Acute type A aortic dissection (AAD) is a life-threatening emergency in cardiac surgery, with high in-hospital (22%) and long-term morbidity and mortality rates.
MethodsData from 134 patients were collected from anamnesis and computed tomography before and after aortic surgery. Data were analyzed using IBM SPSS v23.
ResultsWe found that male patients had better survival than the female (p<0.01) and that age is associated with increased perioperative death (p<0.001). Similarly, patients admitted in shock (p<0.001) and undergoing longer extracorporeal circulation (p<0.05) were associated with lower survival. We observed that patients admitted with partial thrombosis of false lumen had increased survival compared to those presenting a patent lumen (p<0.001). Also, we compared the mortality of the different surgical procedures and found that no intervention had a significant association with increased survival (p>0.05).
Among survived patients, we found that an increased pre-operative ascending aorta diameter is associated with the establishment of a chronic partial thrombosis (54.2±4.6 vs 50.5±7.0mm, p<0.05). Moreover, increased pre-operative descending aorta diameter is associated with a longer complete thrombosis time (29.2±6.1 vs 37.2±6.9mm, p<0.05).
ConclusionsWe found that, in our experience, there is no significant difference between the most commonly used surgical procedures. However, we found that age, gender, shock, pre-operative lumen patency and CEC time are important predictors of survival. Moreover, we found that pre-operative aortic diameters are dramatically relevant to determine the time for a complete thrombosis of the false lumen.
La disección aórtica aguda tipo A (DAA) es una emergencia potencialmente mortal en cirugía cardíaca, con altas tasas de morbilidad y mortalidad intrahospitalarias (22%) y a largo plazo.
MétodosSe obtuvieron datos de 134 pacientes antes y después de la cirugía de la aorta incluida anamnesis y tomografía computarizada. Los datos se analizaron con IBM SPSS v23.
ResultadosEncontramos que los pacientes varones tuvieron una supervivencia superior a las mujeres (p < 0,01) y que la edad se asocia con un aumento de la muerte perioperatoria (p < 0,001). De manera similar, los pacientes ingresados en estado de shock (p < 0,001) y sometidos a una circulación extracorpórea más prolongada (p < 0,05) se asociaron con una menor supervivencia. Observamos que los pacientes ingresados con trombosis parcial de falsa luz tenían una mayor supervivencia en comparación con los que presentaban una falsa luz permeable (p < 0,001). Además, comparamos la mortalidad de los diferentes procedimientos quirúrgicos y encontramos que ninguna intervención tuvo una asociación significativa con una mayor supervivencia (p > 0,05). Entre los pacientes supervivientes, el diámetro de la aorta ascendente preoperatoria se asocia con la trombosis parcial crónica (54,2 ± 4,6 frente a 50,5 ± 7,0 mm, p < 0,05). Además, el diámetro de la aorta descendente preoperatoria se asocia con un tiempo de trombosis completa más prolongado (29,2 ± 6,1 frente a 37,2 ± 6,9 mm, p < 0,05).
ConclusionesEn nuestra experiencia, no existe una diferencia significativa entre los procedimientos quirúrgicos más utilizados. Sin embargo, encontramos que la edad, el sexo, el estado de shock, la permeabilidad de la falsa luz preoperatoria y el tiempo de CEC son importantes predictores de supervivencia. Además, los diámetros aórticos preoperatorios son relevantes para determinar el tiempo para llegar a una trombosis completa de la falsa luz.
Acute type A aortic dissection (ATAAD) is a life-threatening emergency in cardiac surgery, with high in-hospital (22%) and long-term morbidity and mortality rates (survival 96.1% and 90.5% after one and three years of hospital discharge, respectively).1 At the state of the art, conservative operative procedures intended to replace the ascending aorta and eliminate the main intimal tear are preferred by an increasing number of surgeons. Nevertheless, some authors showed that a more radical and extensive operation, such as total arch replacement, improved the long-term outcome by decreasing the incidence of residual patent false lumen without an increase of operative morbidity and mortality.2,3 A post-operative residual false lumen in the distal aorta may progress in a partial or total thrombosis or become chronic.4 Residual patent false lumen was recently reported as a potential risk factor for distal aortic enlargement associated with poor long-term outcomes and thus requires surgical or endovascular reoperation.5 In contrast, a thrombosed false lumen exerts a protective role in aortic size growth, reoperation and late mortality.4,6 An intermediate scenario of partial thrombosis is characterized by the concurrent presence of both flow and thrombus in the false lumen. In this case, the thrombus may occlude the re-entry tear, inhibiting the outflow and increasing both pressure inside false lumen and vessel wall tension.7,8
This study aims to determine in patients who underwent surgery for ATAAD which are the predisposing factors for adverse early and late outcomes focusing on patency of the false lumen.
Material and methodsPopulation studyPatients underwent surgical treatment due to acute type A aortic dissection, at Sapienza University Hospital Policlinico Umberto I° of Rome, Italy between January 2009 and December 2016, were included and clinical data were retrospectively collected.
All patients underwent emergency surgery as soon as the diagnosis was done, using pre-operative imaging (CT scanning in 100% and echocardiography), and the operating room was available.
Data about perioperative mortality were retrieved and survived patients were visited yearly at our hospital for a clinical review and a CT scan of the entire aorta and trans-thoracic echocardiography, during a follow-up period after surgery. The mean follow-up period was 39±36 months.
We analyzed the months that were necessary to patients before they displayed a complete thrombosis and defined ‘chronic’ a partial thrombosis above the 95th percentile of years required, i.e. >5 years
Statistical analysisStatistics were performed on SPSS v23 (IBM). Categorical variables were plotted on contingency tables and Chi-square tested.
Means from scalar variables were compared using Student's t-test or one-way ANOVA with Bonferroni correction. The independent variables of this dataset (clinical condition, surgical methodologies) were tested for correlations with the alive/dead binary variable and for the vascular outcome (partial thrombosis/complete thrombosis). The statistically significant (p<0.05) parameters that were found to correlate with early survival were used as possible predictors of the dependent variable “Alive” in a logistic regression. The beta (B) factor of regression was found and reported with its standard error (SE) and its p-value.
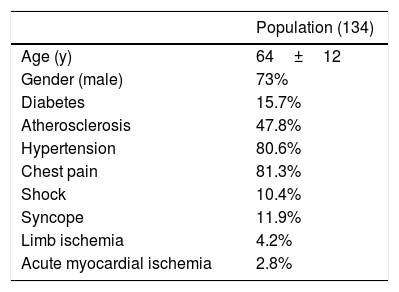
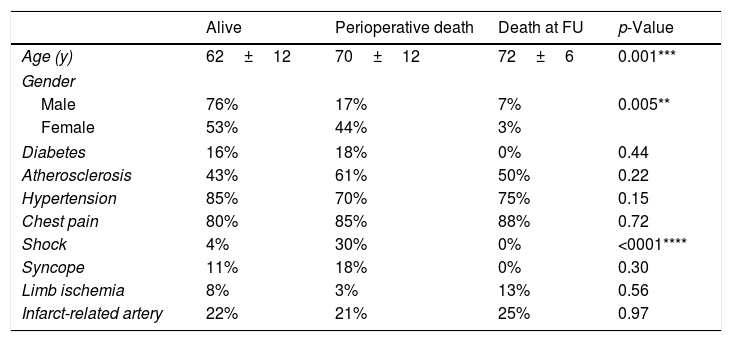
ResultsPopulationThe population object of study was composed of 134 patients enrolled with a mean age of 64±12 years; 73% of these were males. The group presented the following risk factors: diabetes (15.7%), atherosclerosis (47.8%) and hypertension (80.6%). At hospitalization 81.3% declared chest pain, 11.9% presented syncope and 10.4% shock. 4.2% of these suffered from limb ischemia and 2.8% from acute myocardial infarction (AMI) (Table 1).
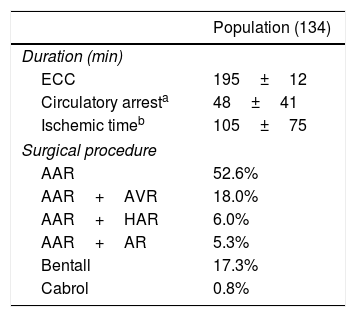
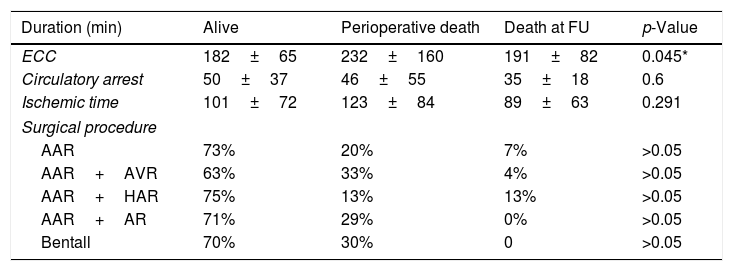
Surgical featuresAll patients underwent operation using extracorporeal circulation, hypothermic circulatory arrest and anterograde cerebral perfusion. 52.6% of the patients underwent ascending aorta replacement (AAR), 18% AAR and aortic valve replacement (AVR), 6% AAR and hemiarch replacement (HAR), 5.3% AAR and arch replacement (AR), 17.3% the Bentall procedure and the 0.8% the Cabrol procedure.
Mean time of extracorporeal circulation (ECC) was 195±12min, mean hypothermic circulatory arrest time was 48±41min long and mean clamping time lasted 105±75min (Table 2).
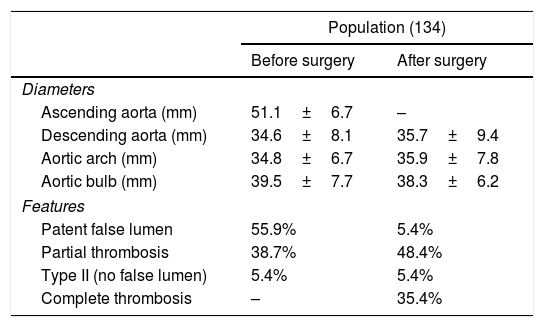
Aneurysm featuresPre-operatively Computed tomography (CT) imaging showed that 55.9% of the AAD had a patent false lumen distal to the ascending aorta, 38.7% displayed a partial thrombosis and 5.4% were classified type II De Bakey, showing no false lumen distal to ascending aorta. Mean aortic sizes were: aortic root 39.5±7.7mm; ascending aorta 51.1±6.7mm, descending aorta 34.6±8.1mm; aortic arch 34.8±6.7mm. After surgery, 5.4% showed a patent false lumen distal to anastomosis, the 48.4% a partial thrombosis and 35.4% a complete thrombosis. The mean post-operative diameters were: aortic root 38.3±6.2mm; descending aorta 35.7±9.4mm; aortic arch 35.9±7.8mm (Table 3).
Maximum aortic diamater surgery-related in the total population object of study.
| Population (134) | ||
|---|---|---|
| Before surgery | After surgery | |
| Diameters | ||
| Ascending aorta (mm) | 51.1±6.7 | – |
| Descending aorta (mm) | 34.6±8.1 | 35.7±9.4 |
| Aortic arch (mm) | 34.8±6.7 | 35.9±7.8 |
| Aortic bulb (mm) | 39.5±7.7 | 38.3±6.2 |
| Features | ||
| Patent false lumen | 55.9% | 5.4% |
| Partial thrombosis | 38.7% | 48.4% |
| Type II (no false lumen) | 5.4% | 5.4% |
| Complete thrombosis | – | 35.4% |
After Type A aortic dissection diagnosis and transfer to the operating room, five of the 134 patients (3.7% of the group) died during the surgical procedure.
All patients were operated on through a midline sternotomy. All operations were done with a standard method of cardiopulmonary bypass, deep hypothermia and selective anterograde cerebral perfusion. Both anterograde and retrograde delivery of cardioplegic solution were used for myocardial protection. Extracorporeal circulation was established by right atrium-femoral artery cannulation in 45 patients (34.9% of the sample), right atrium-axillary in 67 patients (51.9% of the sample), femoral-femoral in four patients (3.1% of the sample), right atrium-aorta in five patients (3.9% of the sample), atrium-anonymous trunk in 6 patients (4.6% of the sample), atrium-subclavian in 1 patient (0.8% of the sample) and femoral-axillary in one patient (0.8% of the sample). Patients reached a systemic body temperature ranging between 20 and 28°C. Surgical techniques for type A aortic dissection usually involved replacement of the ascending aorta and resection of the primary intimal tear (66 patients) with aortic valve replacement (24 patients), a composite replacement of the aortic root (24 patients), proximal arch (8 patients) or total arch replacement (7 patients).
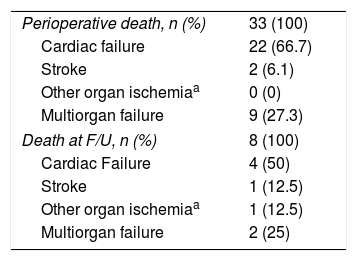
Follow-upDuring the perioperative period, 33 out of 134 patients died, resulting in an in-hospital mortality rate of 24.6%. After surgery, 101 patients underwent clinical and imaging protocols but eight patients died and four patients were lost during the follow-up period.
The remaining 89 patients were studied during the follow-up performing CT scan and trans-thoracic echocardiography after six-twelve months from surgery and then approximately every year since the last check. CT scan images allowed an exhaustive analysis of the anatomical characteristics and changes of the distal thoracic aorta and the eventual residual false lumen. The mean follow-up period was 39±36 months.
Survival analysesWe first divided our population into three groups: alive (94 patients), perioperative death (33 patients) (before the first follow-up) and death at follow-up (8 patients) (FU).
The causes of early death in the perioperative period may be referred in the 66.7% of cases to cardiac failure, followed by multiorgan failure and stroke with 27.3% and 6%, respectively (Table 4).
Instead about death at follow-up, CT post-operative findings could not be retrieved and are not manifest; as reported in Table 4, cardiac failure occurs in the 50% of cases, followed by multiorgan failure with 25%, stroke and other organ ischemia similarly in the 12.5% of cases.
We observed that mean age was significantly lower (p<0.001) in the ‘alive’ group (62±12) compared to perioperative death (PD) and death at follow-up (DF) groups (70±12 and 72±6, respectively). Moreover, female patients displayed higher perioperative mortality compared to males (44% vs 17%, p=0.005). We also found that shock dramatically worsened the prognosis, since its prevalence was 7.5-folds higher in the PD group compared to the ‘alive’ (30% vs 4%, p<0.0001).
Conversely, we found no significant differences in risk factors prevalence among groups (Table 5).
Clinical presentation, comorbidities and characteristics of the three outcome-related groups.
| Alive | Perioperative death | Death at FU | p-Value | |
|---|---|---|---|---|
| Age (y) | 62±12 | 70±12 | 72±6 | 0.001*** |
| Gender | ||||
| Male | 76% | 17% | 7% | 0.005** |
| Female | 53% | 44% | 3% | |
| Diabetes | 16% | 18% | 0% | 0.44 |
| Atherosclerosis | 43% | 61% | 50% | 0.22 |
| Hypertension | 85% | 70% | 75% | 0.15 |
| Chest pain | 80% | 85% | 88% | 0.72 |
| Shock | 4% | 30% | 0% | <0001**** |
| Syncope | 11% | 18% | 0% | 0.30 |
| Limb ischemia | 8% | 3% | 13% | 0.56 |
| Infarct-related artery | 22% | 21% | 25% | 0.97 |
Longer surgical procedures showed increased perioperative mortality (p<0.05), with a mean ECC time of 232±160min in the PD group compared to the others (Alive: 182±65; DF: 191±82). The analyzed surgical procedures showed no significant differences in survival rate (p>0.05). However, the AAR+AVR granted lower chances of survival (63%) compared to the others (all exceeding 70%). Interestingly, AAR+HAR had the lowest perioperative mortality (13%) compared to the others (ranging from 20% to 33%) but the highest dead during the follow-up (13%) compared to the others (below 7%) (Table 6).
Surgical features in the three outcome-related groups.
| Duration (min) | Alive | Perioperative death | Death at FU | p-Value |
|---|---|---|---|---|
| ECC | 182±65 | 232±160 | 191±82 | 0.045* |
| Circulatory arrest | 50±37 | 46±55 | 35±18 | 0.6 |
| Ischemic time | 101±72 | 123±84 | 89±63 | 0.291 |
| Surgical procedure | ||||
| AAR | 73% | 20% | 7% | >0.05 |
| AAR+AVR | 63% | 33% | 4% | >0.05 |
| AAR+HAR | 75% | 13% | 13% | >0.05 |
| AAR+AR | 71% | 29% | 0% | >0.05 |
| Bentall | 70% | 30% | 0 | >0.05 |
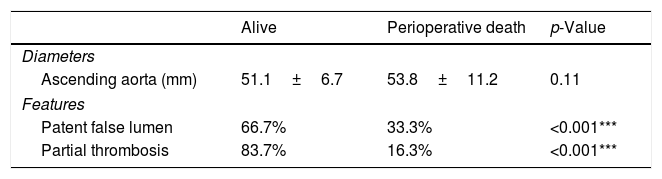
Moreover, we found that the diameter of the ascending aorta did not predict the outcome. Conversely, the eventual thrombosis of the false lumen correlated with survival, with 33.3% of the patients presenting a patent false lumen dying perioperatively compared to the 16.3% of those with a partial thrombosis (p<0.001) (Table 7).
A comparison of ascending aorta maximum diameter and false lumen status before surgery between survived and perioperatively dead patients.
| Alive | Perioperative death | p-Value | |
|---|---|---|---|
| Diameters | |||
| Ascending aorta (mm) | 51.1±6.7 | 53.8±11.2 | 0.11 |
| Features | |||
| Patent false lumen | 66.7% | 33.3% | <0.001*** |
| Partial thrombosis | 83.7% | 16.3% | <0.001*** |
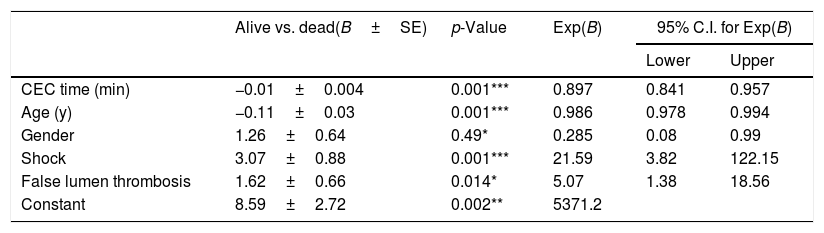
A binary logistic regression including all the factors that were found to be significantly involved in the survival confirmed the exclusion of the used surgical procedure as a predictor. Conversely, the analyses confirmed CEC duration, false lumen eventual thrombosis, age, gender and shock to influence the outcome (Table 8).
Binary logistic regression model of significant predictors of survival (B=regression coefficient; SE=standard error; C.I.=confidence interval).
| Alive vs. dead(B±SE) | p-Value | Exp(B) | 95% C.I. for Exp(B) | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| CEC time (min) | −0.01±0.004 | 0.001*** | 0.897 | 0.841 | 0.957 |
| Age (y) | −0.11±0.03 | 0.001*** | 0.986 | 0.978 | 0.994 |
| Gender | 1.26±0.64 | 0.49* | 0.285 | 0.08 | 0.99 |
| Shock | 3.07±0.88 | 0.001*** | 21.59 | 3.82 | 122.15 |
| False lumen thrombosis | 1.62±0.66 | 0.014* | 5.07 | 1.38 | 18.56 |
| Constant | 8.59±2.72 | 0.002** | 5371.2 | ||
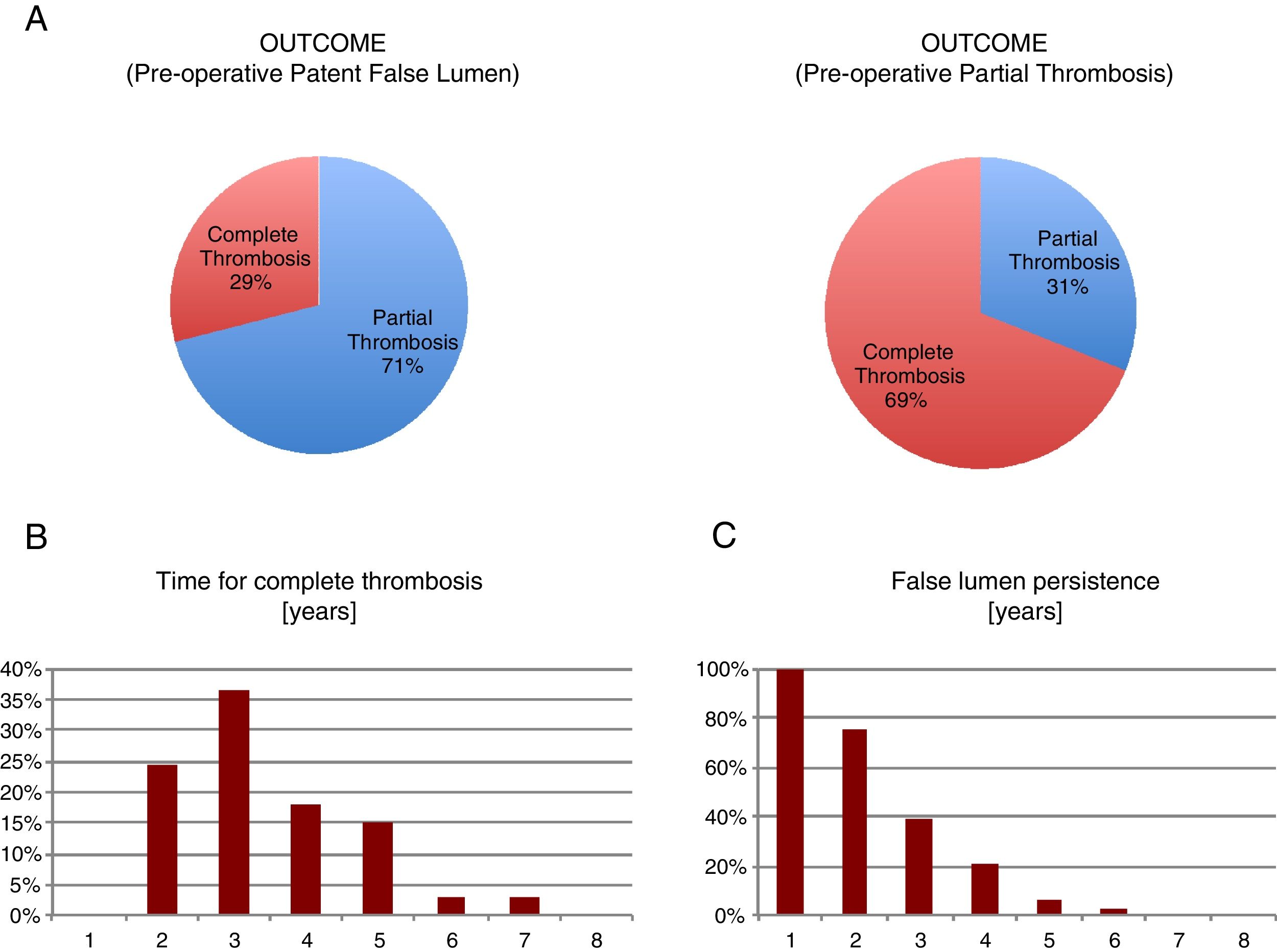
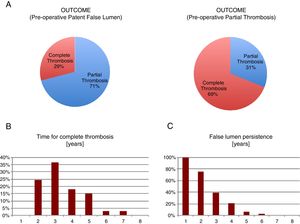
We observed that only 29% of the pre-operative patent false lumen underwent complete thrombosis, while this number was significantly higher (69%) in the group with a pre-operative partial thrombosis (p<0.001). We analyzed the months that were necessary to patients before they displayed a complete thrombosis and defined ‘chronic’ a partial thrombosis above the 95th percentile of years required, i.e. >5 years (Fig. 1).
So, we found that ‘chronic’ patients had an increased (p<0.03) mean pre-operative ascending aorta diameter compared to the other group (54.2±4.6 vs 50.5±7.0), determining a 1.72±0.79 beta coefficient in a bivariate logistic regression (Table 9A, C). Moreover, we found that those patients having a complete false lumen thrombosis above the median value of 34 months displayed a higher (p=0.005) pre-operative mean descending aorta diameter compared to the others (37.2±6.9 vs 29.2±6.1mm) (Table 9B, D)
Correlation between aortic maximum diameters and false lumen status (B=beta regression coefficient; SE=standard error).
| A | Chronic partial thrombosis | Non-chronic partial thrombosis | p-Value |
|---|---|---|---|
| Diameters | |||
| Ascending aorta (mm) | 54.2±4.6 | 50.5±7.0 | 0.028* |
| B | Evolution below median | Evolution above median | p-Value |
|---|---|---|---|
| Diameters | |||
| Descending aorta (mm) | 29.2±6.1 | 37.2±6.9 | 0.005** |
| C | Chronic partial thrombosis (B±SE) | p-Value |
|---|---|---|
| Ascending aorta diameter | 1.72±0.79 | 0.03 |
| D | Evolution above median (B±SE) | p-Value |
|---|---|---|
| Descending aorta diameter | 2.80±1.02 | 0.006 |
Today perioperative surgical outcomes of acute type A aortic dissection have improved with advances in surgical techniques and perioperative care. The long-term fate of residual false lumen after aortic surgery is already not completely clear: in fact, a chance of undergoing re-intervention exists. Since the distal false lumen is not always resected, it could retain patency also from distal suture lines, leading to progressive descending aortic dilation and rupture.4
The incidence of post-operative partial or complete distal false lumen patency is about 31% and 89%, respectively.9 Our data set is in line with this trend. In fact, if we consider the ‘chronic’ variable, which includes only the partial thrombosis lasting for more than five years (the 95th percentile of thrombosis time), we got a 22.7% of partial patency that is likely to increase in the next years of follow-up.
Persistent post-operative perfusion of the distal false lumen can be caused by:
- •
an unresected or secondary entry sites in the distal aorta;
- •
an aortic clamp trauma that may injure the friable dissected aorta and create a new intimal tear immediately distal to the surgical repair;
- •
a leak at the level distal aortic anastomosis.
We associated the aortic pre-operative diameters with chronic false lumen patency and longer thrombosis evolution, in line with Sakaguchi et al. that showed pre-operative hypertension and pre-operative diameter ≥35mm of the descending thoracic aorta, as significant and independent risk factors to develop patent post-operative false lumen.10
Moreover, Zhao et al. have shown that complete false lumen thrombosis at one year from surgery was lower in patients who required post-operative anticoagulation than in those who did not (87% vs 98%, p=0.005).11 Consequently, if the aortic valve cannot be preserved, the prosthetic valve type should be chosen with attention, and the long-term risk of distal aortic complications should be carefully evaluated considering the risk of biological prosthesis deterioration. However, we could not find a correlation between cardiac valve alterations with either survival or false lumen patency.
We define partial thrombosis/patency as the concurrent presence of both flow and thrombus in the false lumen. Tsai and colleagues reported that the natural history of a false lumen partial thrombosis in acute Type B aortic dissection is associated with a worse prognosis than that of the completely patent group.8 Moreover, it was shown that a partial thrombosis of the false lumen after Type A aortic dissection is an independent risk factor for overall survival, re-intervention and rapid increase of aortic diameters. The authors hypothesized that the greater intraluminal pressure in a partially thrombosed false lumen could explain its effect on aortic growth rate.8 Nevertheless, it is worth mentioning that a partially thrombosed false lumen may present multiple entry sites, a condition that reduces the pressure inside the false lumen.12
The presence of a thrombus inside the false lumen is usually associated with an area of low and complex flow, causing blood stasis and endothelial activation. A blood-flow quantification and velocity-mapping study demonstrated the existence of a bidirectional flow within a false lumen that exhibited partial thrombosis.13 Previous studies suggested a direct relationship between intraluminal thrombosis and aortic rupture as the result of arterial wall hypoxia in proximity to the intraluminal thrombus, a condition that leads to increased local inflammation, neovascularization, and wall weakening.14
Coherently, a study showed that growth rate was faster in every segment of the descending thoracic aorta in those patients presenting a partial thrombosis, compared to the others.6
These findings support the concept that extensive surgery for aortic dissection, such as the frozen elephant trunk technique or endovascular strategy, decreases the risk of reoperation. However, the debate is still open. In fact, some authors affirm that total arch repair surgery is associated with a major risk of early mortality and severe neurologic complications.15 On the other hand, a residual intimal tear in the distal aorta is thought to be the major cause of persistent false lumen patency.16,17 Furthermore, excellent results were achieved using the total arch repair elephant trunk technique on patients with Type A acute aortic dissection.18–22 Uchida et al. reported that after five years from the surgical intervention, 29% of the patients that underwent ascending aortic or hemiarch surgery showed a patent false lumen at the proximal descending aorta. On the contrary, the false lumen was completely thrombosed in all patients operated with the frozen elephant trunk.23 An intermediate approach was also proposed to mediate the concerns of both parties.24 In fact, the intimal re-layering technique combines the advantages of arch replacement and elephant trunk technique with the simplicity of a standard hemiarch repair. Also, this technique provides an elephant trunk configuration for an eventual future reintervention.
Our data do not support either of the hypothesis since our correlations suggest that hemiarch replacement is to be considered a safer procedure compared to the entire arch replacement (13% vs 29% perioperative mortality) but may possibly be a non-resolutive procedure in the long period (13% vs 0% death during follow-up). However, this association was not confirmed by our logistic regression.
ConclusionSingularly analyzed surgical procedures have not demonstrated to perform better in the treatment of acute Type A aortic dissection.
Age, gender, shock, pre-operative false lumen patency and CEC time have shown a strong association, acting significantly as predictors of early survival. Moreover, pre-operative aortic diameters play a crucial role to predict the timing for false lumen complete thrombosis, particularly in the descending thoracic tract.
However, major limitations of our dataset are the limited number of patients enrolled, the limited number of data we got from the perioperative dead patients and a consistent part of the population still being followed-up after recent surgery, to date. It is our aim to enlarge and consolidate this study in the next years to provide stronger and possibly new lines of evidence to the debate.
FundingThe authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interestThe authors declare no conflict of interest.
The authors thank Doctor Andreina Brandi for her help and precious advices on statistics.