Chronic idiopathic anal pain (CIAP) remains a diagnosis of exclusion. Its study and management still lack a standardized protocol. The aim of this study is to evaluate the results obtained with the diagnostic-therapeutic protocol established in our service.
Materials and MethodsWe performed a retrospective study of patients diagnosed with CIAP at the Colorectal Unit of the General University Hospital of Elche, between 2005 and 2011.
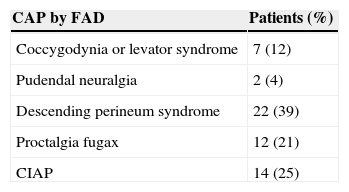
ResultsWe evaluated 57 patients with a diagnosis of chronic anal pain for functional anorectal disease (FAD). After the application of our diagnostic protocol, final diagnosis of chronic anal pain (CAP) was achieved in 43 cases (75%), including 22 cases of descending perineum syndrome, 12 of proctalgia fugax, 2 of pudendal neuritis and 7 of coccydynia. In 14 patients exclusion diagnosis of CIAP was established.
Among the therapies used on patients with CIAP, biofeedback combined with conservative measures improved symptoms in 43% of the cases. Sacral nerve stimulation was assessed in patients who did not respond to other treatments.
ConclusionThrough proper anamnesis, physical examination and complementary tests, a specific diagnosis of the cause of CAP by FAD can be achieved, reducing exclusion diagnosis of CIAP to 25% of cases. Conservative measures combined with biofeedback achieved an improvement in pain in more than 40% of the cases of CIAP in our study. Sacral nerve stimulation can be considered as a treatment option in refractory cases.
En la actualidad, el dolor anal crónico idiopático (DACI) sigue siendo un diagnóstico de exclusión, cuyo estudio y manejo permanece carente de un protocolo estandarizado. El objetivo del presente estudio es evaluar los resultados obtenidos con el protocolo diagnóstico-terapéutico establecido en nuestro servicio.
Material y métodosRealizamos un estudio retrospectivo de los pacientes diagnosticados de DACI en la Unidad de Coloproctología del Hospital General Universitario de Elche entre 2005 y 2011.
ResultadosSe evaluó a 57 pacientes, remitidos con el diagnóstico de dolor anal crónico (DAC) por trastornos funcionales anorrectales (TFAR). Tras la aplicación del protocolo diagnóstico establecido, se llegó a un diagnóstico en 43 casos (75%), incluyendo 22 casos de síndrome del periné descendente, 12 de proctalgia fugax, 2 de neuritis pudenda, 7 de coccigodinia; en 14 casos se realizó un diagnóstico de exclusión de DACI.
Entre las medidas terapéuticas empleadas en los pacientes con DACI, el biofeedback combinado con medidas conservadoras mejoró la sintomatología en el 43% de los casos, valorándose la neuroestimulación de raíces sacras en pacientes resistentes a otros tratamientos.
ConclusiónMediante una protocolizada anamnesis, exploración física y con ayuda de pruebas complementarias pudo especificarse el diagnóstico de DAC por TFAR, reduciéndose el diagnóstico de exclusión de DACI al 25% de los casos. Las medidas conservadoras junto con el biofeedback consiguieron una mejoría de los síntomas en más del 40% de los casos de DACI. En el resto de pacientes debe valorarse de forma individualizada la neuroestimulación de raíces sacras.
The presence of recurrent or chronic anal pain (CAP) at the anal, rectal or pelvic level affects approximately 6.6% of the population, with a high impact on their quality of life.1
There is a lack of clarity in the literature regarding the taxonomy of anal and pelvic pain. CAP is defined within functional anorectal disease (FAD) according to the Rome III2–5 criteria and comprises different diseases, most importantly: coccygodynia or levator syndrome, descending perineum syndrome, pudendal neuralgia and chronic idiopathic anal pain (CIAP).2
The main problem faced in this kind of diseases is their diagnostic difficulty, given that they are characterized by: similar symptoms, frequent coexistence, unknown aetiology and pathogenesis, as well as unpredictable clinical progress and duration. Therefore, a correct differential diagnosis is the basis for a proper diagnosis, which, in the case of CIAP, will be an exclusion one.1,6,7 Moreover, the lack of an effective treatment leads to multiple consultations with different specialists, and treatments are mainly experimental.
The aim of this study is to retrospectively evaluate our experience over the last six years, as a referral coloproctology unit, in the diagnostic-therapeutic management of CAP caused by FAD, with special interest in the diagnostic-therapeutic protocol followed in the patients diagnosed with CIAP.
Patients and MethodsWe conducted a retrospective study of patients who met the Rome III criteria for CAP by FAD, and who were treated at the Coloproctology Unit of the Hospital General Universitario de Elche [Elche University General Hospital], between 2005 and 2011.
All the study patients were treated according to an identical diagnostic protocol. For such purpose, the following diagnostic tests were performed in a sequential way.
A correct anamnesis, investigating comorbidities that could be associated to or justify the clinical picture, was carried out. This was accompanied by a physical examination, including rectal examination, which enabled us to rule out a visible organic disease and confirm the presence of trigger areas or perineal descent.
A rectosigmoidoscopy was requested, for a correct visualization of the anorectal canal, and an endoanal ultrasound, which enabled us to detect non-suspected perianal abscesses, and identify structural-type alterations or abnormalities in the thickness of the internal anal sphincter. Moreover, an anal manometry was performed to measure contraction and relaxation pressures, check the rectoanal inhibitory reflex and perform a rectal volumetry.
Based on the absence of significant findings in the previous tests, a dynamic pelvic nuclear magnetic resonance imaging was performed, which enabled us to rule out structural-type alterations or abnormalities in the thickness of the internal anal sphincter, as well as to assess the degree of perineal descent.
Given the normal results obtained in the previous tests, patients were referred to the Urology and Gynaecology Services to rule out alterations in these systems which could cause pain at the anal level. They were also referred to the Mental Health Unit, where related anxiety-depressive disorders were ruled out.
In case a disease was detected in any of the procedures performed, specific treatment was applied, and these patients were excluded from the study.
The remaining patients were included in the CAP by FAD group, and they were re-examined and interviewed specifically with regard to pain characteristics. Pain was quantified using the visual analogue scale (VAS) and its characteristics were classified according to the specifications set forth by Rome III criteria for FAD.
Patients’ diagnosis was oriented according to pain characteristics towards coccygodynia or levator syndrome, in those patients who had a pain improvement after a posterior digital traction on the puborectalis muscle; towards pudendal neuralgia, in those patients who had a metameric pain distribution, which worsened when sitting down, with positive Tinel's sign and relief after infiltration with lidocaine; towards descending perineum syndrome, in patients who had perineal weakness with an increase in pain after defecation or standing position; and towards proctalgia fugax, in those patients who had recurrent severe pain episodes lasting some seconds or minutes with intervals of pain-free periods. Once the initial diagnostic orientation was performed, patients were further studied based on this orientation for confirmation according to each disease criteria.
If patients lacked pain characteristics typical of each disease according to the Rome III criteria, and had daily nocturnal episodes of continuous severe burning pain in the middle part of the anal canal, with variable radiation, of gradual onset, deep and central, which increased with sitting position (causing a sense of foreign body), and subsided with supine position (generally without interrupting sleeping), they were diagnosed with CIAP.
Therapeutic ProtocolOnce the diagnosis was established, specific treatment was applied for each one of the functional disorders.
In patients diagnosed with CIAP, the following four therapeutic levels were applied:
The first therapeutic level corresponded to the application of conservative measures, among which the following stand out: analgesia, local heat (dry or humid), general tips such as avoiding resting directly on the anal region, oral diazepam for short periods and digital massage on the puborectalis muscle.
Patients who did not show any improvement after the application of the measures corresponding to the first level, continued with the second therapeutic level, which consisted of the application of biofeedback by the personnel of the Coloproctology Unit of our centre.
If patients did not show any improvement after the application of these two therapeutic levels, they were included in the third level, which consisted of their care at the Pain Unit of the referral hospital, where therapies based on the following were applied: electrogalvanic stimulation, repeated block anaesthesia at the sacral nerve level and gabapentin treatment.
Lastly, patients who were resistant to the aforementioned treatments, and after being referred again to our consultation room, were offered the possibility of undergoing sacral root neuromodulation, which accounted for the fourth therapeutic level.
In order to assess and quantify the therapeutic results, pain was quantified by VAS, which was used both in the first consultation and after the end of each therapeutic step.
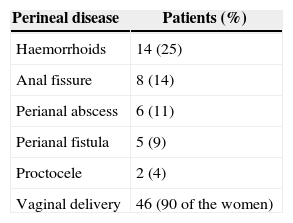
ResultsWe included a total of 57 patients diagnosed with CAP by FAD, according to the Rome III criteria: 51 women (90%) and 6 men (10%) with a mean age of 68 years, with an age range from 14 to 88 years. The mean duration of symptoms before making a consultation at our centre was 36 months (between 2 and 48 months). Mean follow-up time was 48 months (between 6 and 72 months).
Sixty-one percent (61%) of patients had a prior surgical history related to the pelvic area. Perineal disease distribution is shown in Table 1.
After normal complementary tests, the differential diagnosis for CAP by FAD was performed according to the pain characteristics, which was quantified through the VAS. Distribution of patients according to their diagnosis is shown in Table 2.
In 14 patients (25%) pain did not follow any typical pattern and they were diagnosed with CIAP. The characteristics of these patients are shown in Table 3.
Characteristics of Patients Diagnosed With Chronic Idiopathic Anal Pain.
| Characteristics | |
| Age | 70.1 (range: 18–88) |
| Gender | 12 women/2 men |
| Mean symptom onset time (months) | 39 (4–48) |
| Mean follow-up time (months) | 49 (7–72) |
| Initial VAS | 6.2 (5–9) |
| VAS after the first and second therapeutic steps | 4.2 (1–8)1.6 (1–3) among patients who showed an improvement |
| VAS after the third therapeutic step | 3.8 (1–7)1.5 (1–2) among patients who showed an improvement |
| VAS after the fourth therapeutic step | 1.6 (1–2) |
Out of these patients, six had a significant improvement after the application of conservative measures and biofeedback. The remaining eight patients were referred to the Pain Unit of their referral hospital; four of them improved with the treatments received. The four patients who were resistant to treatment with electrogalvanic stimulation, repeated block anaesthesia at the sacral nerve level and gabapentin were referred again to our consultation room, where the possibility of carrying out a sacral root neuromodulation and its procedure were explained to them, which was finally performed in three of these patients; this showed a clear improvement in two of them, and an initial improvement that subsequently worsened in the third case. The fourth patient refused to have further treatments applied.
DiscussionThe literature is not clear about the taxonomy of anal and pelvic pain. The term CAP by FAD is defined according to the Rome III criteria, as a long-course pain or at least 20-min. course recurrent pain episodes at the anal, perianal and perineal level, present for the last three months and at least six months before the diagnosis. When a structural or inflammatory cause that justifies the symptoms is not detected and it does not follow certain clinical patterns, the diagnosis of exclusion of CIAP is established.2–5
The diagnostic difficulty of CAP by FAD lies not only in the absence of a complementary test that provides an objective diagnosis but also in the lack of consensus regarding its pathophysiology and the frequent association with other functional, organic and psychological disorders.5–8
In relation to the diagnostic protocol to be followed in patients with CIAP, since its diagnosis is an exclusion one, a correct differential diagnosis is crucial, but it is too broad and at present it is not well standardized. In a recent study conducted by Chiarioni et al.9 the diagnostic assessment proposed is based on the following: rectal examination, colonoscopy, ultrasound and assessment both by the surgeon and the urologist and gynaecologist, in case of findings that lead to a suspected associated disease at that level. This is similar to the diagnostic protocol applied by our group, where if all the complementary tests are normal and pain does not follow any pattern characteristic of the other FAD, the exclusion diagnosis of CIAP is established.
In the assessment of anal pain, one of the complementary tests that provide more information is endoanal ultrasound. A study conducted by Pascual et al.10 where an endoanal ultrasound is performed to assess patients with spontaneous and postoperative proctalgia, proves that it is an effective test, given that it allows finding an ultrasonographic cause of pain in 81.93% of cases; isolated internal anal sphincter hypertrophy is the most frequently associated ultrasound finding, as stated in other papers.7,11 The presence of incomplete sphincterotomy is the most common finding after anal fissure surgery, which may benefit from an internal lateral sphincterotomy in these cases. Vieira et al.12 also consider it is a necessary test, showing ultrasound findings in 22% of patients, as well as occult organic lesions in the clinical examination in more than half of the cases. We agree with this opinion; for that reason, the endoanal ultrasound is the first complementary test that we request in patients with proctalgia after the proper examination and visualization of the anal canal.
Manometry is supported by some authors who suggest that an increase in anal pressure is observed in patients with anal pain; this would be reflected in an increase in the sphincter tone, which could be related to both hyperalgesia and pelvic floor dysfunction.13–15
Moreover, a greater association with neurotic disorders, depression, anxiety and psychosis was observed compared to the general population.6,16 This makes a psychological assessment necessary, as stated in our protocol, although we should remember that, even though a psychological condition could be the cause of CAP by FAD, it does not rule out a concomitant organic disease.6,17
With regards to treatment for CAP by FAD, in those cases where a certainty diagnosis is achieved, treatment should be specific for each one of the conditions. However, for CIAP, since its pathophysiology is mostly unknown, management is still empirical. In fact, it is aimed at achieving pelvic floor muscle relaxation, based on its potential relation to an increase in muscle tension.1,2,18 That is the reason why our first therapeutic step is focused on the application of conservative measures.
Based on this theory on the pathophysiology of CIAP, Chiaroni9 compared the three most frequently used treatments (biofeedback, electrogalvanic stimulation and massage on the puborectal muscle), showing improvement in 87% of cases with biofeedback, 45% following electrogalvanic stimulation and 22% following massage; this improvement was maintained for 12 months of follow-up. Moreover, such study shows that pain improvement after posterior [digital] traction on the puborectal muscle is a good predictor of the effectiveness of biofeedback, and is especially useful in the levator syndrome of the anus, but also in CIAP. In previous studies, this improvement was also observed.1,2,19 We have seen an improvement through VAS in up to 42% of our patients following the application of conservative measures and biofeedback; this improvement remained for 2–4 years of follow-up.
Since the use of surgical techniques in this kind of patients, far from solving the problem, adds more iatrogenesis, and usually major complications and even subsequent pain,1,16,20 we agree with other groups that the use of surgical techniques should be avoided. This is the reason why we choose sacral root neuromodulation as the last therapeutic level in patients who were resistant to the previous measures. An improvement in the visual scale for pain and quality of life, as well as in the long-term follow-up of patients with CIAP, by using this technique, has been observed in some studies,21,22 although Dudding et al., suggest the contrary in a recent study.23 However, considering the small sample size and the lack of randomized studies on this topic, we think that before adopting more intensive surgical measures, this treatment should be considered in patients with CIAP who have not had any improvement with the previous treatments.
Nevertheless, our results are based on 14 patients only, which is the main limitation given the small sample size. We consider that it is important to conduct a protocolised and uniform study in this kind of patients to achieve an objective and homogeneous assessment by the different Departments, in order to draw generalizable conclusions that contribute to the study and management of this disease, based more on solid criteria and less on experimental-type procedures. For such purpose, to confirm these results, it is necessary to conduct more studies with a higher number of patients in the future.
ConclusionThe application of the diagnostic protocol developed in our Department enabled a reduction in the exclusion diagnosis of CIAP to 25%. Conservative measures together with biofeedback achieved an improvement of symptoms in more than 40% of CIAP cases; the application of sacral root neuromodulation should be assessed on an individual basis in patients who were resistant to other therapeutic measures. However, considering the complexity and low frequency of this kind of disease diagnosis, more research on this topic will be necessary to progress in the adequate management of these patients.
Conflicts of InterestThe authors declare that they do not have any conflicts of interest.
Please cite this article as: Armañanzas L, Arroyo A, Ruiz-Tovar J, López A, Santos J, Moya P, et al. Dolor anal crónico idiopático. Evaluación de resultados diagnóstico-terapéuticos en una unidad de referencia de coloproctología. Cir Esp. 2015;93:34–38.