Diabetic foot is a major health problem all over the world. Approximately 15% of the 200 million people with diabetes worldwide will develop a foot ulcer during their lifetime. Major amputation is a feared complication of diabetes. Many patients who undergo an amputation have a history of ulceration. More than 60% of non-traumatic amputations in the western world are carried out in diabetes patients. Major amputations increase morbidity and mortality and reduce the patient's quality of life. An important prelude to diabetic foot treatment is the differing diagnosis of neuropathic and neuroischemic foot. Treatment of a neuropathic plantar ulcer must correct pathological plantar distribution of pressures. Surgical treatment of deformities, with or without ulcerations, is an effective therapy. Charcot neuroarthropathy is a particular complication of neuropathy wich may lead to fragmentation or destruction of joints and bones. Additionally, in the diabetic population peripheral vascular disease (PVD) is the main risk factor for amputation. If PVD is not diagnosed, treatment of the ulcer cannot be successful. In diabetic patients PVD is distal, but often fully involves the femoral, popliteal and tibial vessels. It can be successfully treated with either open surgical or endovascular procedures. Finally, infection is a serious complication of diabetic foot. Phlegmon or necrotizing fascitis are not only limb-threatening problems, but also life-threatening ones. In this case emergency surgery is mandatory.
Achilles tendon
by-pass grafting
calcitonin gene-related peptide
critical limb ischemia
Charcot neuroarthropathy
deoxypyridinoline
carboxy-terminal telopeptide domain of type I collagen
interleukin-6
medial arterial calcinosis
nitric oxyde synthase
N telopeptides of type I collagen
osteoprotegerin
percutaneous transluminal angioplasty
peripheral vascular disease
NF-kB ligand
TransAtlantic Inter-Society Consensus
transcutaneous oxygen tension
tumor necrosis factor alpha.
Approximately 15% of diabetic patients experience a foot ulcer at some point in their lives.1 More than 60% of non-traumatic amputations in the western world are performed in the diabetic population, with the incidence of major amputation varying from 0.5 to 5 per 1000 patients.2,3 Morbidity and mortality rates are higher in patients with ulcerations. Mortality in the perioperative period is particularly high: reported to be 9% in a Dutch study4 and 10-15% in the UK.5 In a follow-up study of an amputated population, our group reported a 5-year survival rate of 50%.6
Diabetic foot must be considered a complex entity. Two types are recognized: neuropathic foot and neuroischemic foot.7 The two entities have different pathophysiological mechanisms, diagnostic-therapeutic phases, and outcomes. There are different indications, different procedures and different organization of care in diabetic patients with neuropathic and neuroischaemic foot.
The neuropathic footThe association between peripheral neuropathy and foot ulcers is uniform in the literature.8-12 Neuropathy is associated with an eight- to 18-fold increased risk of ulceration and a two- to 15-fold increased risk of amputation.8,9
The mechanisms through which neuropathy acts as a pathogenetic event for ulceration and, thus, amputation are complex and varied.8-12 Above all, the reduction in protective sensitivity (including sensitivity to pain and heat) leads to a reduction in the perception of pain stimuli. Moreover, the motor component of neuropathy involves progressive weakening of the intrinsic muscle component, made up of interosseous and lumbrical muscles. This reveals itself as a deformation in toe flexion and the formation of overloaded plantar areas, identifiable under the metatarsal heads and the tips of the toes. Finally, the autonomous component of neuropathy causes anhydrosis and dry, flaky skin, as well as an increase in arteriovenous shunting, leading to altered skin and bone perfusion.
Proper debridement must follow the evaluation of an ulcer. This should completely remove the callus that surrounds the lesion and all non-healthy tissue, until healthy bleeding edges are revealed. Sharp debridement allows for thorough removal of all necrotic material and diminishes the bacterial load, thus promoting healing.13-16 Although debridement of the ulcer is considered essential for the healing of diabetic foot ulcers, the grade of evidence is low.17 However, this is likely to be related to the lack of studies rather than a lack of effect.
Subsequently, it is necessary to carry out an accurate "probeto-bone" maneuver in order to establish any involvement of deeper structures such as tendons, joint capsules, and bones. In our opinion, in many cases the probe-to-bone maneuver with a sterile blunt instrument is adequate to diagnose osteomyelitis. Therefore, it is only necessary to use more complex methods (such as nuclear magnetic resonance and/or radio-labeled leukocyte scanning) in a small percentage of cases.18-24 A number of recent studies have questioned the reliability of the probe-tobone test.25,26 We entirely agree with the authors of these studies in the case that all patients are being considered and when the probe-to-bone test is being used as a screening tool. However, in "surgical patients" and in those with clinical signs of infection we still believe the maneuver is useful and reliable.
The literature clearly demonstrates that offloading is essential in cases of non-complicated plantar neuropathic lesions. Simple offloading techniques are multifaceted and include casts and boots, sandals, half-shoes, and felted foam dressings.27-37
Armstrong and Frykberg have provided a classification of diabetic foot surgery that correlates classes of treatment with a risk of amputation score.38 The indications for surgical treatment of plantar neuropathic ulcers are essentially as follows:
- •
Coexistence of osteomyelitis.
- •
Plantar exostosis, which puts the healed wound at a high risk of recurrence.
- •
Chronically ulcerated wound resistant to conservative therapy.
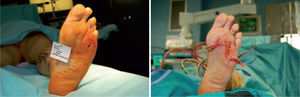
In these situations, surgery allows two important results to be achieved: shorter wound healing time and surgical correction of the pathological overload by means of anatomical correction of the exostosis (figure 1).39
Piaggesi et al. have demonstrated that surgical treatment of a wound (ulcerectomy) accompanied by modification of the pathological overload (exostectomy) in a population of diabetic patients affected by plantar neuropathic ulcers allowed significantly shorter healing times and a lower rate of ulcerative repetition compared with conservative treatment.14
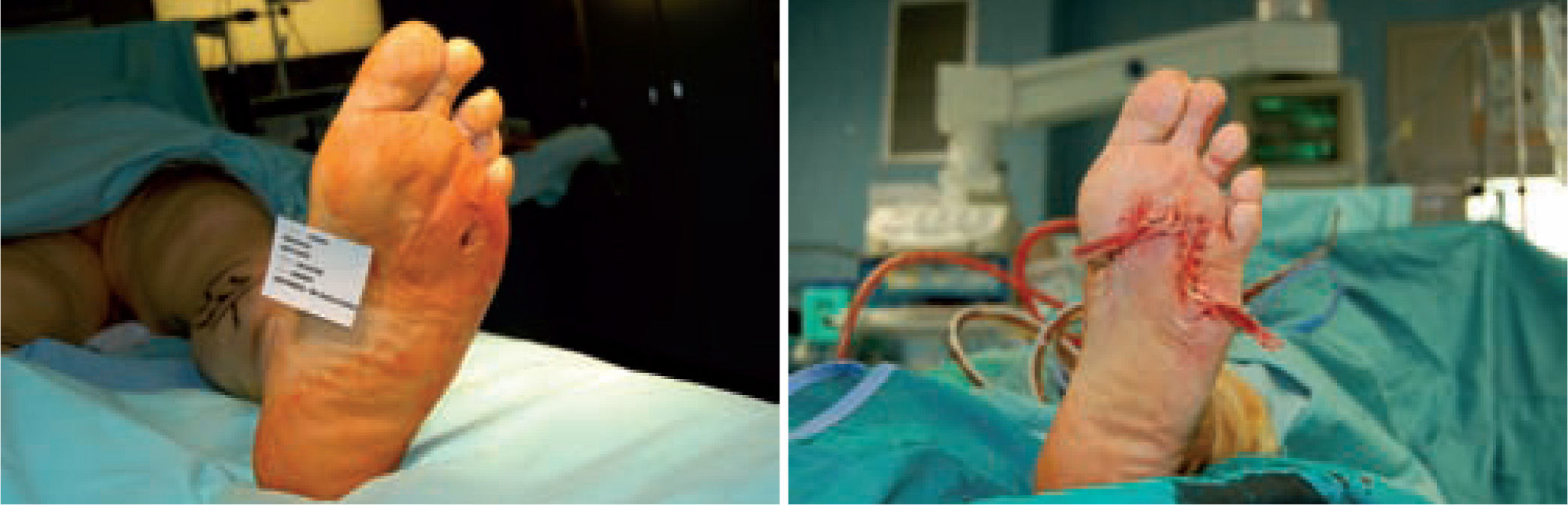
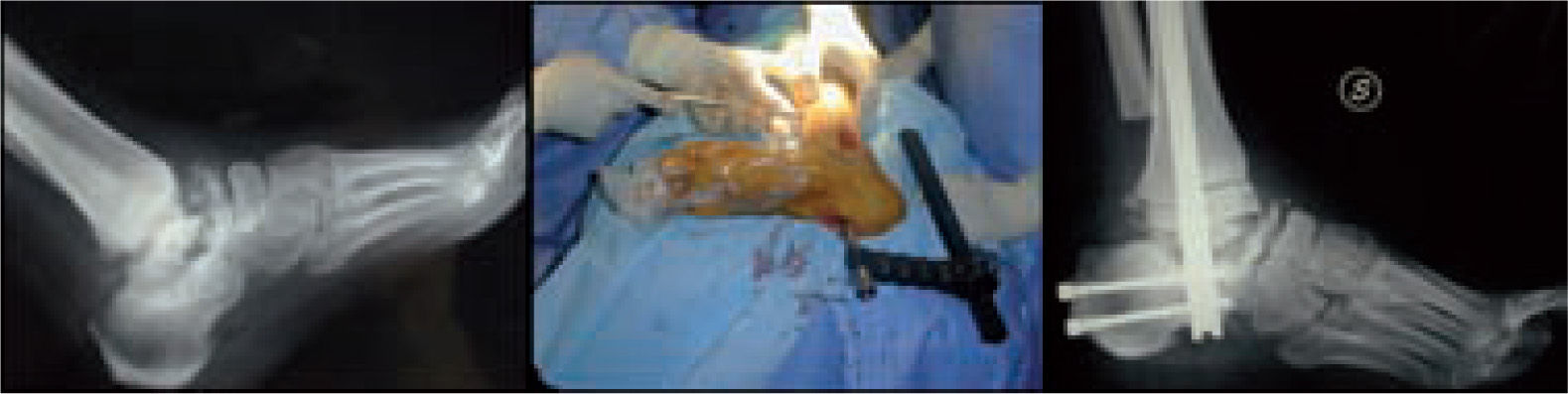
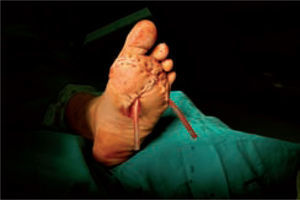
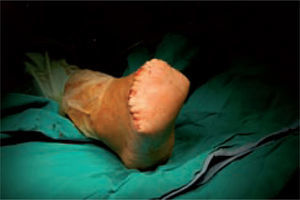
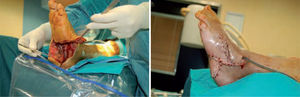
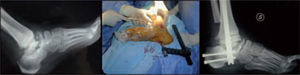
Microbiological assessment is performed to choose the appropriate antibiotic treatment before ulcerectomy. Appropriate specimens are obtained from the wound bed with a biopsy of the deep soft tissue. It is necessary to establish any involvement of bone (such as a metatarsal head) so as to plan the best type of surgery for the wound. Involvement of more than one metatarsal head or the presence of a vast plantar lesion may indicate the need for more complex surgical techniques, such as pan metatarsal head resection or minor amputation (figures 2 and 3).
Surgery should be not only curative but also effective in preventing new ulceration. Treatment of the overload by lengthening the Achilles tendon (AT) has been shown to be effective in reducing both the plantar pressures of the forefoot and the primary risk of ulceration and recurrent infection.40
Charcot neuroarthropathyCharcot neuroarthropathy (CN) is one of the most devastating complications of diabetes and, in its most severe form, it may lead to ulceration, infection, and, subsequently, amputation.41,42 CN has been defined as the simultaneous presence of bone and joint destruction, fragmentation, and remodeling.43 With respect to epidemiology, diabetes is the most common cause of CN.42,43 The prevalence of CN in the diabetic population varies from 0.15% to 6.8% in published series.44-46
The CN has been defined as a disease in which there is the simultaneous presence of bone and joint destruction, fragmentation and remodelling.43
In a more recent study in Manchester the prevalence of CN, evaluated on radiological changes, was 9% in a population of selected neuropathic diabetic patients.47 The initiating event of the Charcot process is often a seemingly trivial injury or unnoticed minor trauma resulting in minor periarticular or major fractures in susceptible diabetic feet.44 Peripheral and autonomic neuropathy together with an adequate blood supply appears to be prerequisites for the development of susceptibility.43,44,48
The reason why seemingly trivial injuries should lead to such major catastrophic destruction of the foot has not yet been completely explained, although reduced bone density in the lower limbs in Charcot patients has been demonstrated which might make such patients at a higher risk for developing these changes.47 It is thought that increased blood flow and arteriovenous shunting as a consequence of autonomic neuropathy, lead to increased osteoclastic activity resulting in bone resorption.48
Together with an increased risk of tripping and falling and repetitive trauma from the higher foot pressures in neuropathic diabetic patients, these factors may then predispose to CN.43,47 To date there have been a few relatively small studies which have both directly and indirectly indicated that there is an increased rate of bone remodelling occurring in Charcot foot disease. Employing a urinary marker of bone resorption such as cross linked N telopeptides of type I collagen (NTX), Edelson et al. have indicated increased levels of collagen breakdown in subjects with Charcot neuroarthropathy.49 More recently Gough et al. assayed both markers of osteoblastic and osteoclastic activity in the foot as well as systematically.50 They found the pyridinoline cross linked carboxy-terminal telopeptide domain of type I collagen (ICTP) was significantly more prevalent in diabetic patients with acute CN. Similarly, we also found an increase in the bone turnover marker urinary deoxypyridinoline (DPD) (both DPD and ICTP are markers of increased bone resorption) in patients with active CN.51 Both these studies also found that alkaline phosphatase was higher in patients with Charcot arthropathy compared to controls. These results suggest that the acute Charcot foot has excessive osteoclastic activity with possible increase in osteoblastic activity suggesting continuous bone remodelling. Recent data have suggested that there may be other underlying pathogenic factors involved in the Charcot process such as involvement of the cytokines tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and also nitric oxyde synthase (NOS) and calcitonin gene-related peptide (CGRP).52,53 Baumhauer et al. demonstrate the presence of TNF-α and IL-6 in more than 90% of osteoclasts.52 These cytokines have also been shown to mediate an acute inflammatory response following bone fracture.54 This suggests the constitutive expression of these regulatory molecules by osteoclasts during the remodelling process associated with Charcot arthropathy. In a separate study LaFontaine et al. showed that in the bones of the Charcot foot (compared to non-Charcot non diabetic individuals) there was reduced expression of CGRP and eNOS, both of which play a role in bone remodelling.53 CGRP impacts osteoblastic activity and eNOS osteoclastic activity.
Therefore with lack of eNOS there is lack of inhibition of osteoclastic activity and therefore increased bone resorption, and lack of CGRP leads to decreased bone formation and osteopaenia, IL-6 and TNF-α are produced in bone marrow cells and in osteoclasts and in combination have important effects on osteoclastic recruitment, proliferation and differentiation and they directly are involved in the regulation of eNOS activity, TNF-α has been shown to downregulate eNOS expression55 but increase iNOS expression. In the latter causing increased bone and joint inflammation54 whereas CGRP reduces TNF-α expression.56 TNF-α by promoting osteoclast differentiation and activation to increase bone resorption can therefore reduce bone density in the Charcot foot.57,58 These processes involve an interaction between receptor activator of NF-kB ligand (RANKL) and its receptor osteoprotegerin (OPG). This pathway has been shown to be unregulated in inflammatory joint disease.59 It has been hypothesized that rankle and OPG play a role in the development of the Charcot process.60 This hypothesis links the inflammatory component of acute CN to the abnormalities in bone metabolism.58 An initial bone fracture or microtrauma may trigger an exaggerated inflammatory response in an insensitive, hyperaemic foot by an excessive release of proinflammatory cytokines such as TNF-α and IL-1β61. These proinflammatory cytokines stimulate overexpression of RANKL, which in turn lead to maturation of osteoclasts, and subsequently leading to a typical osteolysis of CN.60 Therefore these cytokines are inter-related and their up-and/or down regulation can increase bone resorption and reduced bone formation, typical features in the Charcot foot.
Acute CN can be confused with cellulitis, osteomyelitis, and inflammatory arthropathy. Clinical suspicion of the condition is necessary, so that appropriate treatment can be instituted immediately in order to prevent the severe deformities seen in this condition. The first clinical manifestation of the disease is swelling and edema. On examination, the foot is generally warm, and may be inflamed and swollen. Surgical intervention may be present in the patient's history.62 Once the acute phase has subsided, which can take months, the foot progresses into the chronic stage. Chronic CN is painless, without a temperature differential in a deformed foot. CN can infrequently become reactivated if there is further trauma to the foot, in which case, in the presence of an ulceration, differentiating it from osteomyelitis can be difficult.44
There are a number of anatomical classifications of CN. In 1966, Harris and Brand described a pattern of tarsal disintegration in leprosy that is determined by posture and mechanical stress, which is often initiated by unrecognized trauma.63 Eichenholtz64 was the first to codify the phases of CN development in 1966; this classification was modified by Shibata et al. in 1990 with the introduction of stage 0 as the prodromal state (table 1).65
Eichenholtz phases in Charcot foot
|
Modified by Shibata et al.
In 1990, Sanders and Frykberg proposed a five-step classification for patterns of bone destruction.66 In addition, in 1993, Brodsky suggested an anatomical classification of the midand rear-foot.67
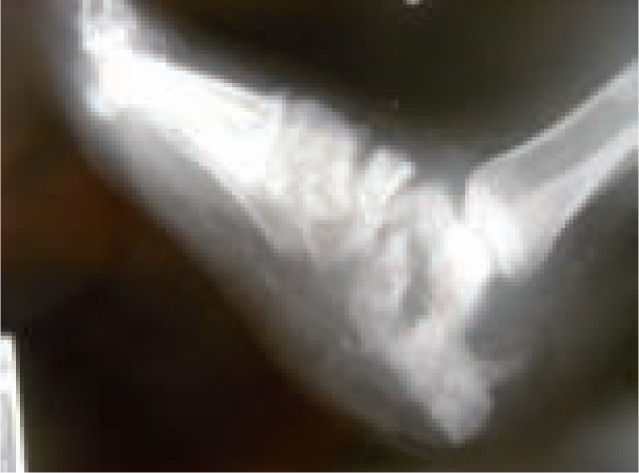
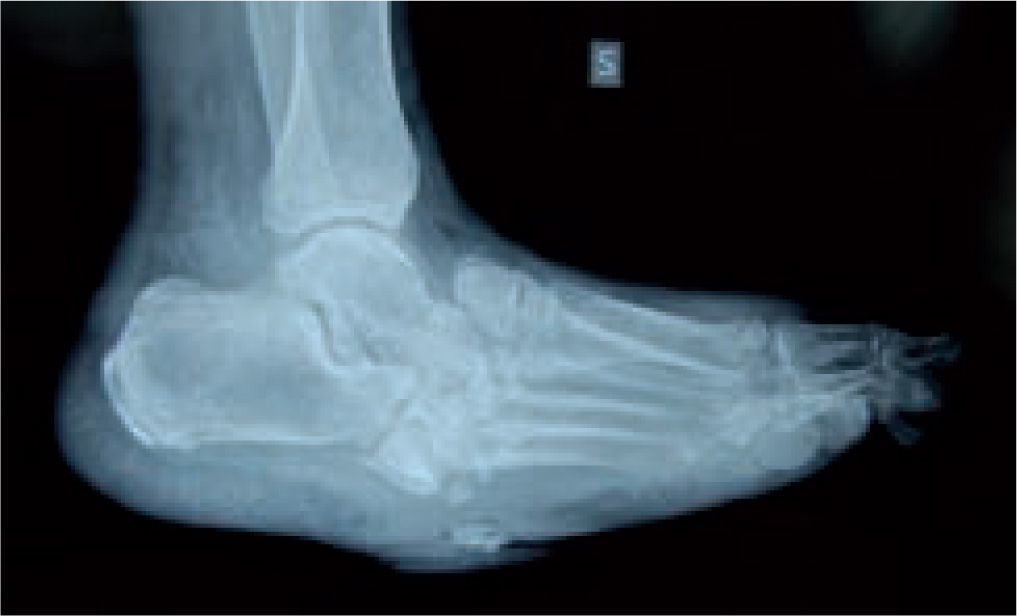
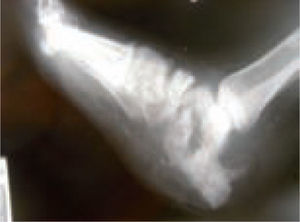
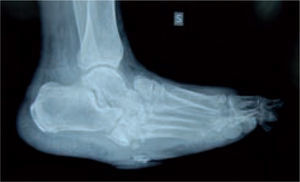
In the majority of cases, CN can be recognized via clinical features and plain X-rays. However, special investigations may be required. Plain X-rays will reveal bone and joint destruction (figure 4), fragmentation, and remodeling in advanced cases (figure 5), but early changes may be subtle or undetectable.68
The aims of treatment in CN are as follows:
- •
To heal fractures.
- •
To prevent deformities.
- •
To create and maintain a plantigrade, stable foot.
- •
To heal an ulceration if present.
The choice of treatment depends on the Eichenholtz stage and the location of the disease. The elective treatment of acute CN is prolonged immobilization. Clinical stabilization is proven by a reduction in skin temperature and the resolution of edema. Radiotherapy and ultrasound have been used in small trials without clear evidence of effectiveness in the treatment of acute CN.69,70
There is no pharmacological treatment approved for the treatment of acute CN. Bisphosphonates are synthetic analogues of inorganic pyrophosphate that inhibit bone turnover by reducing the resorption of bone. They do this directly, by inhibiting the recruitment and function of osteoclasts, and indirectly, by stimulating osteoblasts. Although it has not been demonstrated that bisphosphonates have any beneficial effects on the underlying acute Charcot process, all studies conducted to date have involved a small number of patients and larger randomized trials are urgently needed.71-75
The indications for, and timing of, surgery are controversial, since the surgical management of Charcot deformities of the foot and ankle continues to evolve.76 However, there are some specific clinical conditions that indicate a surgical approach is advisable (table 2). In general, practitioners treating patients with neuroarthropathy of the foot and ankle should be experienced and have the ability to recognize situations in which conservative treatment is likely to fail.
Indications for surgical treatment in Charcot neuroarthropathy
|
Joint dislocation with collapse of the plantar fascia and onset of plantar exostosis can be followed by ulceration. This is usually found at the midfoot level. In the majority of cases, this occurs at the Lisfranc line. The clinical characteristics of these ulcerations are usually persisting exostosis, large size, callus surrounding the wound, torpidity, and lack of healing. In this situation, the risk of osteomyelitis and subsequent amputation is high.77
In the case of superficial wounds (grade I-II in the Texas University Classification),78 the treatment of choice is offloading of pathological pressures together with local dressings, which vary depending on the state of the wound. Once the wound has healed, the decision is taken as to whether to place the patient in a prevention program. Through education, insoles, and rocker-bottom soles, the patient can keep the risk of recurrent ulceration under control.79-82
When the ulcerated wound involves joints and bones (grade III in the Texas University Classification), surgical treatment is indicated, usually entailing removal of the lesion, exposure, and clearing of exostosis. The bones involved are usually those in the medial aspect of the foot, the navicular and/or medial cuneiform and/or base of the first metatarsal; in the lateral aspect of the foot, the bones involved are the base of the fifth metatarsal and the cuboid (figure 6).
The unstable deformities of CN comprise the major indications for arthrodesis, the aim of which is to achieve stability of the joints involved, reducing the risk of further ulceration and the entire collapse of the foot. Reconstruction is considered in patients with deformities and instabilities, in whom surgical stabilization is the only alternative to major amputation.76
In-depth assessments of the international literature have highlighted some key points that must be followed in the surgical treatment of CN.83-85 The literature is oriented to consider surgery only once the quiescent state is obtained, which is characterized by the disappearance of signs of inflammation (reduced edema, normal skin color, lower temperature symmetrical to the contralateral limb). Two studies recently reported good results with early arthrodesis as an alternative to non-operative treatment;86 however, these studies were limited by a lack of follow-up.
The most common localization of CN involves the tarsometatarsal (Lisfranc) joints, but other joints in the midfoot may also be affected.63,66 Instability of the Lisfranc joints often results in a rocker-bottom deformity and plantar-medial ulceration. This is primarily owing to the base of the first metatarsal and medial cuneiform. Plantar-lateral ulcerations can be seen when the cuboid is plantarly extruded as part of a Charcot joint.63 Recently, Bevan and Tomlison have shown that some radiographic measures can predict ulceration in CN of the midfoot.87 Management of these problems requires a flexible approach. Treatment may involve simple exostectomy, with or without a fasciocutaneous flap, or primary arthrodesis of the involved joints to provide stability. Either approach may require concomitant AT lengthening to address ankle equinus.88
Fusion procedures are traditionally carried out with internal fixation. Studies showing positive results can be found in the literature, although with significant complication rates, varying between 30% and 70%.84-86,89-95 Complications include failure of fixation, infections, and sepsis, which may lead to amputation.
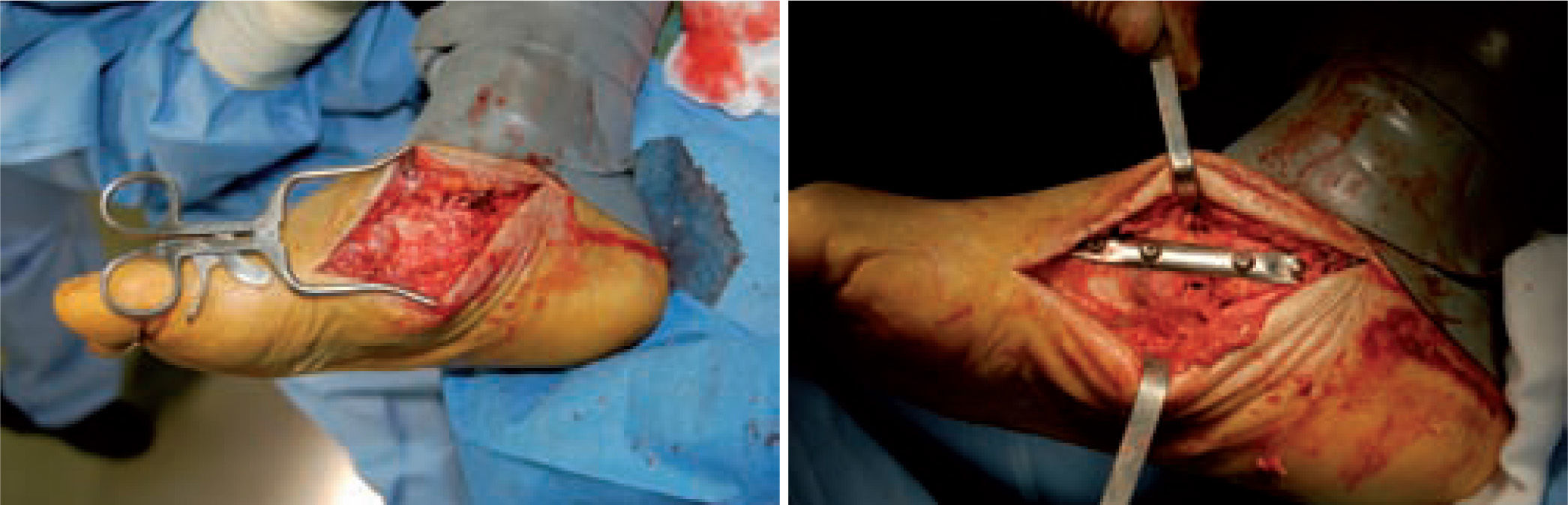
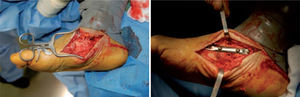
Unfortunately, these studies are not comparable, as they used different methods of patient selection, types of operation, and operative timeframes. When resolution of the Charcot joint process has resulted in significant bone loss, subluxation, joint dislocation, or joint compromise, such that there is significant instability of the medial column of the foot, primary fusion of this joint should be considered. Simple exostectomy may fail in this scenario owing to continued plantarflexion of the bones in this segment if instability remains, resulting in a new bony prominence. Stabilizing this joint in the form of primary fusion is the better alternative. Fixation can be achieved in a variety of ways (figure 7).
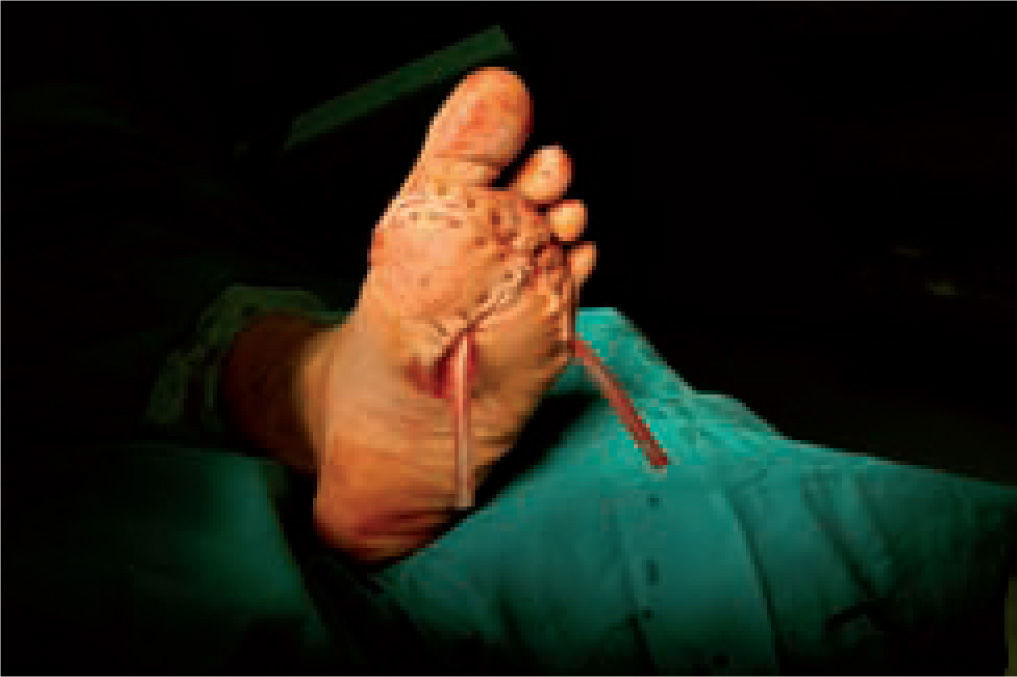
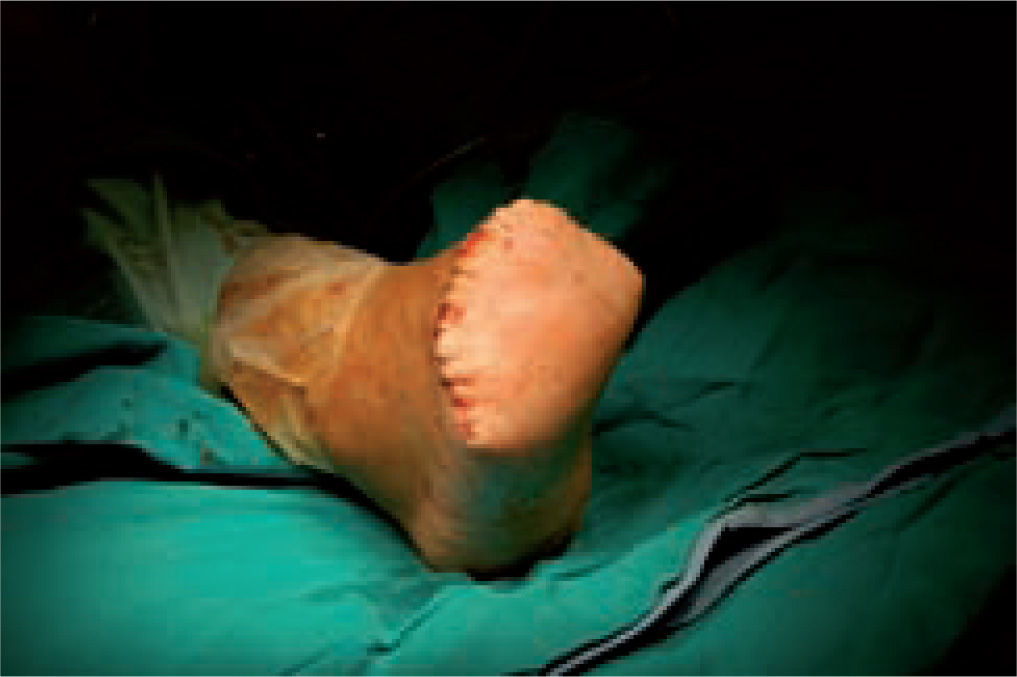
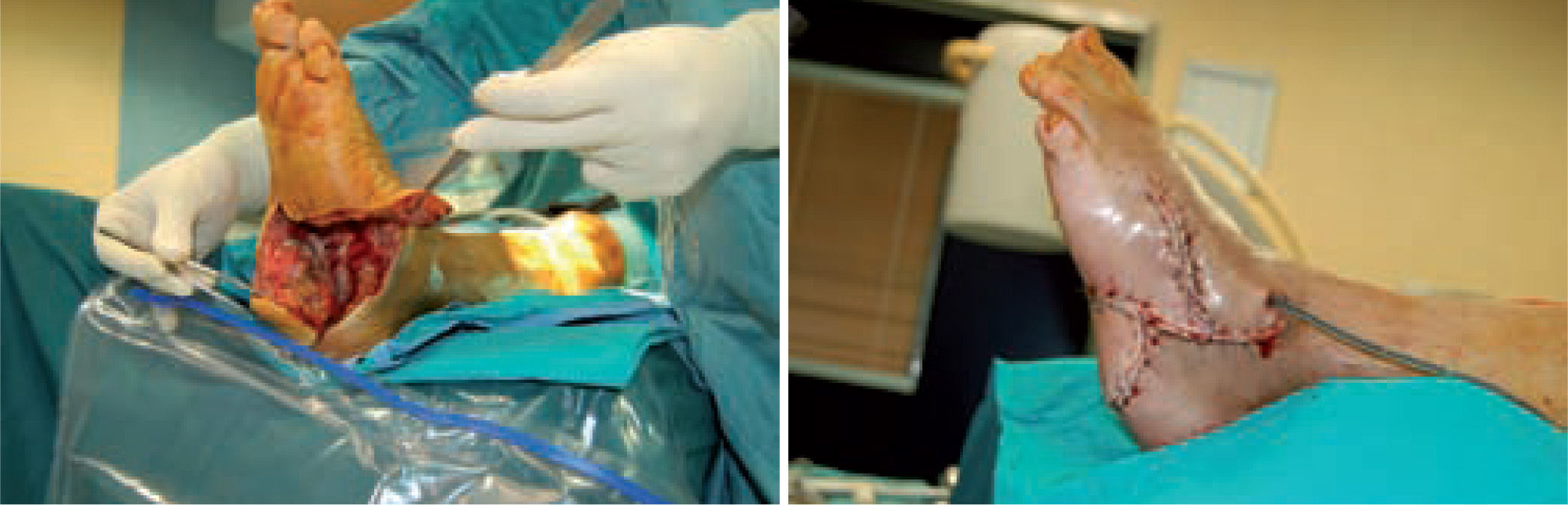
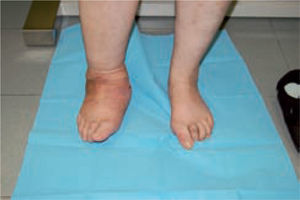
One of the most difficult problems to manage is ulcers located laterally in the midfoot secondary to plantar subluxation of the cuboid bone. This is type 5 in the Harris and Brand classification of Charcot joint disruption (pattern II in the Frykberg/Sanders classification), and has been described as highly resistant to conservative care.63,66 Resolution of these ulcerations often requires surgical intervention. When ulcerations of this type are large, primary excision with closure may be difficult and an alternative technique may be sought.14,38,39 The ulcerectomy and exostectomy procedure may leave a large dead space. This space can be filled with a muscle flap (using the flexor digitorum brevis and/or the quadratus plantae), which will serve two purposes: it will reduce the dead space following the bony resection and it will provide a layer of soft tissue between the underlying bone and the overlying skin. The ulceration itself may be covered by creating a medially based fasciocutaneous flap originating from the arch of the foot and rotated laterally. The donor site in the arch is then covered with a split-thickness skin graft. The postoperative course usually requires a minimum of 6 weeks of non-weightbearing. The foot is then fitted with custom-molded double-density Plastazote orthoses. As the relative shape of the foot has not changed, these patients often require custom-molded shoes as well. Ankle instability is a major challenge in limb salvage procedures (figure 8).
Good results have been reported by our group and others, showing significant percentages of limb salvation with the use of intramedullary nails (figure 9).89-91
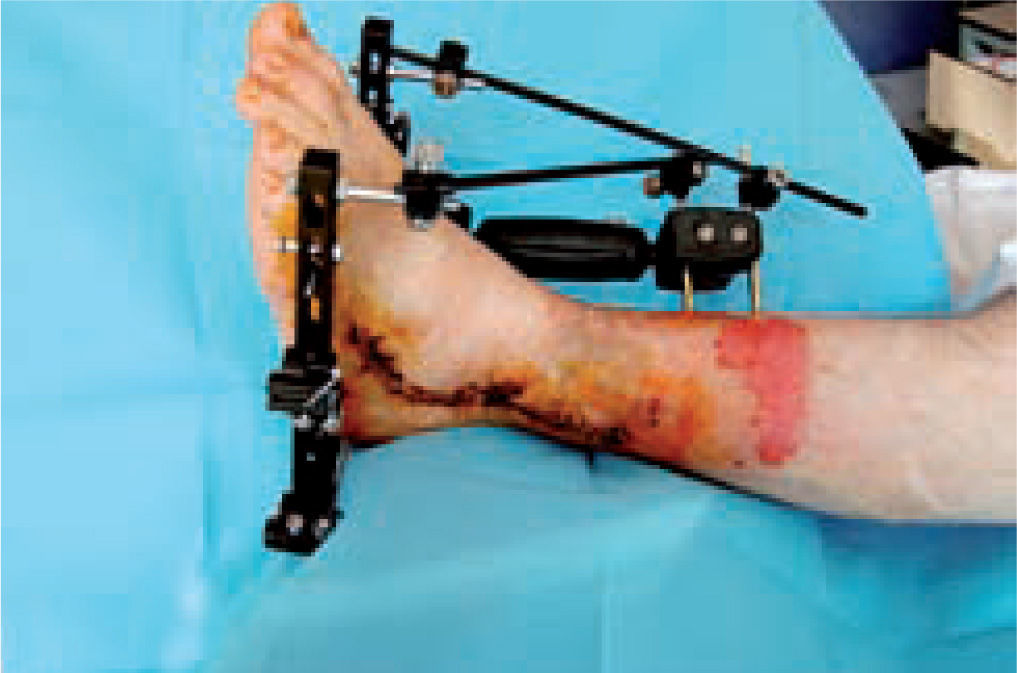
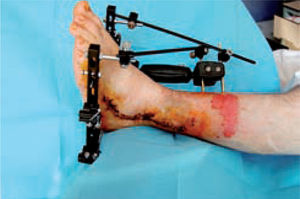
These are used to stabilize the ankle in patients presenting with a high risk of ulceration and, therefore, possible amputation. External fixation is an alternative in patients with osteomyelitis or infective complications from internal fixation (figure 10).92-98 More importantly, external fixation allows earlier mobilization.
Timing and therapeutic protocols in acute and chronic infectionAn ulcerated wound may start as an uncomplicated case, but infection can develop and lead to compromise of soft tissues and even bone involvement. Cases of serious soft tissue destruction, osteomyelitis, and compartment syndrome (progressive infection through plantar and dorsal compartments) constitute true medical and surgical emergencies.99-101 Infection of soft tissues, progressive compromise of deep tissues, and the development of osteomyelitic foci are the factors that distinguish between conservative treatment and a more aggressive surgical approach. Clearly, revascularization must be postponed after acute treatment of infection.102-104
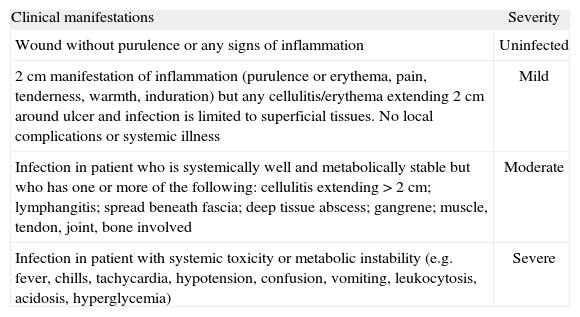
Infections that do not pose an immediate threat of limb loss are defined as "non-limb-threatening", and are generally characterized by the absence of signs of systemic toxicity. In a superficial lesion, cellulitis of >2 cm is generally not present, nor are deep abscesses, osteomyelitis, or gangrene. Infections defined as "limb-threatening" show extended cellulitis, deep abscesses, osteomyelitis, or gangrene. Ischemia characterizes a superficial lesion as limb-threatening.105 Lipsky has provided a more specific classification of infection, shown in table 3.106
Clinical characteristics of diabetic foot infections
| Clinical manifestations | Severity |
| Wound without purulence or any signs of inflammation | Uninfected |
| 2 cm manifestation of inflammation (purulence or erythema, pain, tenderness, warmth, induration) but any cellulitis/erythema extending 2 cm around ulcer and infection is limited to superficial tissues. No local complications or systemic illness | Mild |
| Infection in patient who is systemically well and metabolically stable but who has one or more of the following: cellulitis extending >2 cm; lymphangitis; spread beneath fascia; deep tissue abscess; gangrene; muscle, tendon, joint, bone involved | Moderate |
| Infection in patient with systemic toxicity or metabolic instability (e.g. fever, chills, tachycardia, hypotension, confusion, vomiting, leukocytosis, acidosis, hyperglycemia) | Severe |
In the majority of clinical studies in the literature, antibiotic treatment does not improve the outcome of non-infected ulcers.107 Follow-up, including wound inspection and dressing changes, is necessary to ensure that dangerous signs and/or symptoms of local infection are identified as early as possible.105
The diagnosis of infection is clinical.108 The presence of purulent secretions or two or more signs of inflammation (erythema, warmth, tenderness, heat, and induration) should be used in diagnosing an infection (table 4).
Recommended evaluation of a diabetic patient with a foot infection
|
Faced with a clinical case of non-limb-threatening infection, it is best to start antibiotic treatment early on. For mild and moderate infections, antibiotic therapy is administered orally. Oral treatment is less expensive, easier to manage, and usually sufficient for this type of patient.108 Parenteral treatment can be reserved for selected cases (e.g. those with difficulty in intestinal absorption, gastrointestinal allergies, or isolation of bacteria resistant to oral antibiotic therapy). The chosen antibiotic must reach good serous levels and provide good coverage against gram-positive cocci bacteria.108
Acute infected footAcute infection (phlegmon, abscess, necrotizing fasciitis) is an emergent condition that can threaten not only the limb but also the patient's life. It requires evaluation, and immediate hospitalization and treatment.100,101,104,109 The infection may lead to progressive destruction of soft tissues, involvement of bone, the need for surgical treatment, and possibly amputation.107,110 In many cases, rapid treatment is absolutely essential for effectively treating an acute wound in a diabetic foot.102,111 It is often necessary to turn to debridement surgery in an emergency without considering limiting factors such as metabolic compensation and the patient's nutritional state and vascular condition.104,112-114
In less urgent cases, patients can be treated on the ward or in bed, without the need for anesthesiological support. In cases of wider and deeper infections, an operating theatre is required for adequate debridement and drainage. This is especially true in cases with bone involvement.115,116 Lavery et al. recently detailed independent risk factors for the development of osteomyelitis, such as wounds that extended to the bone or joint, previous history of a wound and recurrent or multiple wounds.117
Recent studies have focused on non-surgical management of osteomyelitis in patients with diabetic foot wounds.118-121 In our practice, we carry out surgical treatment of osteomyelitis when the foot is neuroischemic (after revascularization) or when there are clinical signs of infection. We use to surgically treat all complicated wounds of the forefoot, midfoot, and rearfoot with clinical and/or radiological signs of osteomyelitis. We don't carry out surgery as first approach when we don't have clinical signs of infection in wounds localized on toes with or without radiological evidence of osteomyelitis. Surgical treatment, antibiotic treatment, and support should all be decided after general and local examination of the patient.106
Other than conferring a negative prognosis, infection alone can lead to ischemia through inflammatory and thrombotic mechanisms that involve the terminal digital arteries (increase in oxygen consumption, edema, and septic thromboangiitis).122,123 It is precisely for this reason that debridement allows for reduction of the infected mass and improvement in local circulatory conditions.
Surgical debridement is the physical removal of necrotic material, foreign bodies, and infected tissue from the bed of an acute or chronic ulcer, with an evident secondary reduction in bacterial load. This treatment helps to create a more suitable environment for restoring the physiological processes of tissue repair. An aggressive surgical approach to ulcers, as used in the treatment of acute lesions or burns, can also be beneficial in the treatment of chronic lesions. One of the most important motives for debridement is, in fact, to transform a chronic lesion into an acute wound that has more suitable characteristics for healing.115,116 The timing and the repetition of surgical debridement depends on various factors, such as the length of time the lesion has been present, whether the surface and deep tissues and bone are infected, and whether concomitant vascular disease is present. Steed et al. noted the positive effect of surgical debridement performed routinely on chronic lesions compared with its sporadic use.13
Debridement should be performed in an operating theatre if the extent of the lesion requires locoregional anesthesia or if there is a risk of hemorrhage.104 The procedure should be performed without the aid of a tourniquet to effectively assess bleeding and damage to the soft tissues. The treatment, therefore, requires a balance between the necessary level of aggressiveness to remove anything that may impede tissue repair, and the need to leave healthy tissue intact. The excision of damaged tissue should, in any case, always be complete, and should not stop until it reaches macroscopically healthy, bleeding tissue.
Cleansing is important after debridement, preferably by highpressure irrigation.124 This procedure allows deeper decontamination of both necrotic tissue and the bacterial load, owing specifically to the pressure of the irrigation. If the skin is permanently damaged by the infection or by critical limb ischemia or ischemia secondary to the infection, it should be removed surgically, because both proteases and bacteria accumulate around the edges and underneath the infection, and can cause the necrosis to progress and impede tissue repair. If the margin between healthy and necrotic skin is clearly delineated, the skin should be incised along that margin. We usually use simple gauze dressings soaked in antiseptic agents after surgical debridement. A new antiseptic agent based on superoxidized water has recently been proven to be superior to povidone iodine in the treatment of infected ulcers.125,126
A further key point in debulking infection is the treatment of osteomyelitis.116 Non-bleeding malacic bone should be removed. The instruments used for this procedure are rongeurs and an oscillating saw. The best method for decontaminating small portions of bones (phalanges, metatarsal heads) is to resect thin bone sections until healthy, bleeding bone is reached. The tissue should also be irrigated with saline solution to limit the high temperature caused by using an oscillating saw.
When dealing with osteomyelitis, the problems related to surgical treatment with the removal of bone segment should not be given too much consideration –all of the infected bone should be removed.104 Possible correction of the biomechanical imbalance resulting from the radical treatment of osteomyelitis should be addressed after the infection, and ischemia should be controlled. Microbiological specimens of both clinically infected and clinically healthy bone remain essential to determine the extent of the infection.
Principies of treatment of neuroischemic footThe epidemiological characteristics of peripheral vascular disease (PVD) are more obvious in diabetics than in the general population.127-129 The main characteristic of PVD in diabetics is the morphological and clinical presentation.130 Obstructions are mainly located below the knee; occlusions prevail, compared with stenoses;130,131 painful symptoms are often reduced or absent, due to the co-existence of neuropathic sensitivity, and medial arterial calcinosis (MAC) is common.132 These characteristics make PVD in diabetics more difficult to diagnose and therapy more problematic than in non-diabetics.
They also mean that PVD plays a fundamental role in the prognosis of major amputation. Nevertheless, since the 1990's, revascularization procedures have been proved feasible options compared with initial thinking. Procedures ranging from distal revascularization to angioplasty and by-pass interventions have all been able to change the original prognosis of amputation.133
The fundamental problem of diabetic peripheral obstructive arteriopathy is precision of diagnosis. These patients often suffer little or no pain when walking or at rest. The frequent presence of arterial calcifications is another confusing element, sometimes giving rise to incorrect evaluation of the importance of pressure parameters, as both ankle-pressure and ankle-brachial index. These typical diabetic characteristics are the primary factors that lead to underestimation of the presence of PVD. This mistake plays a major role in delayed wound healing and possible gangrene, and is a contributing factor to many amputations.132 This is true in the case of minor amputations when the foot lacks sufficient blood flow, wounds cannot heal, and an amputation is necessary at a more proximal level.134
The TASC (TransAtlantic Inter-Society Consensus)135 was published in 2000 (table 5).
This Consensus introduced a useful diagnostic tool –transcutaneous oxygen tension (TcPO2)– and gave higher cut-off pressures than those determined previously.136 Some parameters cannot be applied to the diabetic population. Rest pain is often absent and ankle pressure is not feasible or erroneously high. In our experience, around 50% of patients with foot ulcer present incompressible arteries of erroneously high figures, as reported by Gibbons et al.137 The pressure of the big toe is even less useful, not only because calcifications often include inter-digital arteries, but above all because the wound often involves the big toe.
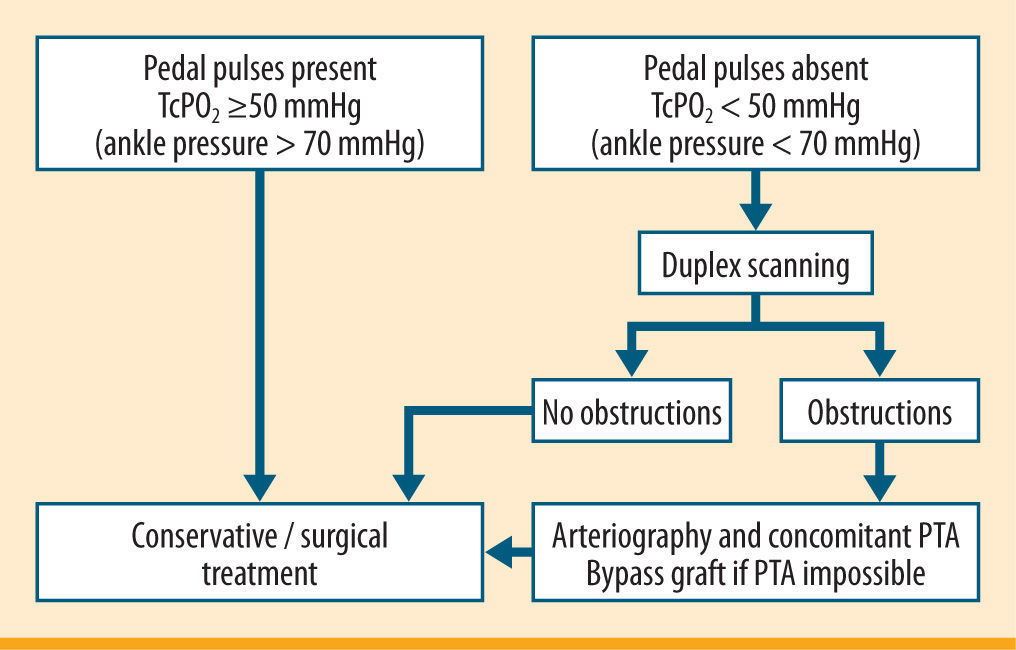
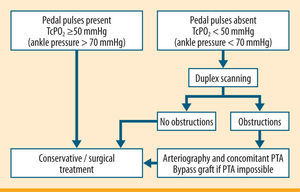
We rely on the TcPO2 in our clinical practice, since it is feasible in all patients. Duplex scanning is an accessible and widespread diagnostic tool, which provides two types of information at the same time. One is morphological and regards the presence of stenosis or occlusions, and one is functional, and deals with the rate of blood flow. This type of diagnostic investigation is highly sensitive and specialised in the large vessels of the thigh. Even without arteriography, some authors consider this examination sufficient for choosing reconstructive therapy. The main limitation of duplex scanning is its dependency on operator ability. All too often defective examinations are made, unreliable for a diagnosis of PVD. Figure 11 shows our clinical and instrumental protocol to assess PVD.103,133,137,139
Arteriography is the gold-standard diagnostic instrument, which fully responds to the need for precise definition of the existence, extent, location and morphology of arterial lesions, even in diabetics. In people with diabetes, arteriography is often described as risking more severe complications compared with nondiabetics. This is true above all as regards the renal toxicity of the contrast medium. From this point of view, current contrast media must be considered. The digital technique and non-ionic contrast medium require lower dosages and concentrations of contrast media. Both literature and our experience have confirmed that hydration procedures, both pre- and post-examination, drastically reduce the risk of renal toxicity.140 In cardiopathic patients with cardiac ejection fraction <40% we use furosemide at the start and end of daily hydration in order to avoid possible effects of overloading liquids. This procedure has allowed us practically to eliminate the risk of nephropathy due to contrast media, even in patients suffering from serious kidney insufficiency.
Vascular investigations with excellent imaging obtained by angio-CT or angio-MRI are currently considered. Despite the high cost and poor accessibility of the equipment, we do not believe that such instrumentation provides a feasible method for routine use at the present time. The double time required when using such methods –the first diagnosis, followed by a second intervention– does not have a place in a procedure like ours. We maintain that arteriography forms a 'bridge' between diagnosis and percutaneous transluminal angioplasty (PTA) therapy (carried out whenever possible) during an angiographic examination. However, we would welcome the double time if it were useful in revascularizing patients with PVD.
Certainly, endoluminal or surgical revascularization is the only treatment capable of reducing the number of major amputations significantly. This is amply shown in literature.103,127-129,133,138,140-152 Revascularization can restore direct arterial flow where it has been interrupted or significantly reduced. This is an indispensable condition for healing a wound in an ischemic foot without resorting to amputation.
This procedure is essential in cases of pain at rest. It is vital when corrective surgery of a wound of part of the foot is necessary. We consider it incorrect to perform surgical amputation without carrying out an exhaustive diagnosis of PVD and (where appropriate) without considering revascularization.153-155
In our protocol PTA was the first-choice revascularization procedure103 yelding outcomes similar to by-pass grafting (BPG).156 It does not require general or spinal anesthetic, is well tolerated, does not pose problems of local surgical treatment of the wound, hospitalization is very short, and the possibility of BPG in the event of failure is not ruled out.137,141-148 In the hands of experts PTA is a feasible approach for distal, long and multiple obstructions,103,138 as noted in recent guidelines.157 However, when critical limb ischemia (CLI) is present any procedure is welcome to save the foot.
It is often believed that PTA is a useless approach, due to immediate and unavoidable restenosis. This is not so since it is necessary to distinguish between clinical and morphological restenosis.158 Morphological restenosis, visible by Duplex scanning or transcutaneous oxygen tension and with ankle-pressure values lower than 15% of pre-PTA values,145 in the absence of returning pain or worsening or relapse of foot wounds, is an irrelevant clinical condition. The absence of a foot wound and of pain when resting does not indicate the need for revascularization. It is clinical restenosis that is important, as it shows itself through investigatory procedures and the reappearance of pain or foot wounds. In our experience, clinical restenosis has a frequency of around 10-14% of treated cases. Efficient PTA is possible in about 80% of cases of restenosis.103,138
In our protocol, PTA is carried out at the same time as angiography.103,138 If PTA is not considered feasible, the angiographic study is used to evaluate the possibility of surgery. PTA may be associated with a BPG at the same time as surgery or immediately afterwards. An example is a femoral-popliteal bypass giving the PTA the task of recanalizing distal (tibial) stenosis. This allows a 'short' bypass to be carried out instead of a 'long' one. The former has a shorter surgical time and better chances of patency in the long term. Distal PTA allows a good run-off to bypass.
In our clinical practice, a surgical bypass is only carried out when PTA is deemed impossible or ineffective. It has been widely shown that distal bypasses are both possible and effective in diabetics.159,160 As in restenosis, non-recanalizable closure during a bypass does not always lead to limb amputation. If bypass closure happens after the wound has healed, in some cases the patient remains asymptomatic.
The objective is always revascularization as regards the foot. We have often seen femoral-popliteal bypasses or PTA of the superficial femoral which have left infragenicular arteries occluded. In a recent analysis of 420 PTAs judged to be technically successful procedures, the probability of major amputation increased 8 times for every non-recanalized infragenicular artery (personal data, unpublished data). In a few cases, recanalization of the peroneal artery was not enough to save the limb. Recanalization of at least one of the tibial arteries is the optimum to salvage a limb.
However, in some diabetic subjects neither endoluminal nor surgical revascularization of the arteries below the popliteal is possible, and amputation is often resorted to although the patency of at least one artery to the foot is an optimal guarantee of limb salvage, revascularization with PTA or BPG of the arteries upstream can lead to an increase in collateral circles, with an improvement in distal perfusion. Such procedures sometimes allow wound healing and pain remission. Even if the foot cannot be saved, a below knee amputation can be performed instead of a above knee amputation, thus creating a distinct advantage for ambulation with a prosthesis.
The extensive use of revascularization undoubtedly allows a very high percentage of limb salvage. The ability to revascularize, with PTA and BPG, is an essential requisite for a diabetic foot center.
ConclusionOver the past years diabetic foot surgery has begun to transform from an ill-defined and not well proven combination of procedures to a much more standardized approach. Indications of surgery are centred on some fundamental variables: 1) the presence or absence of an open wound; 2) the presence or absence of limb-threatening infection; 3) the presence or absence of osteomyelitis; 4) the presence of deformities with an increased risk for ulceration.
The diagnostic paths and treatments examined above are certainly the result of a multidisciplinary approach. The optimal way of improving outcomes in diabetic population with diabetic foot is to create an independent and dedicated multidisciplinary team. In many situations, where the social health impact of the problem has occurred, the decisive step toward facing the problem in a new way has been the creation of specialized centers. The so-called "foot clinics" have various characteristics depending on the healthcare enviroment in which the various specialists work. The organization of care should offer the possibility of treating non complicated wounds in a ward enviroment, using modern offloading techniques, local therapy, and moist dressings. Admittance to a care structure managed by a foot clinic should be arranged for complex wounds. In such a structure, overall treatment should be possible: from revascularization to emergency and/or elective surgical treatment, to rehabilitation.
Potential conflicts of interestThe authors state that here are no conflicts of interest as regards to the content of this article.
References are only available in the online versión of the article (www.avancesendiabetologia.org)