Simultaneous kidney–pancreas transplantation for patients with type 1 diabetes and end-stage chronic renal disease is widely performed. However, the rate of surgical morbidity from pancreatic complications remains high. The aim of this study was to describe the development and results of a new program, from the point of view of the pancreatic surgeon.
MethodsWe analyzed 53 simultaneous kidney–pancreas transplantations performed over a period of seven years (2009–2016), with a median follow up of 39 months (range: 1–86 months).
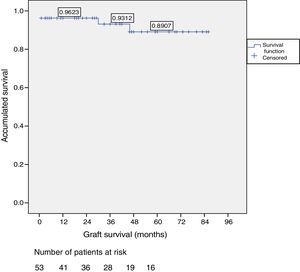
ResultsOut of the total of this series, two patients died: one patient because of cardiac arrest immediately after surgery; and another patient due to traffic accident, complicated by pneumonia. Among the 51 living patients, two grafts were lost: one due to chronic rejection four years after transplantation; and the other due to arterial thrombosis 20 days after transplantation (the only case requiring transplantectomy). In ten patients, one or more re-operations were necessary due to the following: graft pancreatitis (n=4), small intestinal obstruction (n=4), arterial thrombosis (n=1), fistula (n=1) and hemoperitoneum (n=1). Overall patient and graft survival rates after 1, 3 and 5 years were 98%, 95% and 95% and 96%, 93% and 89%, respectively.
ConclusionsThis study has shown that the results of a new pancreas transplant program, which relies on the previous experience of other groups, do not demonstrate a learning curve. Adequate surgeon education and training, as well as the proper use of standardized techniques, should ensure optimal results.
El trasplante simultáneo de páncreas-riñón se encuentra indicado para pacientes con diabetes tipo 1 y enfermedad renal terminal. Los resultados son excelentes aunque el número de procedimientos parece ser un factor que afecta a la supervivencia de paciente e injerto estando en relación con la morbilidad quirúrgica, derivada de complicaciones pancreáticas. el objetivo del estudio es describir el desarrollo de un nuevo programa y exponer los resultados en un centro con un volumen bajo de trasplantes.
MétodosAnalizamos 53 trasplantes simultáneos de páncreas-riñón, en un período de 7 años (2009-2016), con una mediana de seguimiento de 39 meses.
ResultadosDos pacientes han fallecido, uno tras parada cardíaca en postoperatorio y otro tras accidente de tráfico complicado con una neumonía. Entre los 51 pacientes vivos se han perdido 2 injertos, uno por un rechazo crónico tras cuatro años del trasplante y otro por trombosis arterial a los 20 días del mismo, motivo, este último, de la única trasplantectomía realizada. En diez pacientes se han realizado una o más reintervenciones: pancreatitis (n=3), oclusión intestinal (n=4), trombosis arterial (n=1), fístula con peritonitis (n=1) y hemoperitoneo (n=1). La supervivencia del paciente y del injerto a 1, 3, y 5 años fue del 98, 95 y 95% y del 96, 93 y 89%, respectivamente.
ConclusionesLos resultados muestran que un nuevo programa de trasplante pancreático puede conseguir resultados similares a los de grupos con mayor volumen y experiencia. Una adecuada selección de donantes y receptores, una técnica homogénea y el aprendizaje con grupos expertos garantizan estos resultados.
Since the first transplantation performed in 1966, pancreas transplantation has evolved progressively, improving in technical aspects (systemic venous shunt and enteric drainage of exocrine secretion) as well as immunosuppression, which has been reflected in the improved patient and graft survival rates.1,2 Some authors suggest that one of several factors that can affect survival is the number of procedures performed per year, which would be a marker representative of the experience and overall quality of a hospital.3,4 In addition, other teams have published discouraging results related to surgical problems in the early stages of a hospital transplant program, that is, during the theoretical learning period.5,6 For this reason, we consider it of interest to present our results, which are representative of these two circumstances: a low-volume medical center in its initial stage.
In Spain, Professor Fernández-Cruz initiated the first pancreas transplant program in 19837; since then, multiple programs have been established in different institutions. In 2016, 11 adult transplant programs were active, which conducted a total of 70 transplantations. In this study, we present the experience of the pancreas transplant program at the Hospital Clínico Universitario in Salamanca, Spain, over the course of 7 years. In this period, a total of 53 simultaneous pancreas–kidney transplantations (SPK) were performed. We describe the results obtained related with patient and pancreas graft survival in order to evaluate whether these results are comparable to centers with larger transplant volumes and more experience.
MethodsThe study has a retrospective, observational design using a prospective database audited by the Ministry of Health and Consumption, including all transplantations performed (n: 53) during the study period (March 2009–May 2016). Donors: For donor selection, the criteria established in the 1995 Consensus Document by the National Transplant Organization8 were followed. Recipients: For the study and selection of recipients, the recommendations of the Consensus Document by the National Transplant Organization8 were also followed. The geographical areas assigned to the Salamanca group are the Community of Castilla y León (2400000 inhabitants) and, since 2012, the Community of Extremadura (1099000 inhabitants). Since January 2014, we have been accredited as a National Reference Center.
All patients underwent a preoperative SPECT study, followed by coronary angiography. In three patients, pre-transplantation coronary stent placement was necessary. The study of the aortoiliac axis was done by CT angiography. All transplants were simultaneous pancreas–kidney. The study was approved by the Ethics Committee of the Hospital Universitario in Salamanca.
Surgical ProcedureThe team: The team was comprised of 4 surgeons dedicated to and with extensive experience in hepatic and pancreatic surgery. Likewise, the senior surgeon also has experience in liver transplantation and has received specific training in pancreas transplantation at the University of Wisconsin-Madison (Wisconsin, USA) with Professor Sollinger and at the Reina Sofía Hospital (Córdoba, Spain) with Professor Padillo. Donor operation: The team performed the extraction of the pancreas and liver using in situ dissection of the vascular pedicles and isolated extraction of the organs. Perfusion was performed in the first 23 cases with Wisconsin solution (Viaspan, Bristol-Myers Squibb, Madrid, Spain) and subsequently with Celsior (Genzyme SL, Madrid, Spain). The median duration of the procedure was 180min (r: 140–240min). Graft preparation: The surgical technique used meticulously follows the fundamentals previously published by our team.9 Median bench surgery time was 150min (r: 110–220min).
Technique in the recipient. Systemic venous shunt and enteric drainage were performed, and we would like to highlight two technical details that we consider of interest. First, after the portocaval anastomosis, we placed a clamp on the portal vein, which allowed us to remove the one situated on the cava, thus restoring venous return. This enabled us to evaluate the venous anastomosis for leaks and make any repairs, if necessary. Likewise, after the arterial anastomosis, we placed a clamp on the arterial graft and removed the one situated on the iliac artery to release the circulation of the right lower limb and review the anastomosis. Secondly, when initiating reperfusion, we opened the inferior mesenteric vein of the graft, which we have previously marked on the bench with a long ligature, and let flow about 200ml of the recipient's blood. (Personal contribution by Dr. Padillo Ruiz, not previously published). This maneuver not only attempts to wash the graft, but also avoids hyperpressure and possible endothelial and tissue damage in a low-flow organ such as the pancreas.
Enteric drainage was performed by manual biplane anastomosis of the duodenum to a bowel loop 60cm from the angle of Treitz. The median surgery time was 155min (r: 130–180min) and the median cold ischemia time was 11h (r: 6–14h). We did not administer intravenous systemic anticoagulation. Postoperative: Antithrombotic prophylaxis involves the administration of 300mg of acetylsalicylic acid in the preoperative period and then a permanent dose of 100mg/day. Also, until discharge, low molecular weight heparin was added at prophylactic doses. Postoperative monitoring included daily blood, urine and drained fluid analyses, amylase and bilirubin determination, and, in case of suspicion of intra-abdominal infection, culture and antibiotic susceptibility test, abdominal ultrasound and determination of vascular flow. Clinical evolution determined whether further complementary tests were necessary.
The suspicion of fistula (pancreatic, anastomotic or of the ends of the duodenum) or the presence of graft pancreatitis were established in the presence of abdominal pain, fever, leukocytosis, increased amylase levels in the drained fluid, with or without presence of bilirubin and hyperamylasemia. Confirmation was made by the radiological findings of free intra-abdominal fluid, collections around the graft, or the presence of edema and areas of necrosis in the pancreas. When there was clinical suspicion of arterial or venous thrombosis (pain in the area of the implant, hyperglycemia and an abrupt increase in insulin needs), Doppler ultrasound was urgently requested, followed, according to findings, by magnetic resonance angiography and arteriography. No studies were conducted for the diagnosis of possible asymptomatic partial thromboses.
Immunosuppression and follow-up. Induction was performed with thymoglobulin (1.5mg/kg/day for 5 days) and maintenance of immunosuppressive therapy was based on tacrolimus, mycophenolate mofetil, and prednisolone. After discharge, patients often come to the nephrology, endocrinology and surgery consultations with decreasing frequency. Data for immunosuppression, patient and graft survival are sent to the national registry with headquarters in the NTO every 6 months. We have excluded from this present study the data related to medical or urological complications. In the presented group, pancreatic graft biopsies were not necessary.
Complete function of the graft was defined by the absence of diet, insulin or any other type of hypoglycemic agents to maintain glycemia and glycosylated hemoglobin levels within normal ranges. For statistical purposes, death with a functioning graft was considered a graft loss. Mean patient follow-up was 40 months (median: 39); May 30, 2016 was the date established for the analysis of survival data.
Statistical AnalysisThe mean, median, range, standard deviation and interval of all continuous variables were calculated using the SPSS software package (SPSS 20.0 Inc., Chicago, IL, USA); frequencies are reported for dichotomous variables. Patient and graft survival curves were calculated by the Kaplan–Meier method.
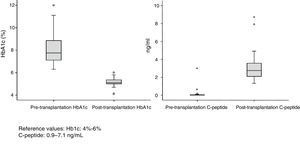
ResultsDonors: The group consisted of 32 men and 21 women with a median age of 34 years (r: 17–52). The most frequent causes of death were acute cerebrovascular accident (30 cases), followed by traumatic brain injury (18) and deaths from different causes (5). The body mass index (BMI) of the donors ranged between 19 and 28. Of the total of 53 recipients (40 men and 13 women) 32 were on hemodialysis, 17 on peritoneal dialysis and 4 on predialysis. Mean renal replacement therapy time was 48 months. Median recipient age at the time of transplantation was 40 years (r: 31–52) and BMI was 23. All patients analyzed had diabetes mellitus type 1 (DMT1), except for one case that showed Maturity Onset Diabetes of the Young (MODY). The mean follow-up of DMT1 was 25 years with an average preoperative HbA1c level of 8%. Median time on the waiting list was 71 days (r: 2–310 days). Median hospital stay during the transplantation process was 16 days (r: 9–49).
Ten patients (19%) required one or more reoperations. Graft pancreatitis was diagnosed in 3 cases. One of these patients presented infected necrosis of the peripancreatic fat instead of the parenchyma itself, which was treated by laparotomy, lavage, debridement and drainage. Another patient had authentic segmental necrotizing hemorrhagic pancreatitis, with maintenance of blood glucose, which required two reoperations for debridement and lavage, resulting in survival of the patient and graft, which continues to function today. The last re-operated patient presented with edematous pancreatitis with peripancreatic collections that were resolved with lavage and drainage. Small bowel obstruction due to adhesions in 4 patients were resolved by laparotomy and adhesion release. One patient was re-operated due to peritoneal bleeding from a collateral of the iliac graft used in the arterial reconstruction, treated with emergency laparotomy and hemostasis. One patient presented arterial thrombosis of the graft 20 days after transplantation with loss of function and additional infection that required an immediate transplantectomy. Last of all, one patient was re-operated 3 times for peritonitis secondary to fistula of the bowel anastomosis, which was initially resolved with laparotomy, lavage, drainage and finally by transformation of the side-to-side duodenojejunal anastomosis to a Roux-en-Y.
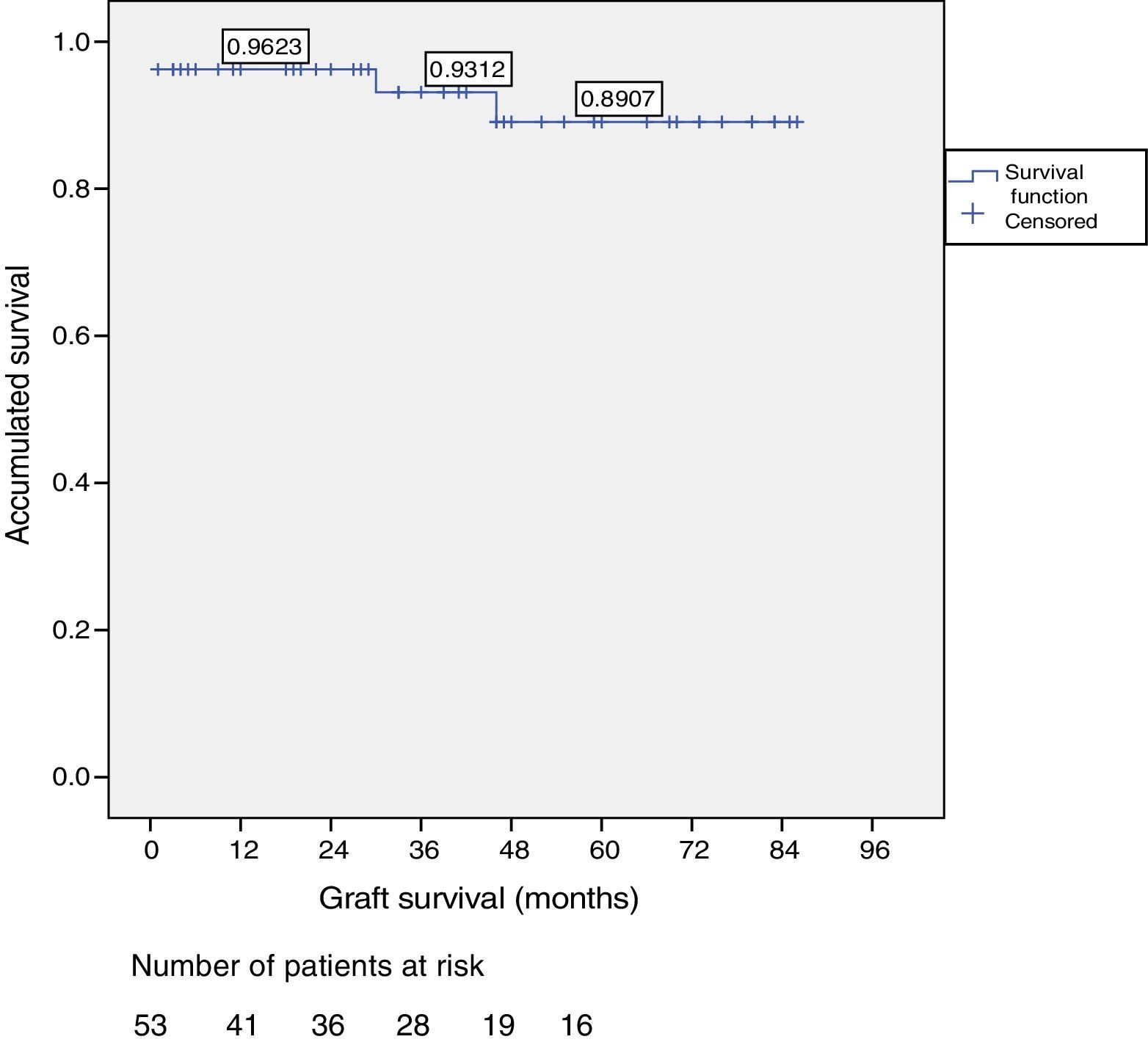
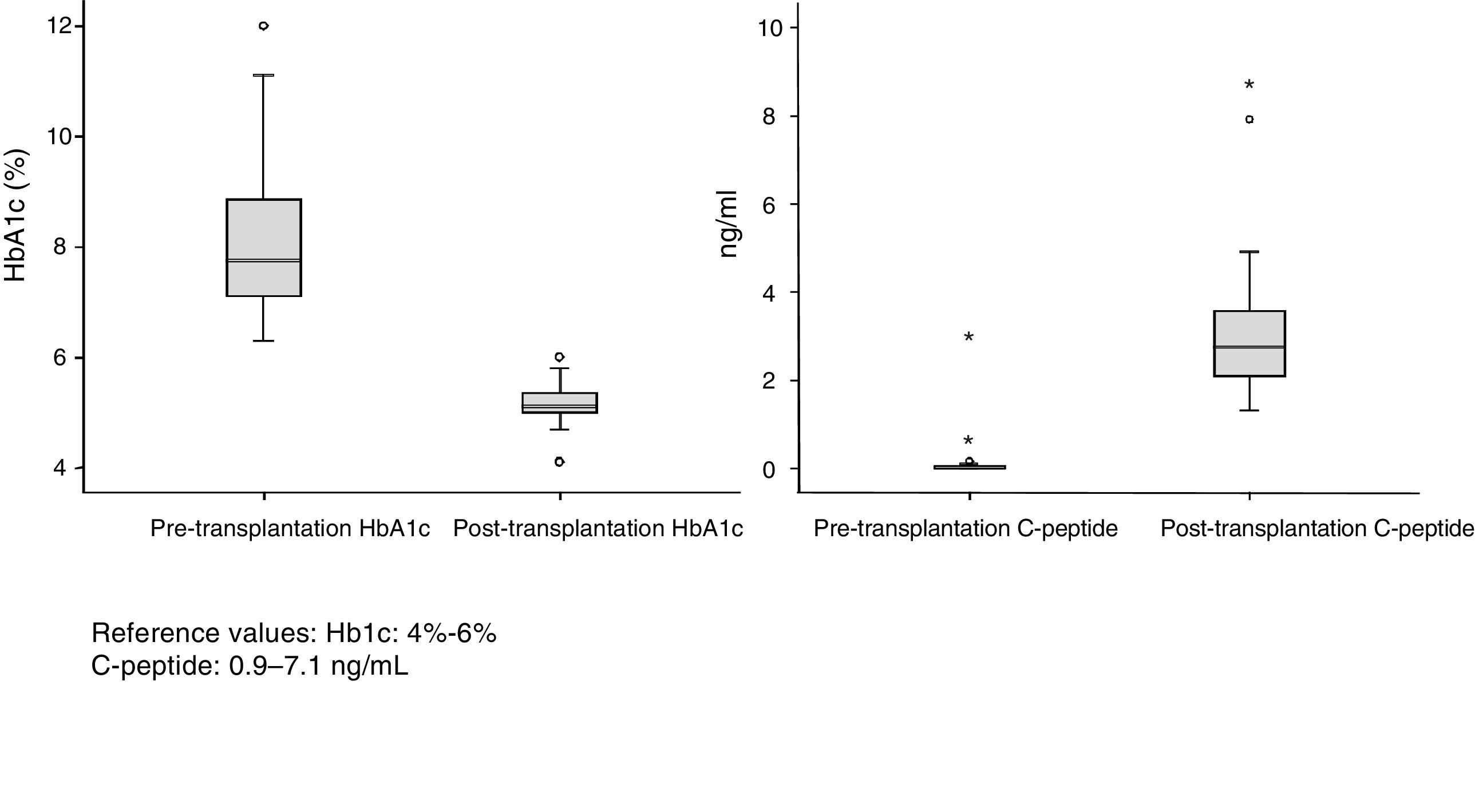
Patient and Graft SurvivalAt the end of the study (May 2016), out of the 53 transplant patients studied, 51 remained alive, 49 of whom conserved complete pancreatic function. Median overall follow-up was 39 months. Patient survival was 98%, 95% and 95% after one, 3 and 5 years, with two deaths, one in the immediate postoperative period due to cardiac arrest and the other, 30 months after the transplant, due to a traffic accident that caused pneumonia that led to death. Graft survival at one, 3 and 5 years was 96%, 93% and 89%, respectively (Fig. 1). The cause of pancreas graft loss was death of the patient with a functioning graft in two cases; the other two losses were due to arterial thrombosis (previously mentioned) and chronic rejection 4 years after transplantation. Fig. 2 shows changes in glycosylated hemoglobin concentrations pre- and post-transplantation, as well as C-peptide behavior.
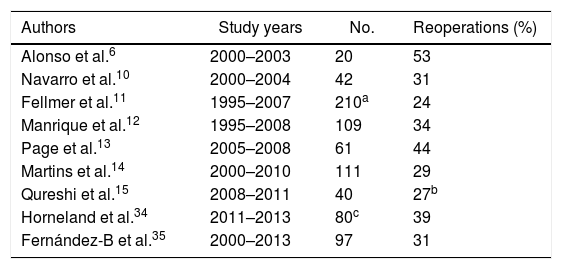
DiscussionThe creation of a pancreas transplant program is often tremendously complex for transplant surgeons. We must overcome the reluctance of nephrologists accustomed to isolated kidney transplantation, with which they have extensive experience. Moreover, the introduction of pancreas transplantation causes certain doubts generated by the high rate of re-operations secondary to pancreatic graft complications reported decades ago. In fact, even today we observe series with re-operation rates related with pancreatic transplantation between 20% and 53% (Table 1) and some hospitals have even reported rates of 83% during their initial period.5
Rate of Reoperations in Different Series of Simultaneous Pancreas–Kidney Transplants With Enteric Drainage.
| Authors | Study years | No. | Reoperations (%) |
|---|---|---|---|
| Alonso et al.6 | 2000–2003 | 20 | 53 |
| Navarro et al.10 | 2000–2004 | 42 | 31 |
| Fellmer et al.11 | 1995–2007 | 210a | 24 |
| Manrique et al.12 | 1995–2008 | 109 | 34 |
| Page et al.13 | 2005–2008 | 61 | 44 |
| Martins et al.14 | 2000–2010 | 111 | 29 |
| Qureshi et al.15 | 2008–2011 | 40 | 27b |
| Horneland et al.34 | 2011–2013 | 80c | 39 |
| Fernández-B et al.35 | 2000–2013 | 97 | 31 |
However, data from the latest international registry for graft loss in the first 90 days after transplantation show a progressive decrease, showing a rate of 6% for patients with SPK transplanted before September 2015.16
Any surgeon who intends to develop a pancreas transplant program must therefore face the reality of a transplant that has an important rate of surgical morbidity, mainly derived from the implantation of an organ with exocrine secretion, when in reality only the endocrine function is needed. Add to this the hopes and expectations of patients and society itself, and we find that the initial development of any transplant program becomes a challenge for the surgical team beyond mere technical reasons. That is why the involvement and complicity of all specialists and the entire hospital are essential. Training of the surgical team and the presence of the senior surgeon in all phases of the transplantation can contribute not only to the standardization of the technique but also to a reduction in complications, as discussed by Sollinger et al.17 Some authors argue that hospital volume also plays a role in the results. With regard to this statement, Mandal et al.3 have shown that medical centers with low pancreas transplant volumes (<10 transplants/year) have worse results in graft survival compared to those with medium (10–20 transplants/year) or high volume (>21 transplants/year). Furthermore, in a recently published study, Alhamad et al.18 confirm that groups with smaller volumes (1–6 transplants/year) have worse pancreatic graft survival rates than those with medium volume (7–13) or high (14–34) volumes. Nonetheless, in their analysis, they found something that could not be explained, which is that a series of groups with low volume had graft survival results that were superior to the hospitals with greater volume. Given these findings, it is suggested that new studies are needed to identify the factors that determine excellent results, regardless of the number of transplants performed. Indeed, our hospital performs a small number of transplants (5–9/year). The results presented here, though, are encouraging and even better than those from hospitals with larger volumes in terms of survival and number of reoperations. This is probably due to the introduction of current immunosuppression regimens, changes in surgical technique, selection of donors and recipients, as well as the fact, not least of which, that it is a recent series.
An analysis of common surgical complications shows that the rate of venous thrombosis is around 5%,19 but when we analyze the series of recent groups, similar to ours, with SKP performed in the last decade, the rates are 10–20%.20–23 Even centers with a large volume of transplantations24 report an incidence of venous thrombosis greater than 9% in transplantations performed since 2008. The possible reasons to explain our absence of cases of venous thrombosis after 53 transplants may be: not using a portal vein extension, opening the cava and removing an oval from it, extreme thoroughness in creating the anastomosis and avoiding making complementary discontinuous sutures once finalized, adequate selection of donors and recipients, and the recentness of the series. Likewise, we believe that avoiding abrupt reperfusion by washing the graft could be a factor to prevent vascular damage. However, our series is short, so this datum should be analyzed with caution. Intra-abdominal hemorrhage continues to be one of the most frequent causes of reoperation, albeit it is in clear decline in recent years with figures between 5% and 11%.12,14,25,26 In our series, there was only one case, and we have followed the practice of the Wisconsin group of no heparinization after reperfusion. We believe that not heparinizing does not increase the risk of thrombosis and, on the contrary, this reduces the possibility of hemorrhage. This fact, along with a meticulous technique both on the bench and in the implant, are able to avoid this complication.
Graft pancreatitis, in its different degrees, is a frequent surgical complication11 that requires reoperation on numerous occasions. In the literature, this complication is difficult to evaluate due to the lack of uniformity in the definition of pancreatitis, manifested in some cases only by the appearance of amylase in the drained fluid, which probably should not be included as it has no clinical relevance and would not be diagnosed if the patient did not have drainage postoperatively. Severe pancreatitis is generally the result of: infectious processes, immunological processes (rejection) or secondary to technical problems, such as graft thrombosis. Complications include: infection, fistulae, abscesses and necrosis, which ultimately lead to the loss of the graft. Multiple factors associated with donors, extraction, ischemia-reperfusion syndrome and the structure of the pancreas itself are considered triggers.
We would like to point out that, in one of our patients, reoperation revealed necrosis of the fat surrounding and even infiltrating the pancreas, which we call graft peripancreatitis. This could have been due to the obesity of the donor and insufficient preparation of the graft on the bench, which led to inadequate perfusion and necrosis of the residual fatty areas. From that moment, we decided to be very strict in accepting obese donors (BMI>30) or those in whom, during extraction, extensive fatty infiltration of the pancreas was observed. Enteric drainage, performed in all the patients in the study, did not seem to influence the rate of technical failures, despite the fact that many publications warn that this technique does not influence graft survival but does cause a higher percentage of technical problems.1,17
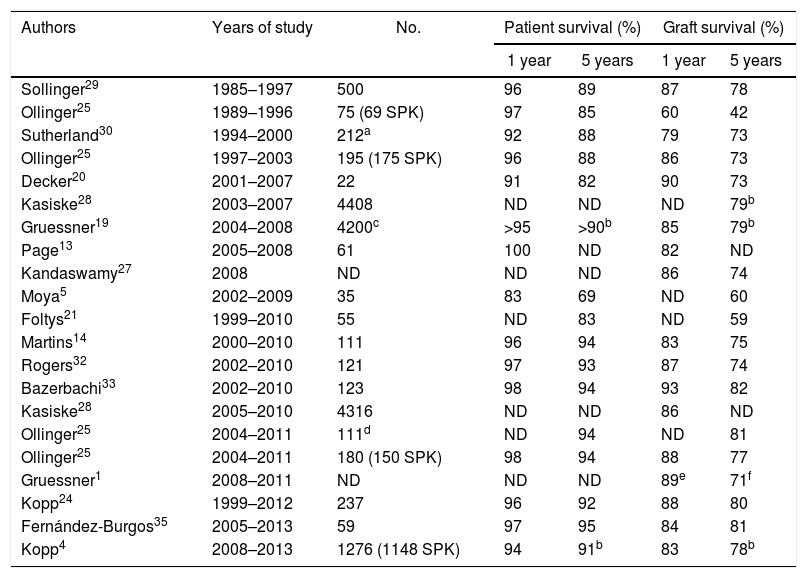
Patient SurvivalThe survival of pancreas–kidney transplant recipients has remained practically stable in recent decades, being greater than 90% after one year and 85% after 5 years. The historical series of large groups or from the international registry (International Pancreas Transplant Registry) referring to the 80s and 90s show survival rates between 92% and 96% after one year and 85%–88% after 5 years (Table 2). Studies published since 2000 show, both in single-center series and in the analysis of the International Pancreas Transplant Registry, survival rates above 95% and 89% after one and 5 years, respectively.16 Our result of 95% survival after 5 years is very satisfactory and concurs with that of large transplant centers.25 In this regard, it does not seem that the possible inexperience or number of procedures is a cause that could affect survival. This contrasts with the analysis of series of emerging groups, similar to that of the present study with transplants performed after the year 2000 or even 2005 using intestinal drainage and the SPK method, which report mortality rates higher than 10% in the first year associated with surgical complications.5,21,22
One- and 5-Year Patient and Graft Survival in Different Series in Simultaneous Pancreas–Kidney Transplantation.
| Authors | Years of study | No. | Patient survival (%) | Graft survival (%) | ||
|---|---|---|---|---|---|---|
| 1 year | 5 years | 1 year | 5 years | |||
| Sollinger29 | 1985–1997 | 500 | 96 | 89 | 87 | 78 |
| Ollinger25 | 1989–1996 | 75 (69 SPK) | 97 | 85 | 60 | 42 |
| Sutherland30 | 1994–2000 | 212a | 92 | 88 | 79 | 73 |
| Ollinger25 | 1997–2003 | 195 (175 SPK) | 96 | 88 | 86 | 73 |
| Decker20 | 2001–2007 | 22 | 91 | 82 | 90 | 73 |
| Kasiske28 | 2003–2007 | 4408 | ND | ND | ND | 79b |
| Gruessner19 | 2004–2008 | 4200c | >95 | >90b | 85 | 79b |
| Page13 | 2005–2008 | 61 | 100 | ND | 82 | ND |
| Kandaswamy27 | 2008 | ND | ND | ND | 86 | 74 |
| Moya5 | 2002–2009 | 35 | 83 | 69 | ND | 60 |
| Foltys21 | 1999–2010 | 55 | ND | 83 | ND | 59 |
| Martins14 | 2000–2010 | 111 | 96 | 94 | 83 | 75 |
| Rogers32 | 2002–2010 | 121 | 97 | 93 | 87 | 74 |
| Bazerbachi33 | 2002–2010 | 123 | 98 | 94 | 93 | 82 |
| Kasiske28 | 2005–2010 | 4316 | ND | ND | 86 | ND |
| Ollinger25 | 2004–2011 | 111d | ND | 94 | ND | 81 |
| Ollinger25 | 2004–2011 | 180 (150 SPK) | 98 | 94 | 88 | 77 |
| Gruessner1 | 2008–2011 | ND | ND | ND | 89e | 71f |
| Kopp24 | 1999–2012 | 237 | 96 | 92 | 88 | 80 |
| Fernández-Burgos35 | 2005–2013 | 59 | 97 | 95 | 84 | 81 |
| Kopp4 | 2008–2013 | 1276 (1148 SPK) | 94 | 91b | 83 | 78b |
ND: no data.
The registry report published in 201527 showed that the mean one-year graft survival was 86%, which concurs with the study published by Kasiske et al.28 analyzing 4316 SPK between 2005 and 2010 with a one-year graft survival of 86%. However, as stated in the report of the latter registry, we must consider graft survival rates with caution as there is no uniformity among groups in the definition of graft failure.16 In this present study, the patients analyzed were type 1 diabetics with end-stage kidney disease who underwent SPK. Currently, we have graft survival rates of 96%, 93% and 89% after one, 3 and 5 years, respectively. The series published with patients transplanted in the 80s and 90s showed graft survival rates between 60% (n=75, 69 SPK)25 and 87% (n=500 SPK)29 after one year of follow-up. Sutherland et al. reported in 212 SPK performed from 1994 to 2000 one-year and 5-year graft survival rates of 79% and 73%, respectively.30
However, in the last decade there has been a significant increase in survival based on technical improvements and changes in immunosuppression. Thus, Ollinger et al.25 analyzed a series of 175 SPK, reaching one-year and 5-year survival rates of 86% and 73%, respectively. In a series of 111 SPK, Martins et al. reported survival rates of 94% and 81% after one and five years of follow-up.14 In the series of patients transplanted after the year 2000, increased pancreatic graft survival was observed in general. This is confirmed by the different analyses of the registry. For example, the study presented in January 2015 and referring to patients transplanted in 2008 reported one- and 5-year survival rates of 86% and 74%, respectively.27 Gruessner et al.19 (a series of 4200 SPK conducted between 2004 and 2008) and Kasiske et al.28 (4316 SPK) presented similar one- and 5-year data at 86% and 80%, respectively.
Series similar to ours, comprised of patients with SPK transplantation, with enteric drainage and performed since 2000, show graft survival rates of 80%–90% after one year and 70%–80% after 5 years.14,20 However, other groups have slightly lower survival rates (60%), mainly due to technical problems5,21 (Table 2). Most grafts are lost in the immediate postoperative period and in the first 3 months after transplantation,16,27 with technical problems and rejection being the most frequent causes.31 The registry shows that the rate of pancreatic graft losses in SPK in the first 90 days after transplantation was 10% in patients transplanted from 2007 to 2008, which decreased to 7% in 2010 and to 6% in 2014–2015.16 In conclusion, the number of annual procedures and possible inexperience do not seem to have had a negative influence on our results. Adequate training, standardization of the technique, homogeneity of the group and the selection criteria for candidates and recipients are factors to be considered. The development of evaluation tools for the results could provide information on the factors that determine variability between groups.
In view of our initial experience, we believe that, with current knowledge and based on the previous experiences of other groups, the development of a pancreas transplant program is safe and offers patients excellent results.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Muñoz-Bellvis L, Esteban MC, Iglesias M, González L, González-Muñoz JI, Muñoz-González C, et al. Desarrollo y resultados de un nuevo programa de trasplante de páncreas en España: la visión del cirujano. Cir Esp. 2018;96:205–212.