Chronic active EBV infection (CAEBV) of T-cell or NK-cell type is an EBV+ polyclonal, oligoclonal or often monoclonal lymphoproliferative disorder (LPD) recognized as representing the spectrum of EBV-associated T-cell and NK-cell LPD with different clinical presentations; one systemic and two cutaneous disorders including hydroa vacciniforme-like T-cell LPD and mosquito bite hypersensitivity. The systemic form of the disease is characterized by fever, persistent hepatitis, hepatosplenomegaly and lymphadenopathy, which shows varying degrees of clinical severity depending on the immune response of the host and the EBV viral load.
Case reportsWe described the clinicopathological findings of two children with CAEBV with a brief review of the literature.
ConclusionsRecognition of the disease is important for adequate management of the patient. EBV analysis should be included in the principal diagnostic tests for febrile children.
La infección crónica activa (CA) de células T o células tipo NK por virus de Epstein-Barr (VEB) es un desorden linfoproliferativo (DLP) VEB+ policlonal, oligoclonal o, frecuentemente, monoclonal reconocido como representación del espectro del DLP de células T y células NK asociado con VEB que tiene diversas presentaciones clínicas: un padecimiento sistémico y dos cutáneos que incluyen el DLP de células T que semeja hidroa vacciniforme y la hipersensibilidad por picadura de mosquito. Los síntomas de la enfermedad sistémica incluyen fiebre, hepatitis persistente, hepatoesplenomegalia y linfadenopatías que muestran diferente grado de severidad clínica, dependiendo de la respuesta inmune del hospedero y de la carga viral del VEB.
Casos clínicosSe describen los hallazgos clínico-patológicos de dos niños con CAVEB y una breve revisión de la literatura.
ConclusionesEs importante reconocer esta enfermedad para proporcionar el manejo adecuado al paciente. El análisis de VEB debería incluirse como una de las principales pruebas diagnósticas en niños con fiebre.
Chronic active Epstein-Barr virus infection (CAEBV) is a clinical term initially defined by Straus as a disease related to chronic or persistent infection of EBV. Suggested criteria for diagnosing severe chronic EBV infection includes the following: 1) severe illness of >6 months duration that began as primary EBV infection and is associated with grossly abnormal EBV antibody titers [IgG to viral capsid antigen (VCA)=∼1:5120; antibody to early antigen (EA)=∼1:640; or antibody to EBV nuclear antigen (EBNA) <2], and 2) histological evidence of major organ involvement such as interstitial pneumonia, hypoplasia of some bone marrow elements, uveitis, lymphadenitis, persistent hepatitis or splenomegaly, and 3) detection of increased quantities of EBV in affected tissues by anticomplementary immunofluorescence for EBNA or nucleic acid hybridization1. In early reports, the cellular lineage of EBV-infected cells has not drawn attention; however, subsequent reports documented that EBV mainly exists in T-cells2,3 but not in B-cells, as revealed by dual staining immunofluorescence analysis3. Just as the accumulation of clinical and laboratory data for CAEBV, it has been recognized that clinical and laboratory findings of CAEBV are of a broader spectrum than initially thought. Because individual cases do not fulfill the diagnostic criteria initially proposed, Kimura and colleagues modified the diagnostic criteria for CAEBV as follows: (1) illness ≥3 months duration [EBV-related illness or symptoms including fever, persistent hepatitis, extensive lymphadenopathy, hepatosplenomegaly, pancytopenia, uveitis, interstitial pneumonia, hydroa vacciniforme (HV), and hypersensitivity to mosquito bites (HMB)]; (2) increased amounts of EBV DNA or grossly abnormal levels of EBV antibodies, for instance, detection of EBV DNA in tissues or peripheral blood by Southern blot hybridization; EBV-encoded small RNA-1-positive cells in affected tissues or peripheral blood, >102.5 copies of EBV DNA/μg of DNA of peripheral blood mononuclear cells; and grossly abnormal levels of EBV antibodies (anti-VCA IgG titers ≥5120 or anti- EA IgG titers ≥640), and (3) no evidence of previous immunological abnormalities or other recent infection that might explain the observed condition.4
Based on the accumulated data, CAEBV of T-cell or NK-cell type has been recently defined as a systemic EBV+ polyclonal, oligoclonal or often monoclonal lymphoproliferative disorder (LPD) characterized by fever, persistent hepatitis, hepatosplenomegaly and lymphadenopathy, which shows varying degrees of clinical severity depending on the immune response of the host and the EBV viral load.5,6
CAEBV is often accompanied by cutaneous lesions such as severe mosquito bite allergy and hydroa-vacciniforme (HV)-like T-cell lymphoproliferative disease. Mosquito bite hypersensitivity (or mosquito bite allergy, MBH) is a unique cutaneous manifestation of CAEBV infection characterized by abnormally intense local reaction at arthropod bite area associated with systemic symptoms and signs like fever, lymphadenopathy and liver dysfunction. EBV genome is mainly found within NK cells of peripheral blood of MBH patients who often show NK cell lymphocytosis.7–10
HV-like LPD is an EBV-associated polyclonal, oligoclonal, or monoclonal cutaneous T-cell lymphoproliferative disease characterized by recurrent vesiculopapular eruptions, mainly on the face and arms. It shows a broad spectrum of clinical aggressiveness and usually a long clinical course with risk to develop systemic lymphoma. As the disease progresses patients develop severe and extensive skin lesions with systemic symptoms including fever, hepatosplenomegaly and lymphadenopathy. Classic HV, severe HV and HV-like T-cell lymphoma constitute a continuum spectrum of EBV-associated HV-like LPD.5,11–14
Recently, the three disorders indicated above have been recognized as representing the spectrum of EBV-associated T-cell and NK-cell LPD with different clinical presentations; one systemic and two cutaneous disorders including HV-like T-cell LPD and MBH.14
2Case reportsThe patients described in the following two case reports depict typical clinical manifestations of CAEBV with MBH in children. Case 2 was reported previously.15
2.1Case 1A 15-year-old Korean boy was admitted to the hospital with chronic intermittent dermatological and various systemic symptoms and signs since he was 5 years old. Birth weight was normal and adequate for gestational age. At a recent visit to a pediatric department, he showed adequate height of 163.7cm for his age, but a low body weight of 39.6kg.
At 5 years old, fever, cough, and abdominal distention suddenly developed. White blood cell count was 12.1×103/μl (lymphocytes 70%, segmented neutrophils 21%, eosinophils 1%, and monocytes 8%). Qualitative fluorescent antinuclear antibody was negative. Following a clinical diagnosis of intestinal perforation, he underwent a subtotal colectomy and ileostomy. One month after surgery, lymphocyte subset counts by flow cytometry showed low numbers of B- and T-cells, with markedly increased NK-cells (CD16+CD56+=68%, normal range 5.6-31%); 1,579/μl (normal range 100-430/μl).
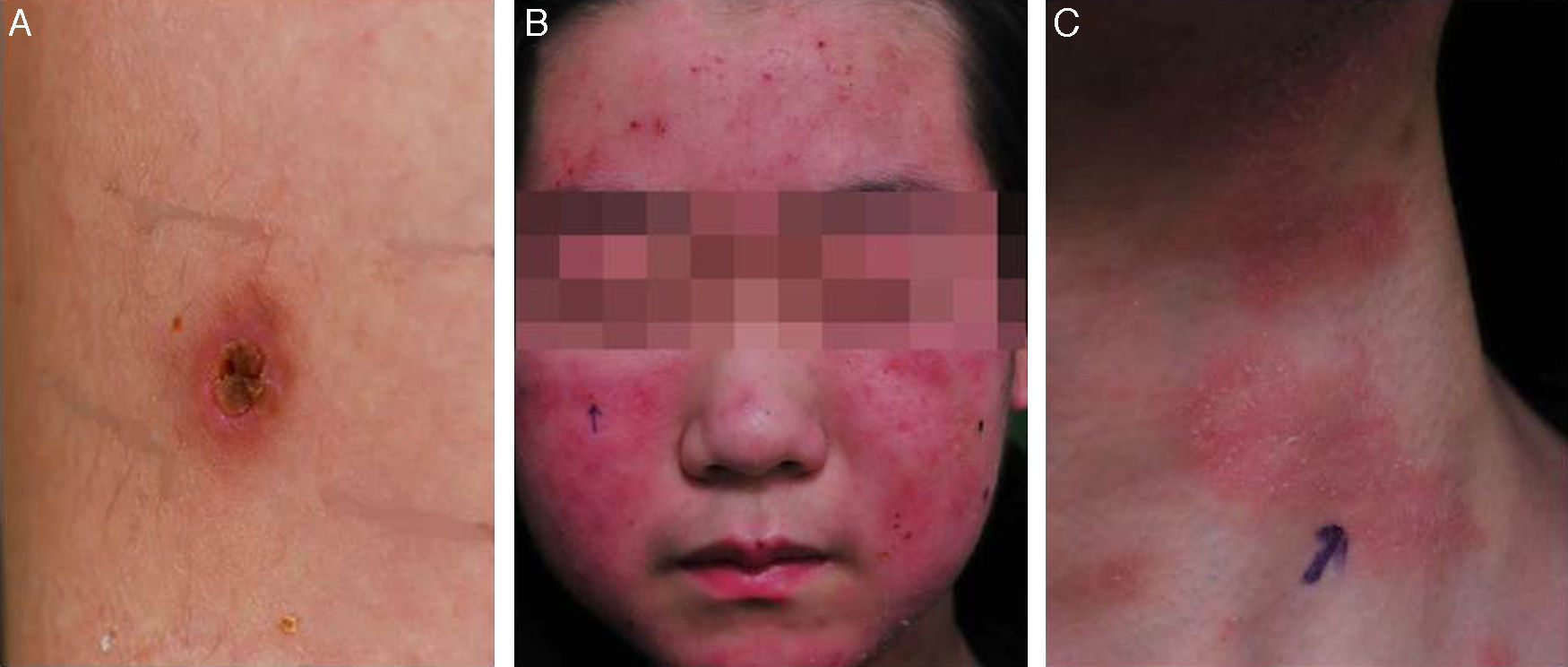
He was subsequently hospitalized five times in 3 years because of acute pharyngotonsillitis, upper respiratory infection, bronchiolitis, and pneumonia and experienced recurrent fever two or three times per month. At 10 years old, he was hospitalized because of upper respiratory tract infection. Splenomegaly of 10.76cm was found incidentally during workup. From the age of 10, intense skin responses manifested at mosquito- or other bug-bitten sites that were characterized by an initial bullous change followed by ulceration, crust formation, and scarring with pigmentation (Fig. 1). Most lesions were <1cm in diameter and were frequently accompanied by fever. Such cutaneous lesions tended to occur in the summer and on the upper and lower extremities. Laboratory tests performed at the time of severe mosquito bite allergy showed mild elevation of aspartate transaminase/alanine transaminase (AST/ALT) with significant elevation of alkaline phosphatase (200 U/l) and globin (4.5 to 4.8g/dl).
A skin biopsy obtained from a lesion was reported as indicating a possibility of Sweet's syndrome. Abdominal sonography indicated splenomegaly. Differential counts of peripheral blood showed low B- and T-cell populations, and NK-cell lymphocytosis [CD16+CD56+=62%; 3520/μl (normal range=70-1200/μl)]. The serum IgE level was increased to 20,370 U/ml although no specific response to various allergen stimuli was identified. Despite no proven family history, a clinical impression of hyper-IgE syndrome was made as the first differential diagnosis. A genetic study that included STAT3 sequencing was performed but failed to find the expected mutation. In considering the clinical course and NK lymphocytosis, CAEBV or EBV-associated lymphoproliferative disease was considered as other differential diagnoses and relevant tests were established.
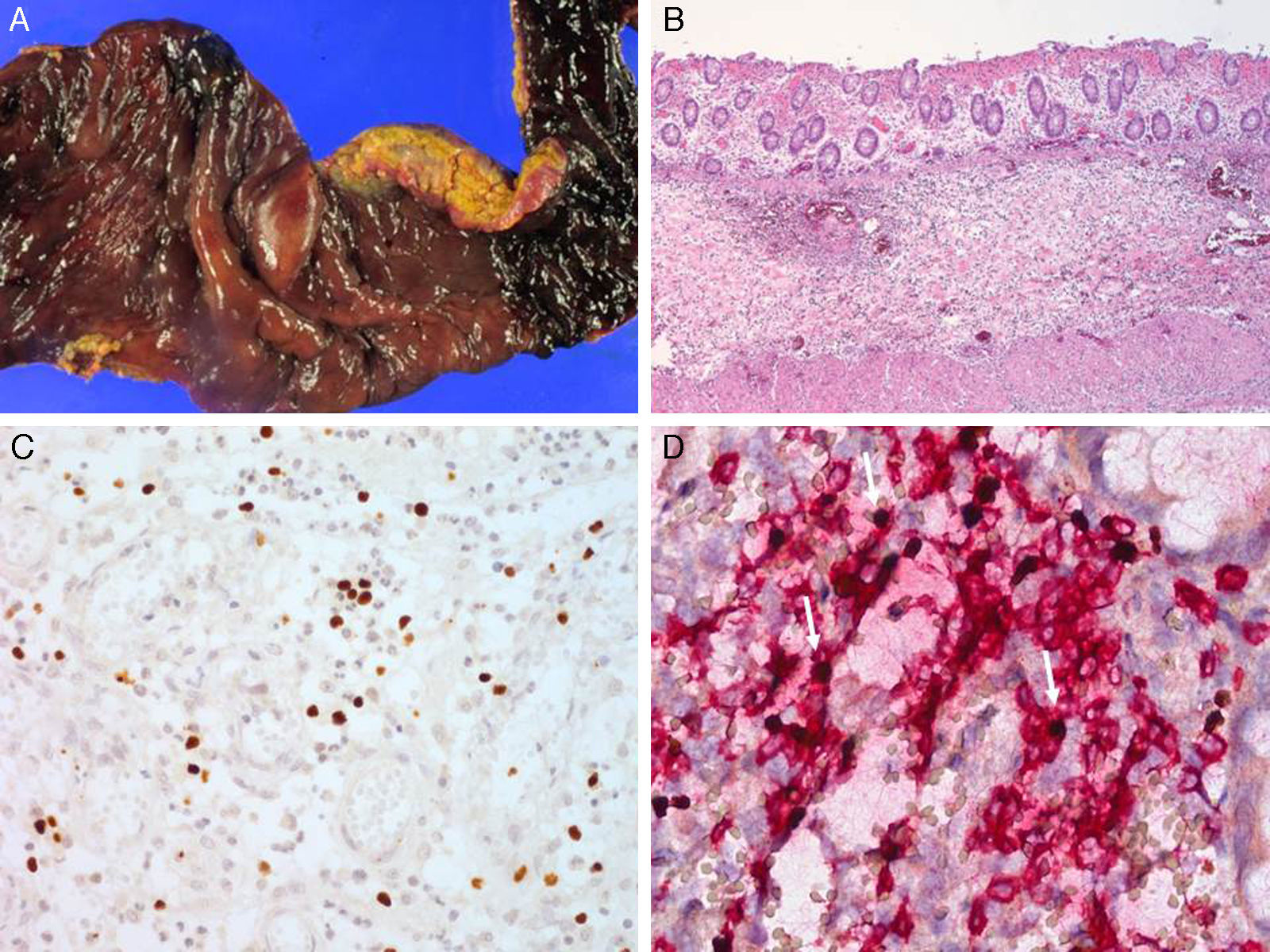
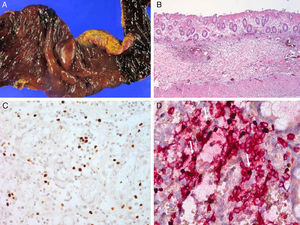
Serologic tests showed positivity of EBV VCA-IgG, EBV-early antigen, and negativity of EBV VCA IgM. Using a whole blood sample, real-time quantitative PCR for EBV DNA was performed to determine the viral load in blood (11,450 copies/5μl of whole blood). Subsequently, he underwent a regular checkup of viral genome copy number, which was found to be consistently high, ranging from 212.5 copies/μl to 1,562 copies/μl of whole blood. His skin problem associated with mosquitoes or bugs had occurred intermittently ever since, while intermittent episodes of febrile illness devoid of skin lesion had also persisted. Nonspecific skin problem persisted for 15 months. Physical examination revealed erythematous to brownish scaly patches with yellowish crusts on his face, neck, scalp, upper chest, and upper back. He was treated with topical steroids and oral antihistamines under impression of seborrheic dermatitis for 11 months (Fig. 1). Two months prior to examination, his symptoms became aggravated and he visited a dermatology clinic. Two skin biopsies from his face and neck were taken and EBV in situ hybridization was performed. The biopsies showed increased lymphoplasma cells in the superficial and deep dermis with occasional periadnexal distribution. There was no clear atypia in most lymphoid cells. Immunohistochemistry showed many CD56+ lymphoid cells. There were numerous lymphocytes showing a nuclear positive reaction to EBER in situ hybridization. Retrospectively, EBER in situ hybridization was applied to both the skin biopsy of the lower leg taken for severe mosquito bite allergy and that of the perforated intestine resected 10 years ago (Fig. 2). Many lymphoid cells with a positive reaction were identified, not only in the skin but also in the intestinal wall and sinusoids of the liver and lymph node, which confirmed CAEBV infection with mosquito bite allergy and NK lymphocytosis. The patient was treated conservatively. Two years later he was readmitted because of hemophagocytic lymphohistiocytosis (HLH). He was treated according to the HLH-2004 treatment guideline16 and responded transiently, but later developed bowel perforation and died of sepsis.
2.2Case 2A 10-year-old Korean boy presented to the Department of Dermatology complaining of intense skin reactions at mosquito bite sites. He exhibited multiple scattered, punctuated ulcerations on bilateral lower extremities. In addition to the marked cutaneous reaction, he also endorsed systemic symptoms including fever following insect bites throughout his childhood. A skin biopsy was taken and local treatment for the lesion was administered without further systemic evaluation. At 16 years of age, the patient admitted with a palpable mass located on the left neck of 23 weeks’ duration. On physical examination, a conglomerate of large lymph nodes was present in the left neck, the largest of which measured 7×5cm. Due to his history of skin lesions, careful inspection of his legs was performed, revealing multiple shallow ulcers with healing scars. Computed tomography and positron emission tomography scan showed multiple poorly enhancing and variable sized homogeneous lymph nodes involving the left cervical region and bilateral inguinal areas. In addition, there were multifocal lesions demonstrating increased 2-deoxy-2-[fluorine-18] fluoro-D-glucose uptake in the skin and subcutaneous layer of bilateral cheeks and buttocks. On hematologic examination, blood cell counts and lactic acid dehydrogenase level were within normal limits. Anti-VCA IgG, anti-EA and anti-EBNA IgG were positive and anti-VCA IgM was negative. The EBV DNA copy number was 529.8 copies/μl of whole blood.
Lymph node biopsy was performed revealing widening of the paracortex with infiltration of heterogeneous inflammatory cells including small lymphocytes, histiocytes and many eosinophils. The inflammatory infiltrate was punctuated by scattered large mononuclear or multinuclear Reed-Sternberg (RS)-like atypical cells, which had large eosinophilic nucleoli and thick nuclear membrane. Using immunohistochemical study, the RS-like cells demonstrated a strong membranous stain for CD30 and perinuclear staining for CD15 and were positive for PAX-5 and LMP-1, but negative for CD20, CD3, and EBNA-2. Atypical mononuclear and multinuclear RS-like cells showed a positive signal by EBER in situ hybridization. Aside from the RS-like cells, many small lymphocytes scattered in the background were also positive for EBER in situ hybridization. No abnormal findings were noted in the bone marrow biopsy except for a few EBV-positive small lymphocytes. The skin biopsy from the region of mosquito-bite allergy in 2002 was retrospectively reviewed, revealing severe necrosis of the epidermis and upper dermis. There was infiltration of small lymphocytes, histiocytes and many eosinophils around the blood vessels and hair follicles and the blood vessel lumens were often obliterated by red blood cells (RBCs) and fibrin. Immunohistochemical analysis revealed that the infiltrating cells were heterogeneous in their lineage; some were positive to CD3 and CD4 or CD8 helper or cytotoxic T cells, whereas others were NK cells positive for CD56. Many EBV-positive cells were documented by EBER in situ hybridization. The diagnosis of chronic active EBV infection with mosquito bite hypersensitivity and polymorphic lymphoproliferation simulating Hodgkin's lymphoma was made. The patient was treated with ABVD (Adriamycin, Bleomycin, Vinblastin, and Dacarbazine) for six cycles with complete remission. Currently he remains free of disease for 7 years after treatment.
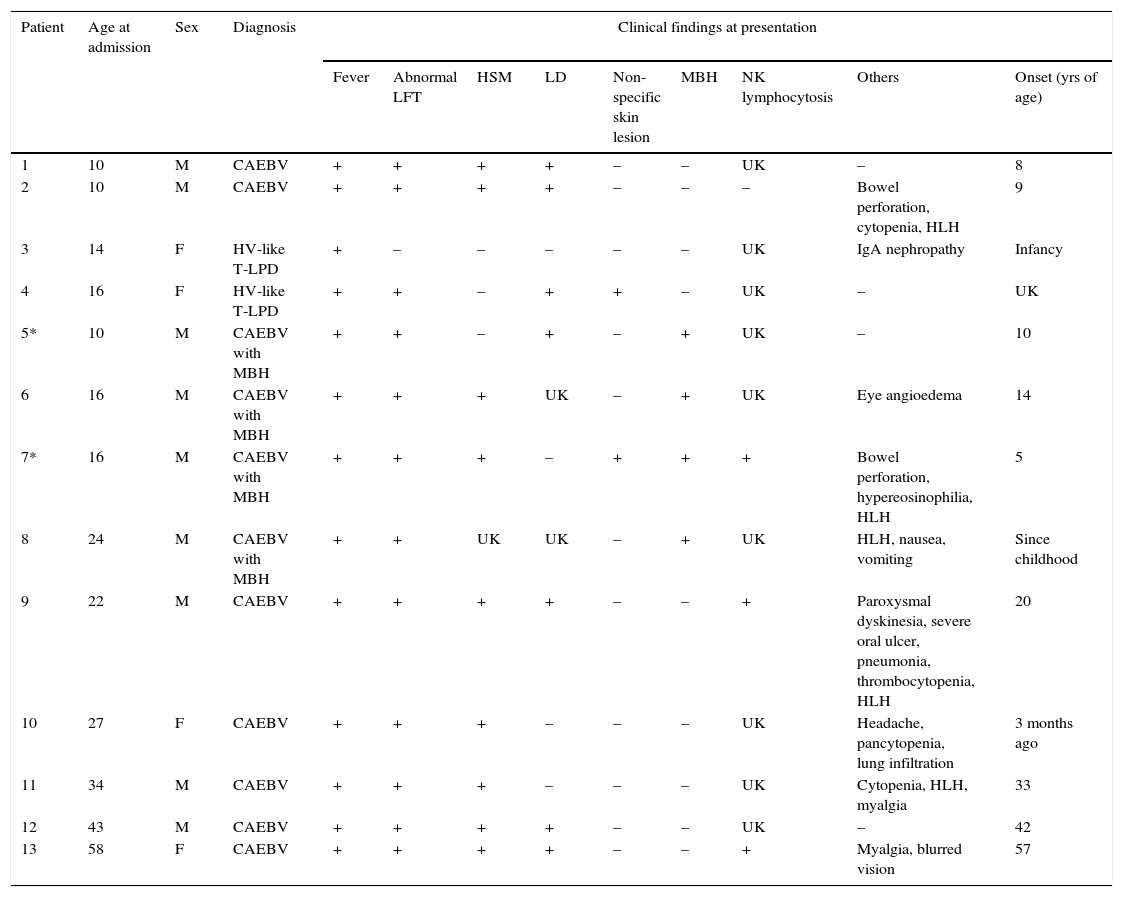
3DiscussionThe clinical course of CAEBV patients depends on the balance between EBV-related factors and host immune function and can be smoldering, progressive, or aggressive. Some patients develop EBV+ T/NK cell lymphoma/leukemia.4,17 From 1998 to 2014, 13 patients aged 10–58 years (median age 22 years, nine males and four females) were diagnosed with CAEBV disease at Samsung Medical Center, Korea (Table 1). The common clinical findings included fever (13/13), hepatosplenomegaly (9/12), lymphadenopathy (7/11), NK lymphocytosis (3/4), MBH (4/13), and HV-like LPD (2/13). Some patients presented with bowel perforation, chorea, or brain infarction. In the median follow-up of 36 months, seven patients (54%) died of the disease, two patients (15%) had persistent disease, and two patients (17%) were free of disease. Two patients were lost to follow-up. The causes of death were hemophagocytic syndrome and organ failure in four patients, EBV-positive T cell lymphoma in one patient, and aggressive NK cell leukemia in one patient. B-lineage lymphoproliferation mimicking Hodgkin's lymphoma as seen in case 2 is rare but has been previously reported.18
Clinical and laboratory findings of 13 patients with chronic active EBV infection related disease.
| Patient | Age at admission | Sex | Diagnosis | Clinical findings at presentation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Abnormal LFT | HSM | LD | Non-specific skin lesion | MBH | NK lymphocytosis | Others | Onset (yrs of age) | ||||
| 1 | 10 | M | CAEBV | + | + | + | + | – | – | UK | – | 8 |
| 2 | 10 | M | CAEBV | + | + | + | + | – | – | – | Bowel perforation, cytopenia, HLH | 9 |
| 3 | 14 | F | HV-like T-LPD | + | – | – | – | – | – | UK | IgA nephropathy | Infancy |
| 4 | 16 | F | HV-like T-LPD | + | + | – | + | + | – | UK | – | UK |
| 5* | 10 | M | CAEBV with MBH | + | + | – | + | – | + | UK | – | 10 |
| 6 | 16 | M | CAEBV with MBH | + | + | + | UK | – | + | UK | Eye angioedema | 14 |
| 7* | 16 | M | CAEBV with MBH | + | + | + | – | + | + | + | Bowel perforation, hypereosinophilia, HLH | 5 |
| 8 | 24 | M | CAEBV with MBH | + | + | UK | UK | – | + | UK | HLH, nausea, vomiting | Since childhood |
| 9 | 22 | M | CAEBV | + | + | + | + | – | – | + | Paroxysmal dyskinesia, severe oral ulcer, pneumonia, thrombocytopenia, HLH | 20 |
| 10 | 27 | F | CAEBV | + | + | + | – | – | – | UK | Headache, pancytopenia, lung infiltration | 3 months ago |
| 11 | 34 | M | CAEBV | + | + | + | – | – | – | UK | Cytopenia, HLH, myalgia | 33 |
| 12 | 43 | M | CAEBV | + | + | + | + | – | – | UK | – | 42 |
| 13 | 58 | F | CAEBV | + | + | + | + | – | – | + | Myalgia, blurred vision | 57 |
| Patient | TCRγ Gene rearrangement | EBV load in whole blood/Serology | Treatment | FU (yrs) | Outcome | Causes of death |
|---|---|---|---|---|---|---|
| 1 | Monoclonal | 1143 copies/5 μl | HLH 2004 protocol | 3 | Died | Acute hepatic failure, HLH, hepatic encephalopathy |
| 2 | polyclonal | 7.5 copies/5 μl | Allo PBSCT | 3 | Alive | |
| 3 | polyclonal | ND | CHOP | 13 | Died | Sepsis, EBV+ T-cell lymphoma at larynx |
| 4 | polyclonal | ND | None | 5 | Alive | |
| 5* | polyclonal | EA+, VCA IgM+, 35 copies/5 μl | ABVD | 13 | Alive | |
| 6 | ND | 7,000 copies/μl/EA+, VCA IgM+ | None | 2.5 | Alive | |
| 7* | polyclonal | 1,562 copies/5 μl, VCA IgM/IgG (–/+), EA+ EBNA+ | HLH 2004 protocol | 11 | Died | HLH, hematochezia |
| 8 | ND | ND | IMVP16 | 10 | Died | ANKL |
| 9 | polyclonal | 150 copies/5 μl | CHOP, ICE/DEXA | 2 | Died | HLH |
| 10 | polyclonal | 6936 copies/5 μl | UK | 1.5/lost to FU | Alive | |
| 11 | ND | 17,230 copies/5 μl VCA IgG+, -VCA IgM- EA+, EBNA+ | None | 1 | Died | UK |
| 12 | ND | EA+, VCA IgG+, EBNA+ | None | 0.5 | Died | HLH |
| 13 | ND | 326.40 copies/5 μl | CVP | lost to FU | Alive |
5*: Case 2, 7*: Case 1.
MBH, mosquito-bite hypersensitivity; LFT, liver function test; LPD, lymphoproliferative disease; FU, follow up; ND, not done; ANKL, aggressive NK cell leukemia; HSM, hepatosplenomegaly; LD, lymphadenopathy; PBSCT, peripheral blood stem cell transplantation; HV, hydroa vacciniforme; HLH, hemophagocytic lymphohistiocytosis; UK, unknown.
CAEBV infection is almost always accompanied by varying degrees of lymphoproliferation. The clonality of EBV and EBV-infected T- or NK-cells varies and may be polyclonal, oligoclonal, or monoclonal. As the disease progresses from polyclonal lymphoproliferation to monoclonal disease, histological atypia increases. Ohshima et al. proposed the categorization of CAEBV into three groups—polymorphous and polyclonal, polymorphous and monoclonal, or monomorphic and monoclonal-based on clonality and histological changes.19 In the series reported by Ohshima et al., 8/48 patients with CAEBV infection were polyclonal for TCR gene rearrangement and the infiltrated cells displayed polymorphic histomorphology; 15 patients showed polymorphic morphology and biclonal or monoclonal TCR gene rearrangement; and 25 patients showed monomorphic histomorphology and monoclonal TCR gene rearrangement. Patients with the monomorphic and monoclonal type of CAEBV infection had poorer prognoses than those with polymorphic polyclonal or polymorphic monoclonal disease. The survival of the polymorphic/polyclonal and polymorphic/monoclonal groups did not differ significantly. Monomorphic/monoclonal groups of CAEBV by Ohshima et al. may correspond to systemic T-cell LPD according to the 2008 WHO classification.20
In summary, CAEBV is a rare EBV-associated LPD of predominantly T-cell or NK-cell lineage with a broad spectrum of clinical presentation. Recognition of the disease is important for adequate management of the patient. EBV analysis through real-time PCR of a blood sample or EBV in situ hybridization in the affected tissue is important to avoid overlooking these patients and should be included in the principal diagnostic tests for febrile children.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interest of any nature.