To determine the efficacy of switching to ranibizumab in patients with diabetic macular oedema refractory to treatment with bevacizumab, and to evaluate the outcomes when switching back to bevacizumab.
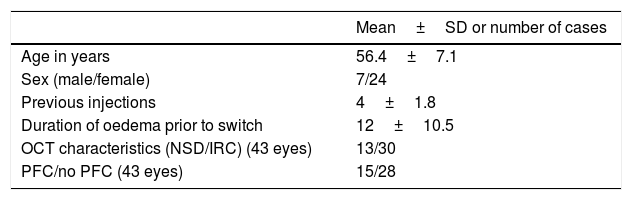
MethodsA prospective study was conducted that included 43 eyes of 31 patients refractory to previous bevacizumab treatment. The patients were switched to ranibizumab, and optical coherence tomography was performed one month post-injection. Patients showing improvement (>10% reduction in central sub-field thickness) were switched back to bevacizumab, and optical coherence tomography was performed one month post-switch back.
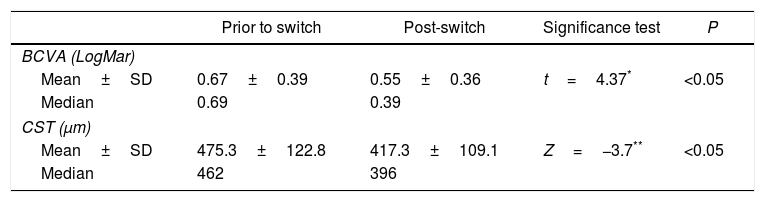
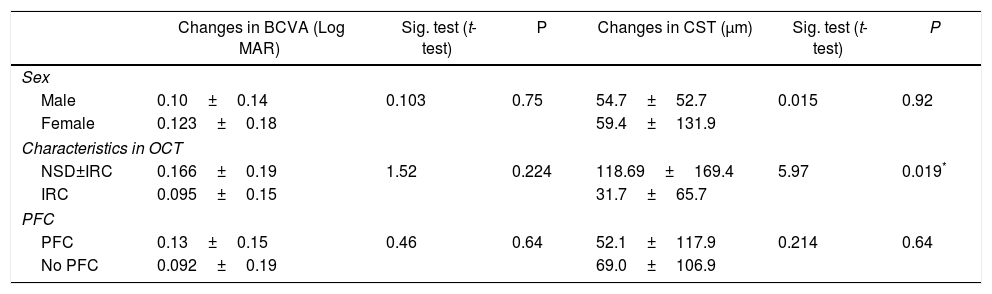
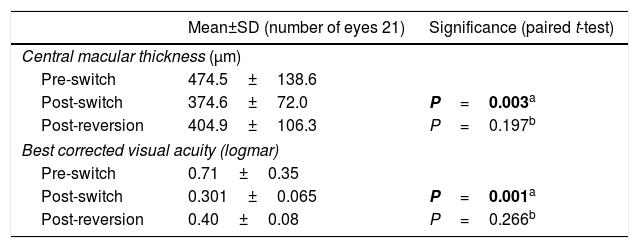
ResultsThe 34 eyes switched to ranibizumab showed a statistically significant improvement in mean best corrected visual acuity from 0.67±0.39 logMAR to a mean of 0.55±0.36 logMAR (P<0.05). In addition, there was a statistically significant decrease in central subfield thickness (CST) from a mean of 475.3±122.8 to a mean of 417.3±109.1 (P<0.05). In the 21 eyes that were switched back to bevacizumab, there was no significant difference either in the change in CST or in the change in best corrected visual acuity post-switch back.
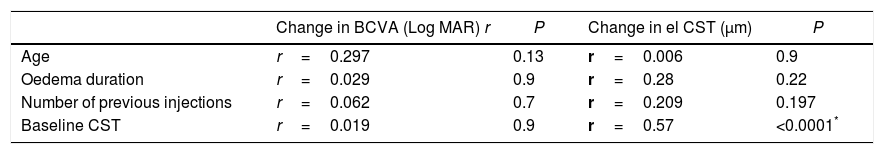
ConclusionSwitching to ranibizumab in patients improves both the best corrected visual acuity and CST in diabetic patients refractory to previous bevacizumab treatment. This effect is pronounced in patients with increased CST prior to the switch. Switching back to bevacizumab adds no further improvement.
Determinar la eficacia del cambio a ranibizumab en pacientes con edema macular diabético refractario a bevacizumab y, posteriormente, evaluar los resultados tras volver al tratamiento inicial con bevacizumab.
MétodosSe llevó a cabo un estudio prospectivo que incluyó 43 ojos de 31 pacientes refractarios al tratamiento previo con bevacizumab. Se realizó el cambio a ranibizumab y se controló mediante tomografía óptica de coherencia un mes después de la inyección. A aquellos que mostraron mejoría (reducción >10% en el espesor macular central) se les realizó una reversión del cambio a bevacizumab y fueron evaluados con tomografía óptica de coherencia un mes después de este cambio.
ResultadosLos pacientes tratados con el cambio a ranibizumab mostraron una mejoría estadísticamente significativa en su mejor agudeza visual corregida, con una media de 0,67±0,39 a 0,55±0,36 logMAR (P<0,05). Asimismo, hubo una disminución estadísticamente significativa en el espesor macular con una media de 475,3±122,8 a 417,3±109,1 (P<0,05). A 21 ojos se les realizó una reversión del cambio a bevacizumab. No hubo una diferencia significativa en el espesor del subcampo central (ESC) ni en la mejor agudeza visual corregida tras este cambio.
ConclusiónEl cambio a ranibizumab mejoró tanto la agudeza visual como el ESC en pacientes diabéticos refractarios al tratamiento previo con bevacizumab. Este efecto fue más marcado en pacientes con aumento del ESC antes del cambio a ranibizumab. Volver al tratamiento con bevacizumab no supuso ninguna mejoría.