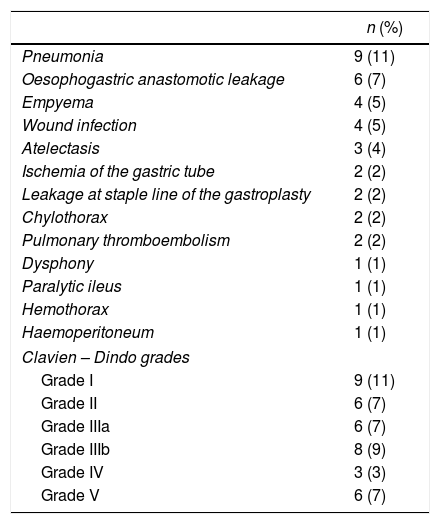
Overall postoperative morbidity was 45%. The most frequent complication was pulmonary infection in 9 cases (11%). Cervical fistula was observed in 6 patients (7%), two of them (2%) with a type 4 leakage21 (necrosis of the gastric tube) requiring plasty remove. The rest of the patients were treated conservatively with broad spectrum antibiotics, fasting diet and enteral nutrition. The etiology of 5 of the 6 patients with leakages was oesophageal neoplasm.
Postoperative mortality was in 6 patients (7%) (Table 3).
Postoperative Morbidity.
| n (%) | |
|---|---|
| Pneumonia | 9 (11) |
| Oesophogastric anastomotic leakage | 6 (7) |
| Empyema | 4 (5) |
| Wound infection | 4 (5) |
| Atelectasis | 3 (4) |
| Ischemia of the gastric tube | 2 (2) |
| Leakage at staple line of the gastroplasty | 2 (2) |
| Chylothorax | 2 (2) |
| Pulmonary thromboembolism | 2 (2) |
| Dysphony | 1 (1) |
| Paralytic ileus | 1 (1) |
| Hemothorax | 1 (1) |
| Haemoperitoneum | 1 (1) |
| Clavien – Dindo grades | |
| Grade I | 9 (11) |
| Grade II | 6 (7) |
| Grade IIIa | 6 (7) |
| Grade IIIb | 8 (9) |
| Grade IV | 3 (3) |
| Grade V | 6 (7) |
When we compared the incidence of anastomotic leakage between the groups that had undergone complete versus incomplete conditioning, we observed non-significant differences, with the six patients experiencing leakage belonging to the group with complete conditioning.
DiscussionIn the reconstruction of the oesophageal conduit by gastroplasty, partial gastric devascularisation and mobilization is necessary to perform it, generating a potential ischemic risk.1,4 This fact means that the oesophogastric anastomosis is considered to be at a high risk for dehiscence.
In 2002, Schröder et al. studied the changes in gastric microcirculation associated with the formation of the gastric tube in an animal model with pigs,3 showing a significant reduction (P<.0001) of tissue perfusion and partial tissue oxygen pressure at the gastric fundus level of the gastric tube that was created.
As a solution to the problem, numerous studies have been carried out on ischemic conditioning of the gastric conduit. Investigations in animal models have reported on its benefits when performed prior to the creation of the gastric tube. In 1995, Urschel et al. showed a significant and gradual recovery of gastric tissue perfusion, of up to 81% of the baseline value at 14 days after vascular ligation (delay phenomenon) in rats.22
Moreover, actually the role of gastric conditioning is questioned. In a recent meta-analysis19 of 1215 patients, there were no differences in anastomotic dehiscence between gastric conditioning group vs control group, although they observed (but it was not been studied thoroughly) that patients with gastric conditioning showed less severe leakages and a reduced need for reinterventions. The authors suggested to use this technique only in patients with a higher average risk for postoperative leakage (aortic calcification or when intraoperative measurements show poor perfusion of the gastric fundus after arterial ligation). However, analyzing the 5 studies that comprise 245 patients on which the results of the arteriographic conditioning are based, they include esophagectomies with cervical and intrathoracic anastomosis. Our series includes cervical anastomosis exclusively, because it is where we believe that the benefit of the arteriographic conditioning would be greater.
There is debate with regard to the best time to construct the gastric tube. Our group (Lamas et al.) situated this at least 15 days after the conditioning,23 which is consistent with the results described by Urschel et al.22 In line with those findings, all of the patients in our series were operated a minimum of 15 days after the embolization, with a mean of 20.4±5.0 days in patients with neoplastic pathology. However, other groups consider the formation of the gastric tube to be safe and efficacious at 3–7 days.12 Kechagias et al.19 observed better results with arteriographic gastric conditioning comparing with laparoscopic arterial ligation, suggesting that maybe it may also partly explained by the short interval between gastric conditioning and esophagectomy in laparoscopic conditioning group.
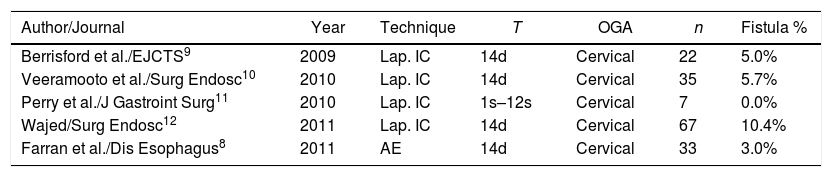
In 2011 our group published the results on morbi-mortality and rate of anastomotic leakage in 33 patients following ischemic conditioning for gastroplasty with cervical anastomosis.24 Overall morbidity associated with the conditioning was 18%, with 3% of cervical fistula. Here, morbidity has descended to 13%, probably due to the increased skills of the angioradiologists Deshiscence stands at 7.1%, without having modified the gastric conditioning procedure or the surgical technique. These results can be superimposed onto those of other groups, whose reports range from 0%11 to 10%11 (Table 4). Boshier et al. performed a meta-analysis of 1777 patients undergoing transhiatal oesophagectomy with cervical anastomosis without gastric conditioning, and the incidence of anastomotic dehiscence was 17%.25
Publications on Ischemic Gastric Conditioning with Cervical Anastomosis.
| Author/Journal | Year | Technique | T | OGA | n | Fistula % |
|---|---|---|---|---|---|---|
| Berrisford et al./EJCTS9 | 2009 | Lap. IC | 14d | Cervical | 22 | 5.0% |
| Veeramooto et al./Surg Endosc10 | 2010 | Lap. IC | 14d | Cervical | 35 | 5.7% |
| Perry et al./J Gastroint Surg11 | 2010 | Lap. IC | 1s–12s | Cervical | 7 | 0.0% |
| Wajed/Surg Endosc12 | 2011 | Lap. IC | 14d | Cervical | 67 | 10.4% |
| Farran et al./Dis Esophagus8 | 2011 | AE | 14d | Cervical | 33 | 3.0% |
AE: arteriographic embolization; IC: ischaemeic conditioning; OGA: oesophogastric anastomosis; T: time.
Recently some groups are developing other techniques to evaluate intraoperatively the vascularization of gastric plasty, such as indocyanine green. The goal is not to improve the vascularization of the plasty, but to check its vascularization and to choose the most optimal place to perform the anastomosis. In this sense, although some authors26 have published hopeful results (dehiscence of 0%) in 30 patients. a recent review27 that includes 214 patients reports leakage rates of 14%, without randomized studies that objectify the real benefit of this technique. It is a promising technique, but it is necessary randomized control trials to analize the benefit of this technique.
Previous studies with cervical anastomosis used the laparascopic technique for ischemic conditioning (Table 4). We believe that ischemic conditioning via arterial embolization is a safe procedure with an acceptable rate of associated morbidity (Table 2) and high efficacy (1% failures). It is true that after coil embolization, a local periarteritis originates, which makes it difficult to dissect the arterial trunks during surgery. However, there was no greater intraoperative morbidity, only a more laborious dissection without more blleeding because the arteries are occluded. Moreover, laparoscopic gastric conditioning requires two surgical procedures.
The limitations of this study is that it is a non-comparative descriptive study. Randomized trials are needed to respond to the actual role of embolization in the prevention of cervical anastomotic dehiscence.
The most interesting contribution of the present study may reside is that is the series published with the highest number of patients arteriographically embolized cervical, with low morbidity of this technique. Unlike the studies described previously, our series focuses on patients undergoing gastroplasty with cervical anastomosis, as this is an anastomosis with a higher risk of leakage and deshiscence.25
ConclusionsIn our series the incidence of anastomotic leakage in patients undergoing gastroplasty with cervical anastomosis following angiographic ischemic conditioning is 7%.
Ischemic conditioning of the gastric conduit via angiographic arterial embolization is a procedure with acceptable morbidity.
Further randomized prospective studies are needed to demonstrate its benefits in the prevention of oesophogastric anastomotic dehiscence.
Authors’ ContributionsAll the authors, except Monica Miro, Leandre Farran, and Maica Galan collected the data for this study. Monica Miro, Fernando Estremiana, Humberto Aranda, and Carla Bettonica made analyzed the data. Based on that Leandre Farran and Maica Galán design the study. Humberto Aranda and Carla Bettonica gave interpretation of the results. Jordi MiqueL took care of design of the manuscript. Leandre Farran and Maica Galán made the critical review. Monicka Miro checked the final version. Monicka Miro and Leandre Farran wrote the content.
Please cite this article as: Miró M, Farran L, Estremiana F, Miquel J, Escalante E, Aranda H, et al. ¿Puede el acondicionamiento gástrico disminuir la incidencia de dehiscencia anastomótica esofagogástrica cervical? Cir Esp. 2018;96:102–108.