Balloon angioplasty (BA) for ‘native’ coarctation of the aorta (CoA) in infants and young children remains controversial. In this study, the aim is to evaluate the immediate and mid-term outcome of BA.
MethodsA prospective, interventional study conducted in a tertiary, high volume cardiac centre, over a 30-month period, ending May 2017. BA was preceded by preoperative clinical examination, and a detailed cardiac ultrasound and catheterization assessment. A statistical analysis was performed – including logistic regression and Kaplan–Meier tests.
ResultsThe study included 74 patients with a median age of 10 months (inter-quartile range – IQR, 5–61) BA was ‘successful’ in 66 (89%) cases, with a decrease in peak pressure gradient (PG) and an angiographic improvement of 59% (95% confidence interval – CI, 56–63; P<0.001) and 85% (95% CI, 83–87; P<0.0001), respectively. Recurrence was reported in 19 (26%) cases. Long-segment CoA was the best predictor of recurrence (adjusted OR, 72; 95% CI, 4–1295; P=0.004). BA was most ‘durable’ in those older than 12 months – 88% of those older than 12 months had freedom from recurrence. The mean time-to-event was 25 months (95% CI, 22–28). Ileofemoral artery occlusion was the most common complication (n=5; 7%), followed by dissection (n=4; 5%).
ConclusionsBA is a feasible option to treat native CoA in infants and children, with good immediate and mid-term outcomes. This study contributes to the growing body of information, supporting the role and effectiveness of BA in this setting.
La angioplastia con balón (BA) en la coartación de la aorta (CoA) de nacimiento en bebés y niños sigue siendo controvertida. El objetivo de este estudio es evaluar el resultado a corto y medio plazo de la BA.
MétodosEstudio prospectivo e intervencionista realizado en un centro terciario cardiológico de gran volumen, durante un periodo de más de 30 meses, que finalizó en mayo de 2017. La BA fue precedida por un examen clínico preoperatorio, así como una ecografía cardiaca detallada y una valoración de cateterización. Se realizó un análisis estadístico, incluyendo regresión logística y pruebas de Kaplan-Meier.
ResultadosEl estudio incluyó a 74 pacientes con una edad media de 10 meses (rango intercuartílico [RIC] de 5 a 61). La BA fue exitosa en 66 casos (89%), con descenso del pico de gradiente de presión y mejora angiográfica del 59% (intervalo de confianza del 95% [IC 95%] de 56 a 63; p<0,0001) y 85% (IC 95%: de 83 a 87; p<0,0001), respectivamente. Se reportó recidiva en 19 casos (26%). La CoA de segmento largo fue el mejor factor predictivo de recidiva (OR ajustado: 72; IC 95%: de 4 a 1.295; p=0,004). La BA fue más duradera en los niños mayores de 12 meses; el 88% de los mayores de 12 meses reflejaron libertad de recidiva. El tiempo medio transcurrido hasta el evento fue de 25 meses (IC 95%: de 22 a 28). La oclusión de la arteria ileofemoral fue la complicación más común (n=5; 7%), seguida de disección (n=4; 5%).
ConclusionesLa BA es una opción factible para tratar la CoA de nacimiento en bebés y niños, con buenos resultados a corto y medio plazo. Este estudio contribuye a incrementar el corpus informativo, respaldando el rol y efectividad de la BA en este contexto.
Coarctation of the aorta (CoA) is the fifth most common congenital heart disease (CHD), with an incidence of 1 in 2500 births, and account for 4–6% of all congenital heart defects.1,2
The first reported surgical repair of CoA was done by Crafoord and Nylin in 19443 and involved resection and end-to-end anastomosis. It remained the only viable option for around 40 years until Singer et al. performed balloon angioplasty (BA) in 1982.4 Since then, BA was regarded as safer and less invasive, and thus widely adopted for CoA.
Both BA and surgical repair for CoA are well-studied and widely available treatment options.5–8 Furthermore, BA, nowadays, has become routine for re-coarctation and is widely accepted, along with implantation of stents, as a preferable alternative to surgical correction for ‘native’ CoA in older children, adolescents, and adults.9–11
Having said that, BA for ‘native’ CoA in neonates, infants, and young children remains controversial and there is no general consensus.6 In this study, we aim to evaluate our immediate and mid-term outcome of BA in that set of children and to contribute this research to the growing body of information in this context.
MethodsDesign, setting and participantsIn this prospective, interventional study, conducted at a tertiary, high-volume cardiac centre, we enrolled all patients who were diagnosed with ‘native’ CoA and scheduled for balloon angioplasty upon decisions taken by the attending paediatric cardiologist, over a 30-month period ending May 2017.
The presence of one or more of the following seven ‘inclusion criteria’ was considered an indication to enrolment in this study: upper-to-lower limb systolic pressure gradient ≥20mmHg, upper limb hypertension plus CoA flow turbulence on echocardiography (echo), peak instantaneous systolic pressure gradient (PG) ≥40mmHg, mean Doppler echo PG ≥20mmHg, peak-to-peak catheterization (cath) PG ≥20mmHg, diastolic ‘tailing’ of Doppler echo flow across CoA segment and, lastly, left ventricular (LV) dysfunction with CoA regardless of the PG.
On the other hand, the ‘exclusion criterion’ included CoA cases associated with cardiovascular lesions requiring surgical intervention. These include univentricular heart (single ventricle), atrioventricular (AV) canal defects, and D-transposition of great arteries (D-TGA).
Informed consent was taken from the patients’ parents. The study was approved by the Institutional Ethics Committee of the Iraqi Board for Medical Specializations (Issue No. 2745; 27 July 2017).
Once recruited, initial, preoperative history was taken. In addition, the following characteristics were recorded: patient's age, gender, body weight (BW), height (Ht), height centile, limbs pulses (including brachio-femoral delay), blood pressure (BP) in all four limbs, oxygen saturation (SPO2) in the upper and lower limbs, and cardiac auscultation.
Different references and charts have been used. Height centile was measured using the Centers for Disease Control (CDC) growth Charts.12 For BP measurement and interpretation, we used the technique and analysis recommended by the working group of the National High Blood Pressure Education Program (NHBPEP) in 2004.13 Both the brachial and popliteal arteries were used. Hypertension is said to be present when the systolic and/or diastolic BP was greater than the 95th percentile for age and sex on at least three occasions. While hypotension is present when these readings were less than the 5th percentile.
Initial trans-thoracic echocardiography (TTE) was done to ‘all’ recruited patients. We used a GE Vivid E9 ultrasonic machine (General Electric, Norway), with 3 and 5MHz probes. We evaluate the aortic arch with 2D mode using suprasternal, high parasternal long axis, and subcostal view – with special consideration to the CoA length, diameters of the ascending aorta, arch, isthmus, and descending aorta. We used continuous wave (CW) Doppler (peak and mean) to assess the severity of CoA. Further, LV function assessment – using an ejection fraction (EF) in the parasternal long axis view – and search for any additional lesion were performed. LV dysfunction was graded as mild, moderate, and severe when the EF is 45–55, 30–44, and <30%, respectively.14
Initial preoperative imaging (like CT scan or MRI) of the aortic arch was not routine. It was ordered only in cases were echo was inconclusive.
ProcedureInitially, we perform BA procedure as described by Holzer et al.15 We took angiographic measurements of different parts of the aorta. The diameters of the CoA site, transverse aortic arch (just proximal to the left subclavian artery), aortic isthmus (just distal to the left subclavian artery), and the descending aorta (at the level of the diaphragm) are measured.
The following definitions are considered. ‘Discrete’ CoA when the angiographic length is ≤5mm. Any length more than this was regarded as a ‘long-segment’ lesion. Furthermore, isthmus and aortic arch hypoplasia were present when the isthmus and the transverse arch were less than 40% and 50%, respectively.16
Once the anatomy has been clarified well, a balloon is chosen. We used a low-profile balloon (Tyshak II, NuMED) whose diameter is three times the CoA segment; not exceeding the descending aorta, at the diaphragm,17 or 1–2mm larger than the aortic isthmus whichever is smaller. To optimize the results, we did three inflations, for 10–15s each. After deflation, the balloons are withdrawn carefully, outside the body. All the manipulations of the catheters and balloons are done over guidewires kept across the CoA site during the procedure.
‘Immediate procedural success’ is present when one or both of the followings were achieved – a decline in PG (echo or cath) to less than or equal to 50% of the initial preoperative reading, and ≥90% angiographic improvement at the CoA.18
We actively searched for postoperative complications. An ‘aortic aneurysm’ was said to be present when there is a discrete protrusion, at the intervention site, outside the aortic adventitial plane by more than three millimetres, or 50% of the descending aorta, detected by postoperative aortogram. To detect a ‘dissection flap’, we depend on angiogram initially. However, in the case of uncertainty, we do echocardiography or CT scan.
Follow-upOnce the procedure was finished, we re-assess BP and check for symptoms of hypertensive crises. We defined a patient as having ‘hypertensive urgency or emergency’ when systolic and diastolic BP ≥95th centile plus 12mmHg, or ≥140/90mmHg, whichever is lower.19 For any patient, the lower limbs are examined carefully. We check the vascular access site (for bruises or haematoma formation), distal pulses and temperature.
Echocardiography is performed in the first postoperative day. Special attention is paid to LV function, and to the CoA site for any residual narrowing or development of complications. All the patients – then after – would be scheduled for the next clinical (including echo) evaluation at 1 month, 3 months, 6 months, and 1–2 years after the procedure. Spiral CT scanning is performed routinely to all postoperative patients at 1 month after the procedure and every 1–2 years then after, accordingly. ‘Recurrent CoA’ is defined as re-stenosis after an initially successful BA.
StatisticsWe performed the statistical analysis using IBM SPSS Statistics package, version 25. Univariate, bivariate, and multivariate analyses have been done. Suitable graphs and tables were used to describe the data. P value <0.05 is considered significant. For a normally distributed, continuous variable, we report mean, SD±95% confidence interval; while for non-Gaussian distributed variables, we present median, and inter-quartile range. For categorical ones, on the other hand, we use frequency and percentages.
To compare two outcome means, we used student, two-tailed t-test. However, Chi-square, Fisher's exact and Kendall's τ (tau) tests have been used to assess relations between variables and frequency distribution.
Risk estimates have been evaluated using Chi-square and presented in the form of crude (unadjusted) odds ratio. After that, the results were re-validated, for multicollinearity, using multivariate logistic regression, and presented as ‘adjusted’ OR. Recurrence's mean time and freedom rate, in different age groups, have been evaluated using the Kaplan–Meier survival method.
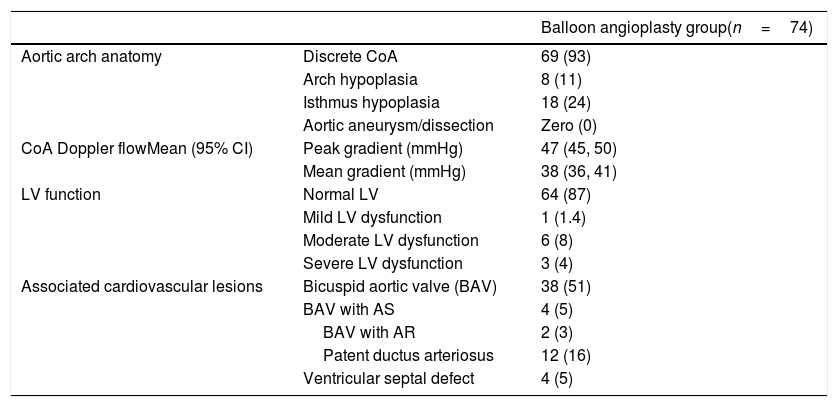
ResultsPreoperativeA total of 74 patients aged 1 month to 13 years were enrolled. The means for the patients’ age, body weight, and height are 34 months, 12kg, and 86cm, respectively. Other patients’ baseline characteristics and preoperative echocardiographic evaluation are listed in Tables 1 and 2.
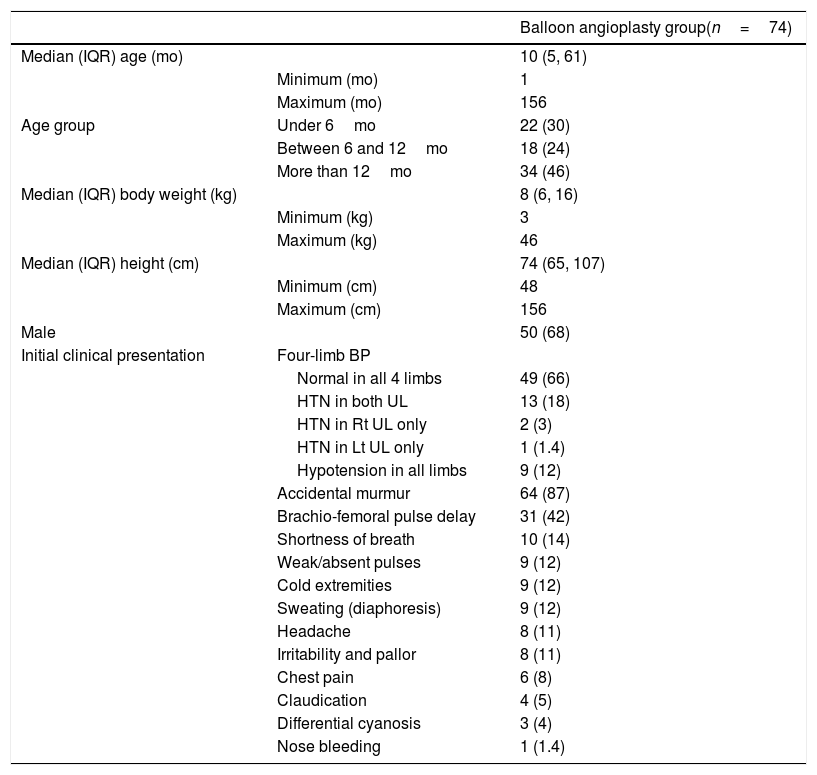
Patients’ baseline characteristics.a
| Balloon angioplasty group(n=74) | ||
|---|---|---|
| Median (IQR) age (mo) | 10 (5, 61) | |
| Minimum (mo) | 1 | |
| Maximum (mo) | 156 | |
| Age group | Under 6mo | 22 (30) |
| Between 6 and 12mo | 18 (24) | |
| More than 12mo | 34 (46) | |
| Median (IQR) body weight (kg) | 8 (6, 16) | |
| Minimum (kg) | 3 | |
| Maximum (kg) | 46 | |
| Median (IQR) height (cm) | 74 (65, 107) | |
| Minimum (cm) | 48 | |
| Maximum (cm) | 156 | |
| Male | 50 (68) | |
| Initial clinical presentation | Four-limb BP | |
| Normal in all 4 limbs | 49 (66) | |
| HTN in both UL | 13 (18) | |
| HTN in Rt UL only | 2 (3) | |
| HTN in Lt UL only | 1 (1.4) | |
| Hypotension in all limbs | 9 (12) | |
| Accidental murmur | 64 (87) | |
| Brachio-femoral pulse delay | 31 (42) | |
| Shortness of breath | 10 (14) | |
| Weak/absent pulses | 9 (12) | |
| Cold extremities | 9 (12) | |
| Sweating (diaphoresis) | 9 (12) | |
| Headache | 8 (11) | |
| Irritability and pallor | 8 (11) | |
| Chest pain | 6 (8) | |
| Claudication | 4 (5) | |
| Differential cyanosis | 3 (4) | |
| Nose bleeding | 1 (1.4) |
Abbreviations: BP, blood pressure; cm, centimetre; HTN, hypertension; IQR, interquartile range; kg, kilogram; Lt, left; mo, month; Rt, right.
Preoperative echocardiographic features.a
| Balloon angioplasty group(n=74) | ||
|---|---|---|
| Aortic arch anatomy | Discrete CoA | 69 (93) |
| Arch hypoplasia | 8 (11) | |
| Isthmus hypoplasia | 18 (24) | |
| Aortic aneurysm/dissection | Zero (0) | |
| CoA Doppler flowMean (95% CI) | Peak gradient (mmHg) | 47 (45, 50) |
| Mean gradient (mmHg) | 38 (36, 41) | |
| LV function | Normal LV | 64 (87) |
| Mild LV dysfunction | 1 (1.4) | |
| Moderate LV dysfunction | 6 (8) | |
| Severe LV dysfunction | 3 (4) | |
| Associated cardiovascular lesions | Bicuspid aortic valve (BAV) | 38 (51) |
| BAV with AS | 4 (5) | |
| BAV with AR | 2 (3) | |
| Patent ductus arteriosus | 12 (16) | |
| Ventricular septal defect | 4 (5) |
Abbreviations: AR, aortic regurgitation; AS, aortic stenosis; BAV, bicuspid aortic valve; CI, confidence interval; CoA, coarctation of aorta; LV, left ventricle.
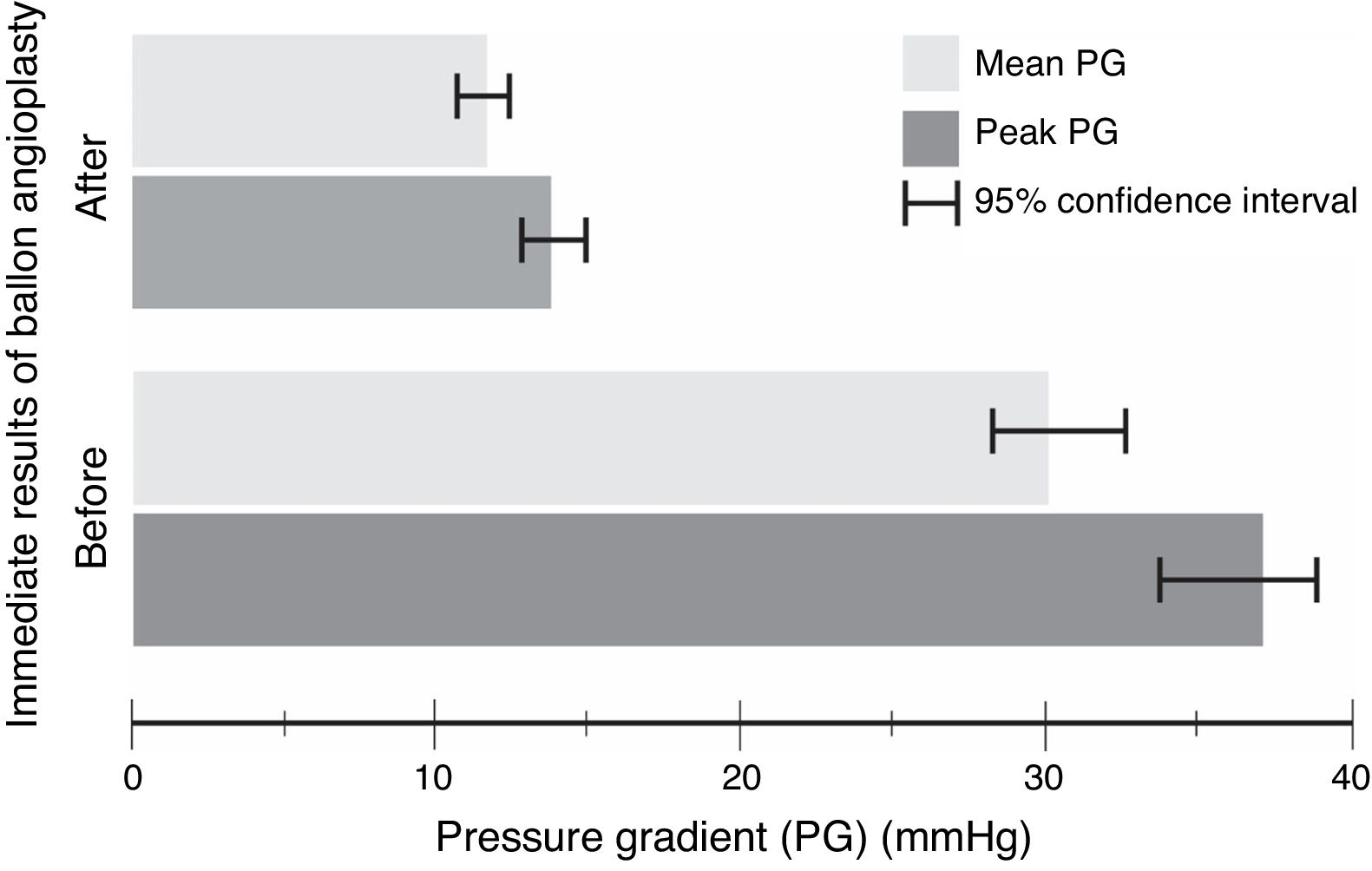
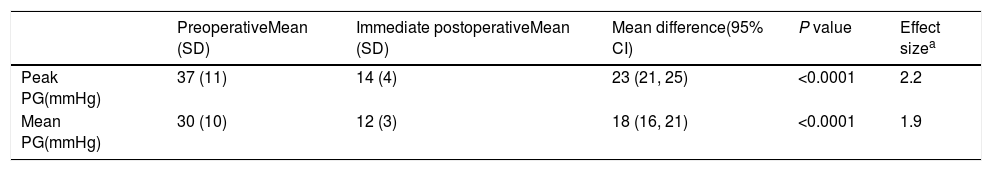
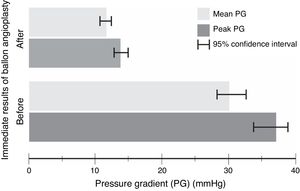
The procedure had an initial (immediate) success in 66 (89%) cases, with a mean procedural time of 65 (95% CI, 63–67)min, and fluoroscopy time equals to 20.6 (95% CI, 20–21)min. It resulted in immediate decline in ‘peak’ PG by 23 (95% CI, 21–25)mmHg and ‘mean’ PG by 18 (95% CI, 16–21)mmHg, in the catheterization laboratory, with statistical significance and substantial effect size (Table 3 and Fig. 1). However, if we compare percentages, BA resulted in a decrease of 59 and 57%, for the peak and mean PG, respectively. The angiographic dimension of the CoA segment, on the other hand, has increased by 85% (Table 4).
Paired t-test model – comparison between pre- and postoperative pressure gradient (PG) change with balloon angioplasty (n=74).
| PreoperativeMean (SD) | Immediate postoperativeMean (SD) | Mean difference(95% CI) | P value | Effect sizea | |
|---|---|---|---|---|---|
| Peak PG(mmHg) | 37 (11) | 14 (4) | 23 (21, 25) | <0.0001 | 2.2 |
| Mean PG(mmHg) | 30 (10) | 12 (3) | 18 (16, 21) | <0.0001 | 1.9 |
Abbreviations: CI, confidence interval; PG, pressure gradient; SD, standard deviation.
One-sample t-test model – percentage change in haemodynamic and angiographic measurements with balloon angioplasty (n=74).
| Per cent change(95% CI) | Standard deviation(SD) | P value | Effect sizea | |
|---|---|---|---|---|
| Peak PG | −59 (−56, −63) | −14 | <0.0001 | 4.3 |
| Mean PG | −57 (−54, −61) | −17 | <0.0001 | 3.4 |
| Angiographic dimensions | 85 (83, 87) | 7 | <0.0001 | 12.8 |
Abbreviations: CI, confidence interval; PG, pressure gradient; SD, standard deviation.
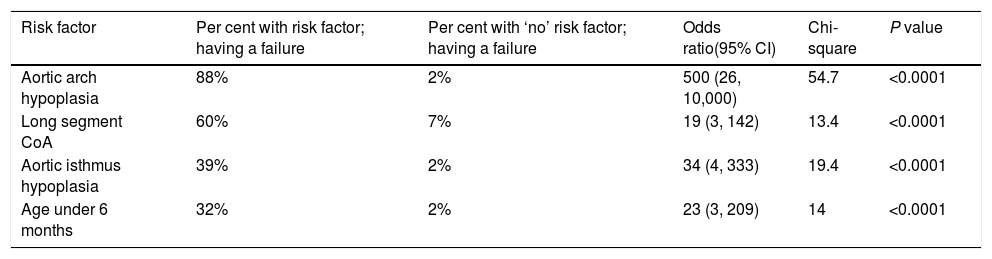
Immediate ‘failure’ of the procedure has been encountered in 8 (11%) cases. Aortic arch hypoplasia was found to be the strongest predictor of failure (OR 500; 95% CI, 26–10,000; Chi-square 54.7; P<0.0001) (Table 5). Having said that, upon multivariate logistic regression, arch hypoplasia had an ‘adjusted’ OR of 100 (95% CI, 11–1000; Nagelkerke R square, 0.56; P<0.0001). Strong multicollinearity with other variables, like isthmus hypoplasia, age under 6 months, and long-segment CoA, has been noticed. They inflated the standard error (SE) by more than 10% and made the regression model unstable. As such, we considered them important ‘confounders’ and exclude them from the analysis.
Chi-square risk estimate model – predictors of immediate ‘failure’ of balloon angioplasty (BA) (n=8).
| Risk factor | Per cent with risk factor; having a failure | Per cent with ‘no’ risk factor; having a failure | Odds ratio(95% CI) | Chi-square | P value |
|---|---|---|---|---|---|
| Aortic arch hypoplasia | 88% | 2% | 500 (26, 10,000) | 54.7 | <0.0001 |
| Long segment CoA | 60% | 7% | 19 (3, 142) | 13.4 | <0.0001 |
| Aortic isthmus hypoplasia | 39% | 2% | 34 (4, 333) | 19.4 | <0.0001 |
| Age under 6 months | 32% | 2% | 23 (3, 209) | 14 | <0.0001 |
Abbreviations: CI, confidence interval; CoA, coarctation of aorta.
The median follow-up duration of the patients was 8 (IQR, 3–16; minimum 0.25; maximum 30) months.
Some postoperative complications have been encountered. They were, in order of frequency, vascular access blockage (n=5; 7%), aortic dissection (n=4; 5%), life-threatening condition, necessitating resuscitation (n=3; 4%), blood loss, needed blood transfusion (n=2; 3%), vascular access haematoma (n=1; 1.4%), and lastly, death (n=1; 1.4%). However, no aortic rupture or hypertensive emergencies have been reported in our cohorts.
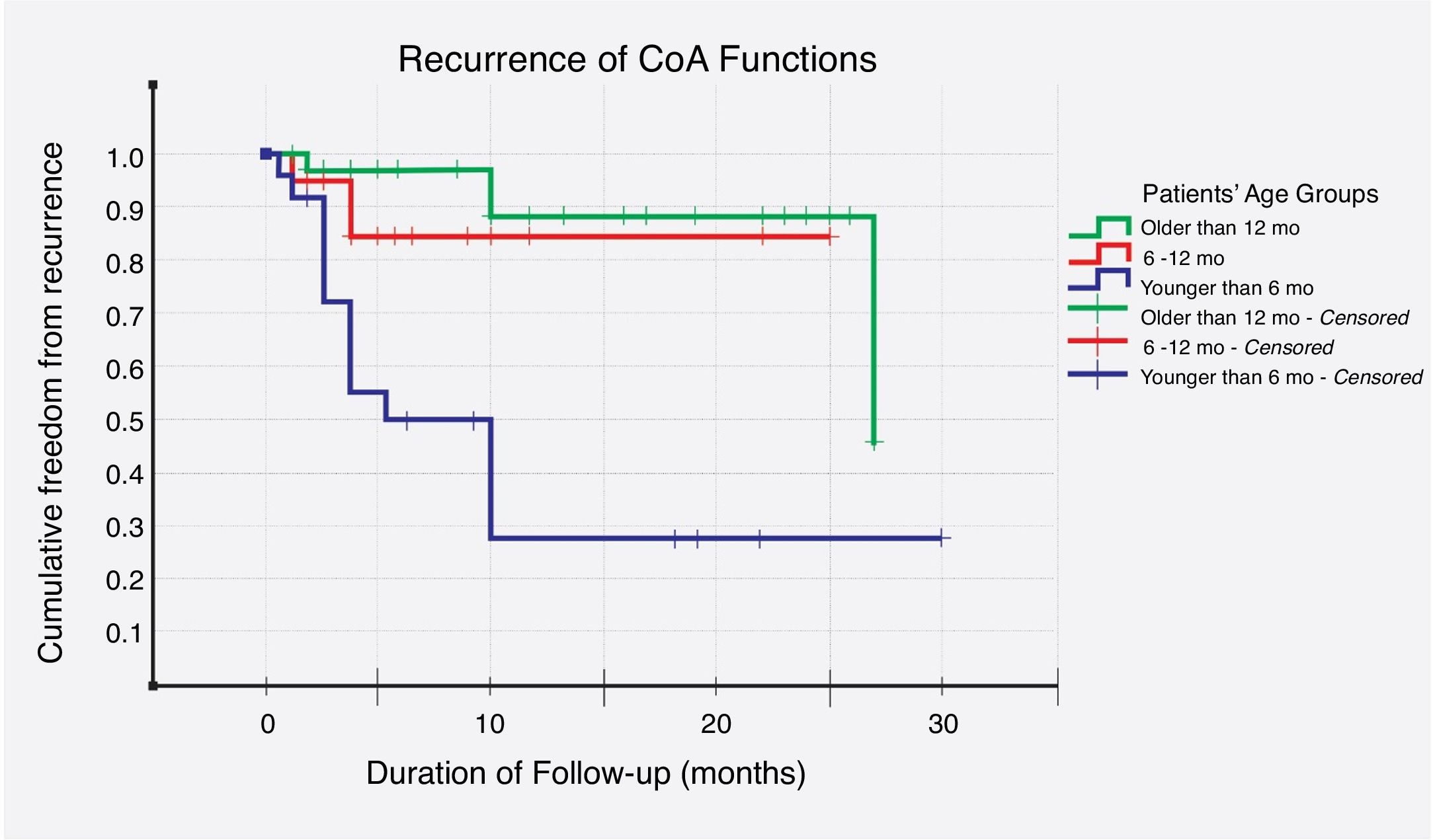
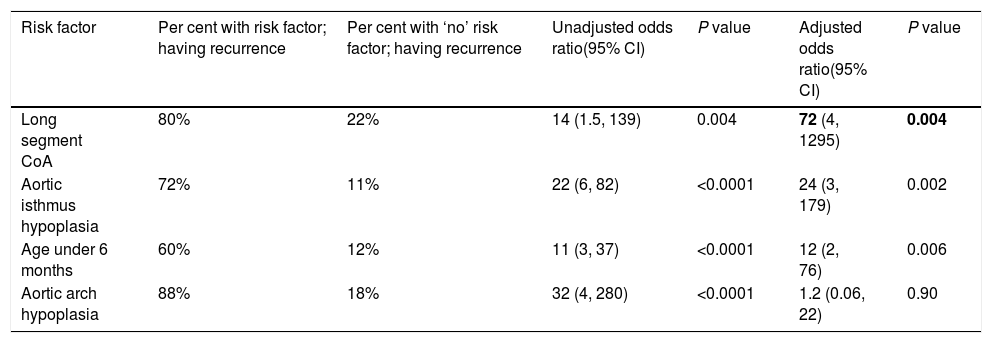
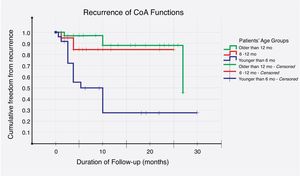
Recurrence was noticed (detected with echocardiography and confirmed with CT scan) in 19 (26%) cases. Multivariate logistic regression analysis has shown that long-segment CoA, rather than arch hypoplasia, was the strongest predictor of recurrence in this study (adjusted OR, 72; 95% CI, 4–1295; P=0.004) (Table 6). After that, we evaluated the recurrence rate in different age groups, and the time to this event, using the Kaplan–Meier test (Table 7). This analysis indicated that freedom from recurrence rate for those under 6 months, between 6 and 12 months, and older than 12 months were 29, 86, and 88%, respectively. The Log Rank (Mantel–Cox) test showed that there was a statistically significant difference between the three survival rates (P<0.0001). Collectively, these data suggest that BA results are most durable in those older than 12 months (Fig. 2).
Multivariate logistic regression model – predictors of ‘recurrence’ of CoA after an initial procedural success (n=19).
| Risk factor | Per cent with risk factor; having recurrence | Per cent with ‘no’ risk factor; having recurrence | Unadjusted odds ratio(95% CI) | P value | Adjusted odds ratio(95% CI) | P value |
|---|---|---|---|---|---|---|
| Long segment CoA | 80% | 22% | 14 (1.5, 139) | 0.004 | 72 (4, 1295) | 0.004 |
| Aortic isthmus hypoplasia | 72% | 11% | 22 (6, 82) | <0.0001 | 24 (3, 179) | 0.002 |
| Age under 6 months | 60% | 12% | 11 (3, 37) | <0.0001 | 12 (2, 76) | 0.006 |
| Aortic arch hypoplasia | 88% | 18% | 32 (4, 280) | <0.0001 | 1.2 (0.06, 22) | 0.90 |
Abbreviations: CI, confidence interval; CoA, coarctation of aorta.
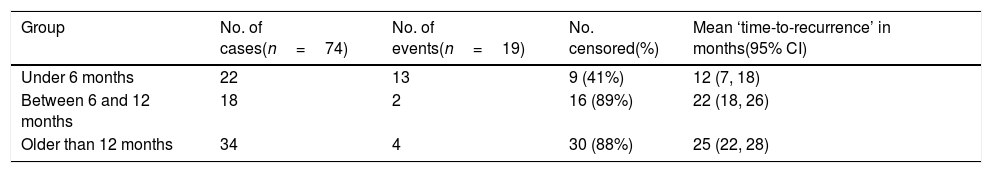
Kaplan–Meier survival model – freedom from recurrence (rate and time) in three different age groups (n=19).
| Group | No. of cases(n=74) | No. of events(n=19) | No. censored(%) | Mean ‘time-to-recurrence’ in months(95% CI) |
|---|---|---|---|---|
| Under 6 months | 22 | 13 | 9 (41%) | 12 (7, 18) |
| Between 6 and 12 months | 18 | 2 | 16 (89%) | 22 (18, 26) |
| Older than 12 months | 34 | 4 | 30 (88%) | 25 (22, 28) |
Abbreviation: CI, confidence interval.
The results of this study have shown that balloon angioplasty (BA) has resulted in ‘immediate’ procedural success in the majority of cases with a substantial decline in pressure gradient (PG), and an increase in angiographic dimensions of the coarctation of aorta (CoA) segment.
Currently, in older children and adults, BA with or without stent implantation have acquired preference over surgical treatment.6,11,14,20,21 In infancy, on the other hand, a great deal of debate exists in the cardiology community about the role of BA in ‘native’ CoA, though it is the option of choice for postoperative recurrent CoA.6,11,22
This dichotomy approach, in infancy, stems from a ‘long-standing belief’ that BA, in this age-group, shows an increased risk of complications (such as aortic rupture, aneurysm, and major vascular injuries) or, in the best circumstances, suboptimal results.10,22–24 Furthermore, BA, for postoperative recurrent CoA, is considered safer because of the presence of surgical adhesions and scar tissues, and thus, reduced risk of the theoretical aortic rupture in comparison to re-do surgical angioplasty. McCrindle et al., however, have demonstrated equivalent immediate results of BA in native and recurrent CoA. Therefore, the distinction between native and recurrent CoA, in the treatment strategy, is no longer justifiable.
Recently, Wu et al. published a meta-analysis, aimed to compare the outcome between BA and surgery for paediatric native CoA.25 Ten studies (9 non-randomized and 1 randomized clinical trials) have been included, with overall 723 subjects. They found no difference between BA and surgery in the following three parameters: immediate PG relief (weighted mean difference – WMD, −1.66; 95% CI, −4.23 to 0.9; P=0.2), perioperative complications including aneurysm (OR, 1.77; 95% CI, 0.95 to 3.28; P=0.07), and mortality (OR, 2.57; 95% CI, 0.87 to 7.61; P=0.09). Moreover, BA had significantly shorter hospitalization time (WMD, 19.4; 95% CI, 15.82–22.99; P<0.001). However, surgery was associated with lower incidence of re-coarctation (OR, 0.43; 95% CI, 0.30 to 0.61; P<0.001), and lower PG on the mid- to long-term follow-up (WMD, −0.85; 95% CI, −12.34 to −3.76; P<0.001).
In this study, there was immediate procedural success in 66 (89%) cases. The peak PG, for example, declined by 23 (95% CI, 21–25)mmHg, and the angiographic diameter of the CoA segment increased by 85 (95% CI, 83–87)%. These are similar to other results obtained elsewhere in the world.26–30
Recurrence of CoA, after an initial success, is one of the most important debatable issues with BA. In this study, 19 (26%) recurrence cases have been reported. In the literature, however, reported rates of recurrence after BA vary according to the patients’ age at time of intervention; being up to 50% in infancy,31,32 20–30% in older children,29,33–36 and 8% in adolescents and adults.37
This has driven a lot of researchers and scientific bodies14,16,26,38,39 to recommend against BA in infancy, especially in the first 4 months of life. They argue the trade-off with BA is that it avoids the immediate surgical risk but does so at the expense of increasing likelihood of requiring re-intervention in the near future – an assumption which is absolutely not acceptable in many centres.
According to multivariate logistic regression, ‘long-segment CoA’ was the strongest predictor of recurrence, in this study (adjusted OR, 72; 95% CI, 4–1295; P=0.004). Aortic isthmus hypoplasia, age under 6 months, and arch hypoplasia came next, in order of strength. Furthermore, in regards to age groups, Kaplan–Meier survival analysis has shown that freedom from, and time to recurrence were worse in those under the age of 6 months; where the freedom-from-event rate was 29%, and the mean time to re-CoA was 12 (95% CI, 7–18) months. Rao et al., on the other hand, analyzed 30 variables and identified the following risk factors for recurrence – age less than 12 months, isthmus hypoplasia, CoA segment less than 3.5mm before BA, and 6mm after dilation.36
Aortic dissection is one of the most catastrophic complications of BA. Early and accurate diagnosis and management are crucial for survival. The mortality rate can be as high as 25–30%, even in advanced cardiac centres.40 In this study, we faced four (5%) cases with aortic dissection, with a ‘dissecting flap’ depicted with fluoroscopy, immediately after the balloon deflation. Three of them were under the age of 6 months, and the fourth one was older than 11 years. Although some case series have reported successful treatment with endovascular stenting,41,42 we did not try it and rather referred them directly to surgery with good results, and no mortality.
An aortic aneurysm may develop at the site of prior BA for native or recurrent CoA. It has been studied well, with a reported incidence that ranges from 1 to 9%.37,43–45 It is thought to be the result of an inherent aortic medial wall abnormality, characterized by an increase in ground substances, fragmentation of elastic fibres, and a decrease in the number of smooth muscles cells.46 The most important predictor of aneurysm formation, after BA, is older age at the time of intervention.43,47 This age-dependent propensity explains the absence of aneurysm in our paediatric sample.
Considering all these things, while most long-term studies recommend surgery in infants with ‘native’ CoA, on the contrary, we do believe that BA is still the first line, in some parts of the world. The rationales for this are as follows: first, in our centre, we do not have the necessary surgical expertise to deal with small, critically ill infants – a situation which might be seen in other parts of the world. Second, although the re-intervention rate with BA is still higher than surgery in the first year of life, the re-intervention of choice – later in life – often is another angioplasty. However, even in the worst scenarios, when an invasive surgery is needed, it would be performed when the child is haemodynamically stable, grown-up, and gained some weight. Third, when aneurysms do happen, years after BA, their clinical significance is ‘uncertain’ and many patients are scheduled for conservative follow-up only, rather than being sent to surgery. Lastly, surgery is not 100% risk-free, as many infants are critically ill with lots of morbidities. Thus, complications are reported, even in the well-developed centres.25
ConclusionBased on our review and results, balloon angioplasty is a feasible option to treat native CoA in infants and children, with good immediate and mid-term outcomes. Angioplasty in those below 6-month old is best approached with surgery. However, where surgical expertise is lacking, a centre-dependent approach is the rule; provided that the identified risk factors – herein – are taken into consideration, to reduce failure and recurrence rate. We contribute this research to the growing body of information, supporting the role and effectiveness of BA in the paediatric age group.
Data availabilityThe raw data, required for study reproducibility, are available on the Open Scientific Framework (OSF).48
OSF: Replication Data for: Balloon Angioplasty for Infantile & Childhood Coarctation of Aorta, http://www.doi.org/10.17605/OSF.IO/59U2R.
Data is available under a CC0 1.0 Universal License.
Ethical approvalThe Institutional Ethics Committee of the Cardiology Council, Iraqi Board for Medical Specializations (IBMS) has reviewed and discussed the initial application to conduct this study, informed about its progress, and any revision in the protocol and patient information/informed consent. The Committee has received and approved the final report of the study on July 2017.
None of the investigators participating in this study took part in the decision making and voting for this study.
Patients’ consentInformed written consent was taken from the parents of the children to participate in this study and for publication of the clinical details.
Grant informationThe authors declared that no grants were involved in supporting this work.
Conflicts of interestThe authors have no conflict of interest to declare.