The prosthetic joint infection is the most feared and catastrophic complication for cause severe physical damage to patients and, generates high economic costs.
ObjectivesTo describe the microbiological characteristics and to determine the resistance pattern in prosthetic joint infections in a reference hospital in Mexico.
Material and methodsPatients whose prosthetic devices were withdrawn due to suspicion of septic and aseptic loosening were included. Cultures were performed to identify microorganisms and susceptibility analysis.
ResultsOf the 111 patients included, 55% were diagnosed with prosthetic joint infection, with the most frequent prosthesis being of the hip (43%). Positive cultures were obtained in 97% of the infected cases, of which 75% were monomicrobial infections. The most frequent bacterial species isolated were: Staphylococcus epidermidis (31%), Enterococcus faecalis (16%), Staphylococcus aureus (13%), and Escherichia coli (8%). The resistance patterns for the Staphylococcus genus were: oxacillin (79%), erythromycin (45%) and ciprofloxacin (37%). Enterococcus faecalis showed a high percentage of resistance to erythromycin and clindamycin (86%), and fluoroquinolones (43%). The large majority (86%) of Escherichia coli were extended spectrum beta-lactamases positive, in addition to having high resistance to fluoroquinolones (86%), trimethoprim/sulfamethoxazole (86%) and gentamicin (72%).
ConclusionThe microbiological characteristics found in prosthetic joint infections vary according to the hospitals. In this series, a high proportion of coagulase-negative Staphylococci and Enterococcus spp. were found, as well as a high bacterial resistance.
La infección de prótesis articular es la complicación más temida y catastrófica, por causar severos daños físicos en los pacientes, y generar elevados costos económicos.
ObjetivosDescribir las características microbiológicas y determinar los patrones de resistencia que se presentan en infecciones de prótesis articular en un hospital de referencia en México.
Material y métodosSe incluyeron pacientes a los que se les retiró la prótesis articular por sospecha de aflojamiento aséptico y séptico. Se hizo búsqueda microbiológica y análisis de susceptibilidad.
ResultadosSe incluyeron 111 pacientes, el 55% se diagnosticaron con infección de prótesis articular, siendo la más frecuente la prótesis de cadera (43%). En el 97% de los casos infectados se tuvieron cultivos positivos, el 75% fueron infecciones monomicrobianas. Las especies bacterianas aisladas con mayor frecuencia fueron: Staphylococcus epidermidis (31%), Enterococcus faecalis (16%), Staphylococcus aureus (13%) y Escherichia coli (8%). El patrón de resistencia en las 2 primeras fue: oxacilina (79%), eritromicina (45%) y, ciprofloxacino (37%). Enterococcus faecalis mostró alto porcentaje de resistencia para: eritromicina y clindamicina (86%), y fluoroquinolonas (43%). El 86% de las Escherichia coli tenían betalactamasas de espectro extendido, además de alta resistencia para fluoroquinolonas (86%), trimetoprim/sulfametoxazol (86%) y gentamicina (72%).
ConclusiónLas características microbiológicas encontradas en infecciones de prótesis articular varía de acuerdo a los centros hospitalarios; en esta serie se encontró una proporción alta de Staphylococcus coagulasa negativos y Enterococcus spp., así como una alta resistencia bacteriana.
The implantation of prosthetic joints is a therapeutic option used to improve the mobility and quality of life of those patients who suffer from joint wear and tear1; however, in a small number of cases, fitting the prosthetic material may lead to complications which affect the patient and the surgical outcome. The most common complications associated with arthoplasties are the aseptic loosening of the joint and prosthetic joint infection (PJI), the latter being the most serious catastrophic occurrence since it usually causes irreversible physical sequelae with high economic costs, due to prolonged administration of antimicrobial treatments and constant hospital stays.2,3
In general the most common aetiological agents in prosthetic joint infections are of the genus Staphylococcus, the most common of which is Staphylococcus aureus (S. aureus)4; however, it has been observed that distribution changes, depending on geographical location or hospital centre. For example, Bejon et al.5 described how in the orthopaedic centre in Oxford, United Kingdom, the most frequent species were of the negative coagulase Staphylococci. There are therefore differences between microorganism distribution and also between the patterns of antimicrobial resistence.6,7
In Mexico up to the present day, studies published on prosthetic joint infections do not offer a detailed description of microbiological characteristics and antimicrobial resistance patterns8–10 and it is thus of the utmost importance to gain knowledge of the microbial epidemiology and antimicrobial susceptibility of this type of infection to establish preventative guidelines and optimise empirical antimicrobial treatments to use in the prevention of infections related to prosthetic joints.
The aim of this study was to describe the microbiological characteristics and determine the antimicrobial resistance patterns in prosthetic joint infections which presented or had been referred to the National Rehabilitation Institute – the largest Health Department's referral hospital in Mexico – which specialises in treating musculoskeletal pathologies and in carrying out primary and revision arthroplasties.
Materials and methodsWe conducted an observational, cross-sectional, descriptive study from 20 November 2011 to 23 November 2013. All patients who had had a prosthetic joint removed due to aseptic or septic loosening were included in the study. At least 3 biopsies of periprosthetic tissue for microbiological examination had also been performed.
Definition of prosthetic joint infectionProsthetic joint infection was defined according to when patients met the following criteria:
- 1.
Two positive cultures of periprosthetic tissue or a positive culture of the prosthetic joint, with phenotypically identical microorganisms.
- 2.
Formation of a fistula in the prosthetic joint.
- 3.
Meeting 3 of the following lesser criteria: (a). Elevated serum C-reactive protein levels and globular sedimentation speed. (b) High white blood cell count in synovial fluid. (c) High percentage of polymorphonuclear neutrophils in synovial liquid. (d) Positive histological analysis of the periprosthetic tissue. (e) A positive culture.
The periprosthetic tissues were prepared for transportation and immediately taken to the laboratory. They were then macerated and homogenised in 2ml of sterile saline solution (0.85%). Alliquots of 0.1ml were taken from the maceration solution and plated in the following culture mediums: 5% sheep blood agar, MacConkey agar, phenyl alcohol agar, Sabouraud agar with antibiotic and thioglycolate broth. Culture conditions and times used were as follows: aerobic microorganisms: 37°C, 7 days; fungal microorganisms: room temperature, 30 days: anaerobic microorganisms: 37°C, 2 days.
Prosthetic Joint CultureThe prosthetic joint culture was performed using the sonication technique briefly described by Trampuz et al.4 The prosthetic joint was placed in a sterile polypropylene recipient. 400ml of saline solution (0.85%) was then added to the recipient. This was immediately place in an ultrasonic cleaner (BRANSON 3510, U.S.A.) at 40kHz for 5min. Once the time was up, aliquots of 0.1ml were taken from the sonication liquid, which were then plated and cultivated under the same conditions as the periprosthetic tissues.
Identification and microorganisms susceptibility trialsDeveloped cultures were identified and susceptibility testing took place with the semiautomatic Vitek 2 (BioMériux, France) equipment, in accordance with the manufacturer's recommendations.
Descriptive statistics (average, median and frequencies) using Stata 12.0 software was used for data analysis.
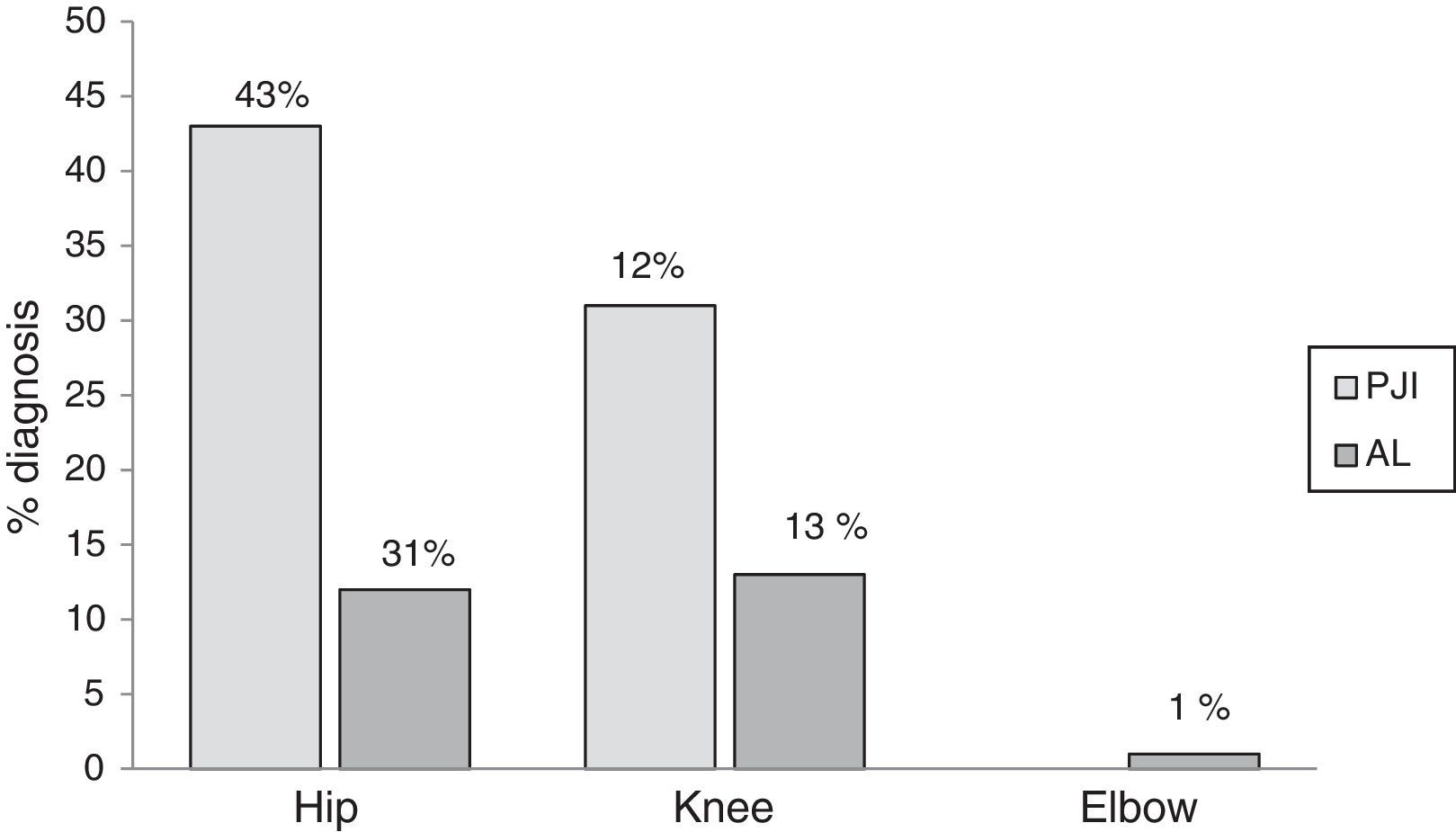
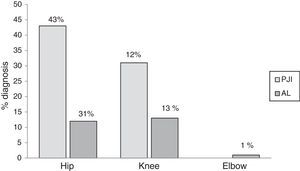
ResultsThe study included 111 patients who had had their prostheses removed. Seventy five (68%) patients were female and the median age was 64 (19–91). The following types of prosthetic joints were removed: hip, 83 (75%); knee, 27 (24%); and elbow, 1 (1%). An average of 3 periprosthetic tissue samples were cultivated for each patient (ranging from 3 to 6 samples). Of the patients included 62 (55%) were diagnosed as prosthetic joint infection, the hip prosthesis was the most infected joint (Fig. 1).
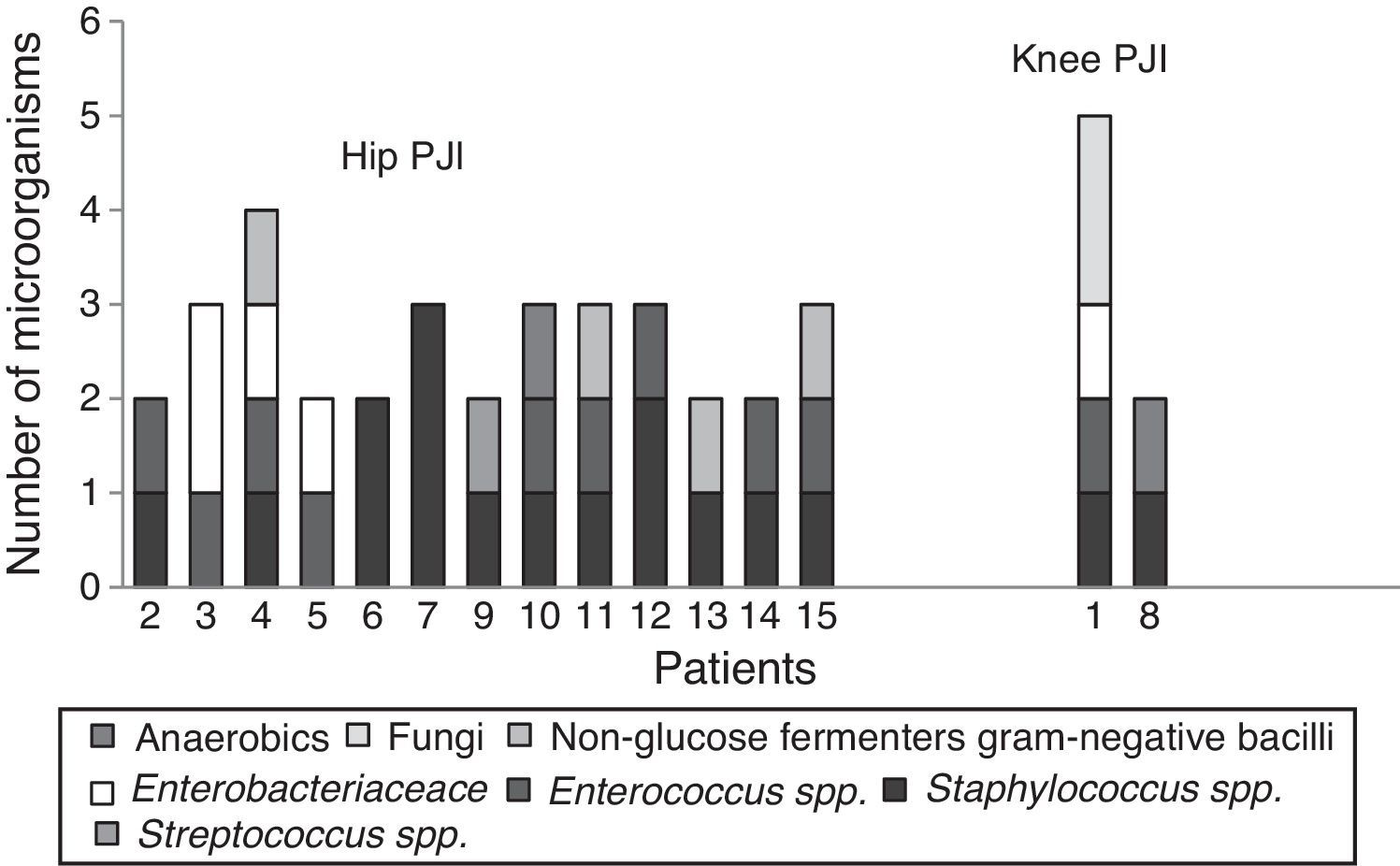
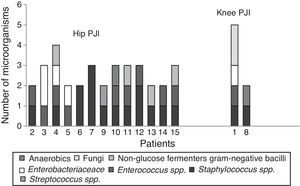
Positive cultures were obtained in 59 (97%) patients with prosthetic joint infection; the 3 remaining patients were clinically diagnosed, due to fistula presence. Forty four (75%) infections were monomicrobial and 15 (25%) were polymicrobial. Of the microorganisms isolated, 70 (80%) were Gram-positive, 14 (16%) were Gram-negative and 3 (4%) were fungal microorganisms (Table 1). The bacterial species isolated with greater frequency were firstly Staphylococcus epidermidis (S. epidermidis), followed by Enterococcus faecalis (E. faecalis), S. aureus and Escherichia coli (E. coli), at 27 (31%), 14 (16%), 11 (13%) and 7 (8%), respectively. In the polymicrobial PJI, the average of isolated microorganisms was 3 species (ranging from 2 to 4 species). Fig. 2 describes the microbiological characteristics of this type of infection.
Distribution of microorganisms isolated in positive cultures.
| Microorganism | n (%) |
|---|---|
| Gram-positive | 70 (80) |
| Staphylococcus spp. | |
| S. epidermidis | 27 (31) |
| S. aureus | 11 (13) |
| S. hominis | 5 (6) |
| S. lugdunensis | 1 (1) |
| S. caprae | 1 (1) |
| S. sciuri | 1 (1) |
| S. lentus | 1 (1) |
| Enterococcus spp. | |
| E. faecalis | 14 (16) |
| E. faecium | 1 (1) |
| E. casseliflavus | 1 (1) |
| Streptococcus spp. | |
| St. anginosus | 2 (2) |
| St. salivarius | 1 (1) |
| St. agalactiae | 1 (1) |
| Others | |
| Gemella sp. | 1 (1) |
| Corynebacterium striatum | 1 (1) |
| Parvimonas micra | 1 (1) |
| Gram-negative | 14 (16) |
| Enterobacteriaceae | |
| Escherichia coli | 7 (8) |
| Salmonella spp. | 1 (1) |
| Klebsiella pneumoniae | 1 (1) |
| Pantoea agglomerans | 1 (1) |
| NGFGRB | |
| Acinetobacter baumannii | 2 (2) |
| Pseudomonas flourescens | 2 (2) |
| Fungi | 3 (4) |
| Candida lipolytica | 1 (1) |
| Candida parapsilosis | 1 (1) |
| Rhodotorula mucilaginosa | 1 (1) |
NGFGRB: non-glucose fermenters Gram-negative bacilli.
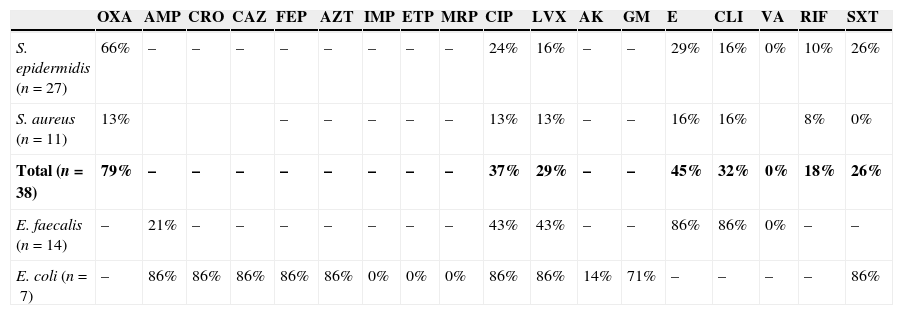
Table 2 shows the percentage of resistance to diverse antibiotics, in the 4 species of microorganisms which were isolated with greater frequency. A high antimicrobial resistance to oxacillin (79%), erythromycin (45%), ciprofloxacin (37%) and clindamycin (32%) was found in S. epidermidis and S. aureus. E. faecalis showed resistance to erythromycin (86%), clindamycin (86%), ciprofloxacin (43%) and levofloxacin (43%). None of the Gram-positive cocci were resistant to vancomycin. 86% of the isolated E. coli strains were positive broad spectrum beta lactamases; they also presented resistance to ciprofloxacin (86%), levofloxacin (86%), trimetoprim/sulfamethoxazole (86%) and gentamicin (71%).
Resistance pattern to different antibiotics of the 4 microorganisms isolated with greater frequency.
| OXA | AMP | CRO | CAZ | FEP | AZT | IMP | ETP | MRP | CIP | LVX | AK | GM | E | CLI | VA | RIF | SXT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. epidermidis (n=27) | 66% | – | – | – | – | – | – | – | – | 24% | 16% | – | – | 29% | 16% | 0% | 10% | 26% |
| S. aureus (n=11) | 13% | – | – | – | – | – | 13% | 13% | – | – | 16% | 16% | 8% | 0% | ||||
| Total (n=38) | 79% | – | – | – | – | – | – | – | – | 37% | 29% | – | – | 45% | 32% | 0% | 18% | 26% |
| E. faecalis (n=14) | – | 21% | – | – | – | – | – | – | – | 43% | 43% | – | – | 86% | 86% | 0% | – | – |
| E. coli (n=7) | – | 86% | 86% | 86% | 86% | 86% | 0% | 0% | 0% | 86% | 86% | 14% | 71% | – | – | – | – | 86% |
AK: amikacin; AMP: ampicillin; AZT: aztreonam; CAZ: ceftazidime; CIP: ciprofloxacin; CLI: clindamycin; CRO: ceftriaxone; E: erythromycin; ETP: ertapenem; FEP: cefepime; GM: gentamicin; IMP: imipenem; LVX: levofloxacin; MRP: meropenem; OXA: oxacillin; RIF: rifampicin; SXT: trimethoprim/sulfamethoxazole; VA: vancomycin.
The global results of sensitivity to the Staphylococcus (S. epidermidis and S. aureus) are in bold.
Over the last few decades joint replacement has become one of the most frequent surgical procedures in industrialised countries.11 This surgery improves the mobility and quality of life of patients suffering from joint wear and tear; however, several cases present complications which may affect patient and surgical outcome. Aseptic loosening and infection are among the most common complications which usually present after a joint replacement procedure.12 In a study published by Zhang et al.13 the authors state that out of 10,000 hip and knee replacements performed in Australia in 2013, 13% were revision arthroplasties caused by problems with the prosthetic joint and of these, 29% were due to aseptic loosenings and 20% to prosthetic joint infection. Allepuz et al.1 calculated that of the 6027 revision arthroplasty replacements performed in 2 referral hospitals in Spain in 1 year, 13% were caused by aseptic loosening and 17% by prosthetic joint infection. The number of aseptic loosenings and prosthetic joint infection we found were much higher than the above-mentioned figures, with the latter being the most frequent, since a little over half of patients whose prosthetic joint was removed presented with infection. Prosthetic joint infection is one of the most serious and catastrophic complications, because in the majority of cases there are usually irreversible physical scars; treatment also generates high economic costs to the health system.14
The most common causes of the development of PJIs are direct contamination of the prosthetic material during surgery caused by: skin bacteria from medical personnel or the patient him/herself; by haematogenous dissemination when the bacteria comes from a different place in the body and through loss of continuity in the skin or when there are infectious processes in the surrounding soft tissues of the prosthetic joint.15,16
The main microorganisms which cause this type of infection are reported to be: firstly Gram positive cocci (70%), followed by Gram-negative bacilli (20%) and under 1% of fungal microorganisms.17,18 Of the first group, the most frequent species are: S. aureus (45%), S. epidermidis (36%) and Enterococcus spp. (10%).19,20 In this study similar percentages of Gram-positive cocci were isolated, but by species we observed that S. epidermidis and Enterococcus spp. were the most frequent; the latter were even higher in number than the S. aureus, which is one of the most important microorganisms in prosthetic joint infection21. The Enterococcus spp. species cause several types of infections, mainly nosocomial infections, endocarditis, urinary tract infections and intra-abdominal and pelvic infections.22 Although it is not a frequent pathogen for orthopaedic infections, there is a large number of reports which show an increase in orthopaedic infections caused by Enterococcus spp.23 Tornero et al.24 conducted a multicentric study to describe the clinical and microbiological characteristics of prosthetic joint infection by Enterococcus spp. The study shows that approximately 9.3% of prosthetic joint infection are caused by these microorganisms, with E. faecalis and Enterococcus faecium being the species which are most isolated, mainly in polymicrobial polymicrobial prosthetic joint infection. As previously mentioned, we found a large number of prosthetic joint infection were caused by Enterococcus spp. and the species we isolated the most were the same as those reported by Tornero et al., in addition to polymicrobial infections having been isolated (69%). In the polymicrobial prosthetic joint infection by Enterococcus spp., we observed that these microorganisms were together with: Staphylococcus spp., Enterobacteriacea and Pseudomonas aeruginosa (P. aeruginosa). Another characteristic observed was that the Enterococcus spp. were isolated mainly in prosthetic hip joints (81%). This may be associated with poor pre-surgical hygiene or proximity to the genital area.
Regarding Gram-negative bacilli in prosthetic joint infection, epidemiologic studies such as that of Rodríguez Pardo and his workgroup25 describe that the most frequent causes of this type of infections are: Enterobacteriaceae (78%), P. aeruginosa (20%) and other Gram-negative bacilli (2%). Of the former, E. coli and Enterobacter spp. are the most common. Zmistowski et al.26 published a similar study and stated that in the majority of patients who presented with prosthetic joint infection due to Gram-negative bacilli primary urinary infections were involved. In our study we isolated this same type of Gram-negative bacteria. However, it was of note that in one case Salmonella spp. was isolated. Prosthetic joint infections due to Salmonella spp. are rare and generally associated with haematogenous contamination, when patients present with gastrointestinal tract infection.27 We were unable to discover the origin of the prosthetic joint infection due to Salmonella spp. as we did not have sufficient clinical patient data.
Prosthetic joint infections are generally due to bacteria and in very few cases involve fungal microorganisms. However, in patients with chronic prosthetic joint infection who have been administered antimicrobial therapy for some time, the risk of the prosthetic joint becoming infected by fungal microorganisms increases.28 In keeping with the frequency of fungal microorganisms in prosthetic joint infection its isolation has been reported in under 1% of cases, Candida spp. being the most common genus and, by species, C. albicans.29,30 The percentage of fungal microorganisms we isolated was high compared with that published previously; of all the species we cultivated, the majority were different from C. albicans.
Anaerobic bacteria are another of the microorganisms of increased relevance in prosthetic joint infection over recent years. They are difficult to isolate and identify because they require specialised culture mediums and lengthy incubation.31 Automated blood culture systems have provided a solution to this problem. Minassian et al.32 use the BD BACTEC™ system as a diagnostic prosthetic joint infection tool; in their study the authors recuperated a large number of anaerobic bacteria through this system, mainly Propionibacterium spp., a bacteria which is isolated in a low percentage and recognised as the cause of prosthetic joint infection.33 We isolated a very small number of anaerobic bacteria, despite having optimised the culture conditions to isolate them, and we used highly specialised culture mediums (thioglycolate broth and phenyl alcohol agar). To increase recuperation of this type of microorganisms it was suggested that molecular biology techniques be used, such as the amplification of the 16S RNA gene, which has proven effective in the diagnosis of prosthetic joint infection.34
Indiscriminate use of antibiotics has led to high percentages of antimicrobial resistance in bacteria.35 This high resistance is the main cause of prophylactic antimicrobial therapies failing to prevent prosthetic joint infection.36 In a multicentre study published by Tornero et al.37 on resistance in isolated S. aureus and S. epidermidis of prosthetic joint infection between 1999 and 2009, it was found that approximately 9% of the S. aureus were resistant to meticillin, whilst resistance to these same antibiotics in S. epidermidis was 60%; resistance to fluoroquinolones in these bacteria was 16% and 35%, respectively. In our study we observed much higher resistance percentages for fluoroquinolones in S. aureus and S. epidermidis. In a similar study, Martínez Pastor et al.38 described the resistance in Gram-negative bacilli, mainly those of E. coli, which were prosthetic joint infection isolated between 2000 and 2007, in the orthopaedic hospital of Spain; in their results these authors mention that 85% of the E. coli they isolated from prosthetic joint infection were producers of broad spectrum beta lactamases and 50% were resistant to fluoroquinolones. The percentage of E. coli strains which were producers of broad spectrum beta lactamases which we found were similar to those documented by Martínez Pastor et al.38 Notwithstanding, resistance to fluoroquinolones was much higher. Resistance to ampicillin in the E. faecalis we isolated was not as high (21%), which is striking, as in recent years it has been observed that clinical strains of E. faecalis isolated in different types of infections show a diminished susceptibility to this antibiotic, and the use of broader spectrum drugs have therefore been required.39 In general the differences in resistance patterns between the different microorganisms isolated in prosthetic joint infection are due to the clonal selection by selective pressure, which is the product of indiscriminate use of antibiotics and to the type of antimicrobial therapies used in different geographical areas and different hospitals.40
Lastly, the greatest contribution of this study is the detailed analysis of the microbiological characteristics and antimicrobial resistance patterns which present in prosthetic joint infection. These results are exponential since the National Institute of Rehabilitation is one of the most important referral centres in Mexico, and these same characteristics may be presented in much smaller centres. A major factor of current consideration is to analyse whether the prophylactic antimicrobial therapies used in Mexico to prevent prosthetic joint infection are effective, since we find much greater antimicrobial resistance in microorganisms that cause this type of infections.
ConclusionThe microbiological characteristics in prosthetic joint infection vary depending on the hospitals; in this series we found a higher proportion of coagulase-negative Staphylococci and Enterococcus spp., and also a high antimicrobial resistance.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Ortega-Peña S, Colín-Castro C, Hernández-Duran M, López-Jácome E, Franco-Cendejas R. Características microbiológicas y patrones de resistencia en infecciones de prótesis articular en un hospital de referencia. Cir Cir. 2015;83:371–377.