To determine the usefulness of myeloperoxidase in discriminating between patients with acute coronary syndrome and patients with chest pain by other causes.
MethodsThe study included all patients over 18 years of age who come consecutively to the emergency department from September 2015 to December 2015 with chest pain of non-traumatic origin. The initial patient evaluation was performed according to the study protocol for patients with suspected acute coronary syndrome (ACS) in our Emergency Department. This included the serial measurement of troponin, and in this case myeloperoxidase, with serialization on admission and at 6h.
For the determination of myeloperoxidase (MPO), a single step sandwich enzyme immunoassay by Siemens, automated on a Dimension analyser, was used.
ResultsStatistically significant differences were observed in the concentration of myeloperoxidase at time 0 among patients diagnosed with ACS: 505 (413)pmol/L, and non-ACS patients: 388 (195)pmol/L (p<.001), as well as at 6h (p<.001).
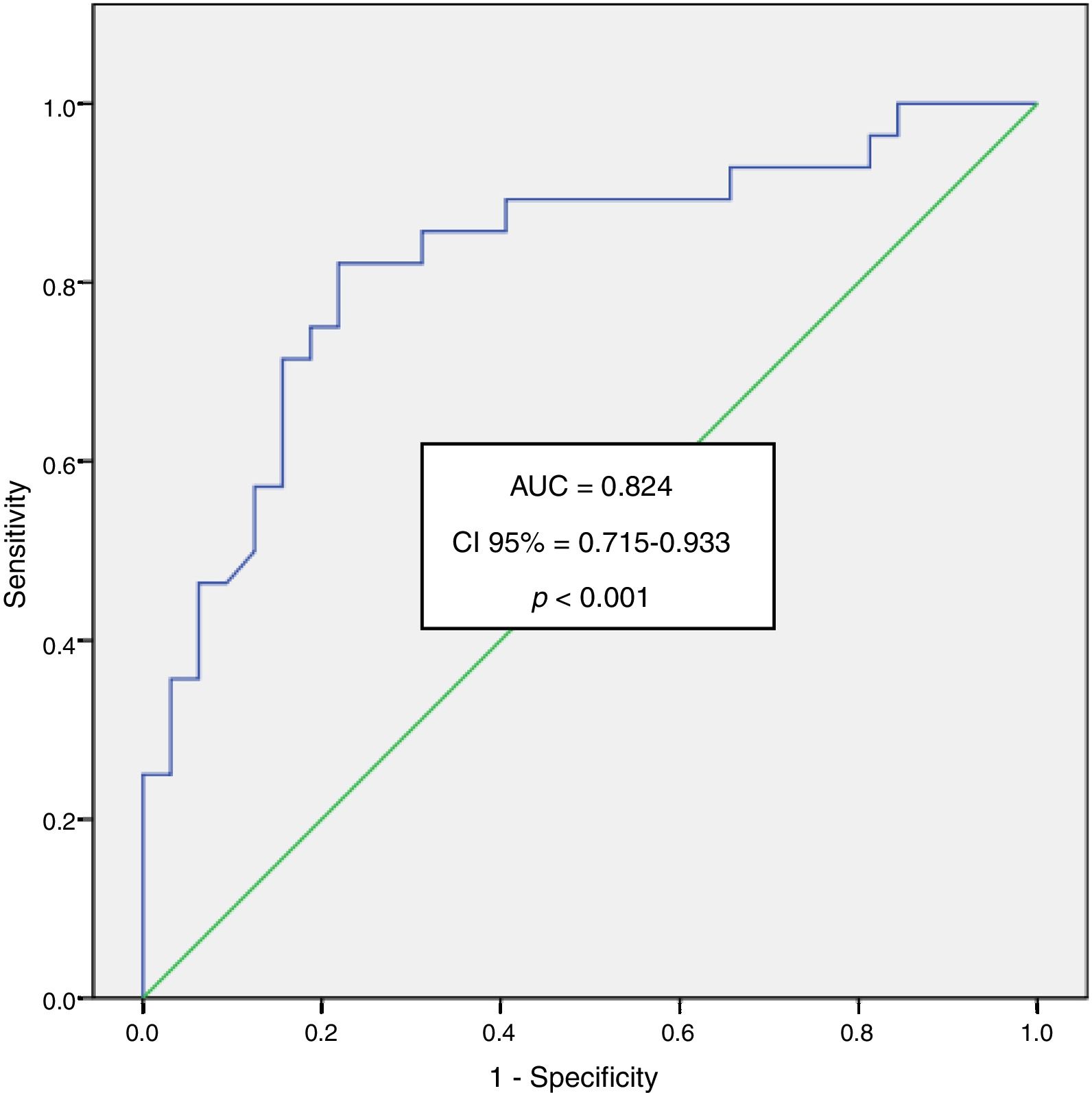
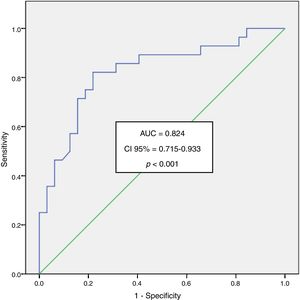
An area under the curve ROC of 0.824 was obtained at 6h for ACS patients, with a confidence interval of 95% from 0.715 to 0.933 and a level of significance of p<.001.
Statistically significant differences were also found in the concentration of myeloperoxidase at time 0 and at 6h among patients with ACS and patients with heart disease other than coronary artery disease.
ConclusionsThe concentration of MPO helps to differentiate between ACS and non-ACS patients, as well as between ACS patients and patients with heart diseases other than coronary artery disease.
Conocer la utilidad de mieloperoxidasa (MPO) para discriminar entre pacientes con síndrome coronario agudo y dolor torácico de otras causas.
MétodosDe septiembre a diciembre de 2015 se incluyeron todos los pacientes mayores de 18 años que acudieron de forma consecutiva al servicio de urgencias con dolor torácico de origen no traumático. La evaluación inicial del paciente se realizó de acuerdo con el protocolo de estudio para pacientes con sospecha de síndrome coronario agudo (SCA) en nuestro servicio de urgencias, que incluye la medición de troponina y en este caso MPO, con serialización al ingreso y a las 6h.
Para la determinación de MPO se utilizó un inmunoensayo enzimático de tipo sándwich, de una sola etapa de Siemens, automatizado en un equipo Dimension®.
ResultadosSe obtuvieron diferencias estadísticamente significativas en la concentración de MPO a tiempo 0 entre los pacientes con diagnóstico de SCA: 505 (413)pmol/l y los pacientes no SCA: 388 (195 pmol/l (p<0.001), así como a las 6h (p<0.001).
Se obtuvo a las 6h un área bajo la curva ROC para pacientes con SCA de 0.824 con un intervalo de confianza del 95% de 0.715 a 0.933 y un grado de significación p<0.001.
También se obtuvieron diferencias estadísticamente significativas en la concentración de MPO tanto a tiempo 0 como a las 6h entre pacientes con SCA y pacientes con enfermedad cardiaca diferente de enfermedad coronaria.
ConclusionesLa concentración de MPO sirve para diferenciar entre pacientes SCA y pacientes que no son SCA, así como entre pacientes SCA y pacientes con otras enfermedades cardiacas diferentes a la enfermedad coronaria.
Cardiovascular disease is the leading cause of death in developed countries and it is expected to be in 2020 the first cause of death in developing countries. On the other hand, acute chest pain of possible coronary origin is one of the most frequent causes of consultation in emergency services.
Early diagnosis of acute coronary syndrome (ACS) is frequently a challenging task, while immediate risk stratification remains crucial for the prompt implementation of appropriate therapy in this setting.
However, the prolonged release pattern of troponins, well established biomarkers of myocardial necrosis, and limited sensitivity of the routine troponin assay make it difficult to diagnose ACS at an early stage.
The ACS is usually caused by the rupture of a coronary plaque producing a sudden and critical reduction in blood flow and includes various clinical manifestations ranging from Acute Myocardial Infarction (AMI) with ST-segment elevation and myocardial infarction without elevation of ST segment to unstable angina.
Myeloperoxidase (MPO) is an enzyme released by the activation and degranulation of polymorphonuclear cells and monocytes that is involved in the production of free radicals, responsible for the LDL cholesterol oxidation. The oxidative modification of LDL leads to the increase of its uptake and degradation by macrophages, resulting in cholesterol deposit and formation of foam cells, the cellular mark of the fatty streaks.
It is also involved in the activation of metalloproteinases and in the instability and plaque rupture1,2 as well as in the bioavailability of nitric oxide, derived of endothelium, thereby altering the tone and certain anti-inflammatory properties of the endothelium.
Some studies show that the concentration of MPO is higher in ACS patients3,4 thus, it has been proposed as a useful risk marker and diagnostic tool in ACS and in patients admitted to the emergency department for chest pain.5
Also, whereas troponin T takes 3–6h to rise following chest pain, MPO levels already rise even within 2h after the onset of symptoms.
But, although it seems that myeloperoxidase can independently predict the onset of coronary artery disease,6 there are few studies comparing the value of the determination of myeloperoxidase and other cytokines that reflect endothelial activity among patients with acute coronary syndrome and patients with stable angina or other causes of non-traumatic chest pain.7
Material and methodsFrom September 2015 to December 2015 we included all patients over 18 years of age who come consecutively to the emergency department with chest pain of non-traumatic origin. Exclusion criteria were: age less than 18 years, active cancer, pulmonary embolism, cerebral infarction or peripheral vascular disease as well as chronic advanced diseases such as cirrhosis and heart or kidney failure, communication difficulties, cognitive impairment, acute infections or inflammatory states. The study design was transversal analytical.
Patients who came to the emergency department 12h after the onset of chest pain were also excluded, since in these patients is not necessary Troponin serial determinations, as well as patients whose first Troponin was positive, because in these cases the diagnosis of Acute Myocardial Infarction (AMI) is already firmly established.
In each patient who participated it was obtained information on epidemiological data such as age, sex and risk factors for cardiovascular disease such hypertension (blood pressure≥140/90mmHg or treatment with antihypertensive drugs), diabetes mellitus, dyslipidemia (when the patient is taking lipid-lowering drugs), smoking, family history in first degree of ischemic heart disease and obesity (BMI>30kg/m2).
In each patient who participated, informed consent was obtained.
The initial patient evaluation was performed according to the study protocol patient with suspected ACS in our emergency department, which includes the measurement of troponin and in this case myeloperoxidase with serialization on admission and at 6h.
After 15min of resting 20ml of blood was extracted, 10ml in a EDTA container for the determination of myeloperoxidase and 10ml in a tube without additives, only with gel separator for determination of serum parameters.
In our study MPO was measured in plasma EDTA samples and the plasma was quickly separated and frozen at -80°C until determination of myeloperoxidase. The preferred sample for determination of MPO is plasma with EDTA, since serum or heparin plasma gave consistently higher values due to MPO leakage from leucocytes.8
For the determination of myeloperoxidase a single step sandwich enzyme immunoassay of Siemens, automated in Dimension equipment, was used.
The measurement interval of myeloperoxidase is between 20 and 5000pmol/L and its detection limit is 13pmol/L.
For a level of myeloperoxidase of ≤20pmol/L a variation coefficient of 20% is obtained.
According to the manufacturer the detection limit for this technique is 1.5pg/ml and shows no cross reactivity with IL-1alpha, IL-1beta, IL-2, IL-3, IL-4, IL-8 and alpha TNF.
Statistical analysis of the dataContinuous variables are expressed as mean±standard deviation or median (interquartile range) according to their distribution, and qualitative variables as frequencies. For comparison of qualitative variables the test of Chi Squared was used and for comparison of quantitative variables the nonparametric Mann–Whitney test was used. For multiple comparisons the Bonferroni method was used. It is considered significant a value of p<0.05. Myeloperoxidase diagnostic performance is calculated by analyzing the area under the Receiver Operating Characteristic (ROC) curve with a confidence interval of 95% (95% CI).
For statistical analysis of the data the SPSS version 20.0 (IBM, USA) has been used.
ResultsDuring the study period, 83 patients were included. The average age of them was 68±15 years and 52% were male. NSTEMI were diagnosed in 15.7% of all patients and 19.3% of they had unstable angina. 13.2% were diagnosed with stable angina. 20.5% of patients had heart disease different of ACS and 31.3% of them were diagnosed with chest pain of non-coronary origin. The classification of patients was done as follows:
Stable angina was diagnosed as chest pain typical of cardiac ischemia on exertion.9
Unstable angina was diagnosed in patients, who had new-onset angina within 2 months after a previous bout; angina with a progressive crescendo pattern or with the angina episodes increasing in frequency and/or duration; or angina that occurred at rest.10
NSTEMI. Patients with persistent or transient ST-segment depression, inversion or pseudo-normalization of T-waves during symptoms and subsequent normalization.
ACS patients. It includes patients with unstable angina and NSTEMI patients.
Patients with heart disease different to cardiac coronary disease (CAD). When CAD was excluded by additional testing.
Non-cardiac chest pain. When a cardiac etiology was excluded.
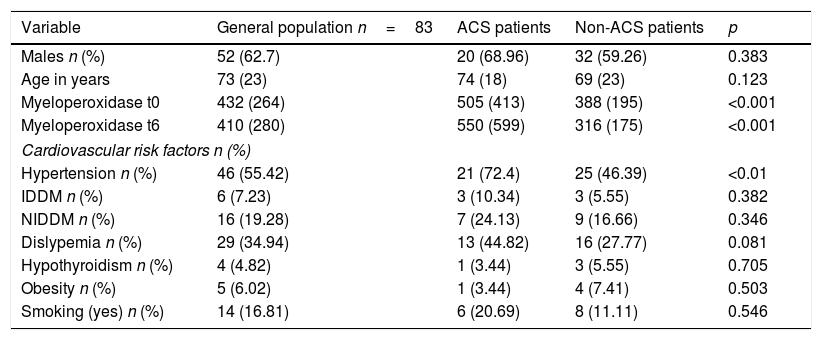
Table 1 shows the general characteristics of the patient sample studied as well as those of the ACS and non-ACS patients.
General characteristics of the sample of patients studied.
| Variable | General population n=83 | ACS patients | Non-ACS patients | p |
|---|---|---|---|---|
| Males n (%) | 52 (62.7) | 20 (68.96) | 32 (59.26) | 0.383 |
| Age in years | 73 (23) | 74 (18) | 69 (23) | 0.123 |
| Myeloperoxidase t0 | 432 (264) | 505 (413) | 388 (195) | <0.001 |
| Myeloperoxidase t6 | 410 (280) | 550 (599) | 316 (175) | <0.001 |
| Cardiovascular risk factors n (%) | ||||
| Hypertension n (%) | 46 (55.42) | 21 (72.4) | 25 (46.39) | <0.01 |
| IDDM n (%) | 6 (7.23) | 3 (10.34) | 3 (5.55) | 0.382 |
| NIDDM n (%) | 16 (19.28) | 7 (24.13) | 9 (16.66) | 0.346 |
| Dislypemia n (%) | 29 (34.94) | 13 (44.82) | 16 (27.77) | 0.081 |
| Hypothyroidism n (%) | 4 (4.82) | 1 (3.44) | 3 (5.55) | 0.705 |
| Obesity n (%) | 5 (6.02) | 1 (3.44) | 4 (7.41) | 0.503 |
| Smoking (yes) n (%) | 14 (16.81) | 6 (20.69) | 8 (11.11) | 0.546 |
ACS, acute coronary syndrome; IDDM, insulin dependent diabetes mellitus; NIDDM, non-insulin dependent diabetes mellitus.
No statistically significant differences were found between ACS and non-ACS patients in age, gender, and cardiovascular risk factors except for hypertension.
There are a greater proportion of hypertensive patients in the group of patients with ACS (p<0.01) (Table 1).
This shows that the differences in MPO concentration between the two groups are not influenced by these potentially confounding variables.
No statistically significant differences between the concentration of myeloperoxidase at time 0: 432 (264)pmol/L and at 6h: 410 (280)pmol/L were obtained in the total group of patients (p=0.812). We obtained statistically significant differences in the values of myeloperoxidase at time 0 among patients diagnosed with ACS (NSTEMI patients and unstable angina patients): 505 (413)pmol/L and non-ACS patients: 388 (195)pmol/L (p<0.001) as well as at 6h: 550 (599)pmol/L in patients ACS vs. 316 (175)pmol/L in non-ACS patients (p<0.001).
The analysis of the area under the curve Receiver Operating Characteristics (ROC) for myeloperoxidase in the SCA patients at time 0 was of 0.755 with a confidence interval of 95% from 0.644 to 0.866 and a degree of significance p<0.001. For the time 6 we obtained an area under the curve of 0.824 with a confidence interval of 95% from 0.715 to 0.933 and a degree of significance p<0.001 (Fig. 1).
When we refer to differences in the concentration of myeloperoxidase among different groups according to their pathology, we can appreciate the following:
- •
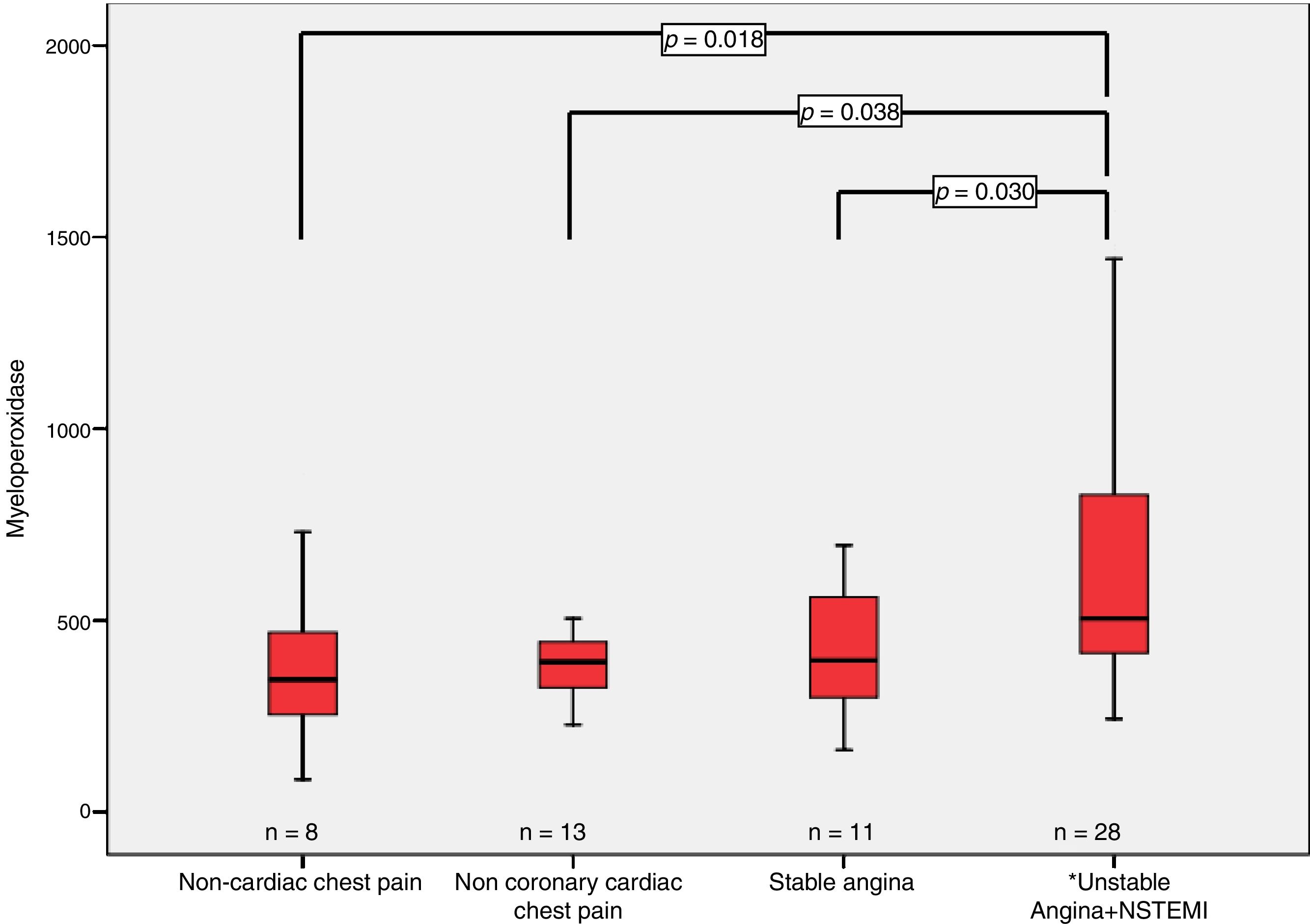
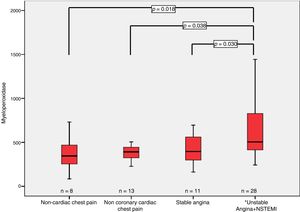
We find statistically significant differences in the concentration of myeloperoxidase when comparing the group of patients ACS with the patients with chest pain of non-coronary origin, at time 0: 505 (413)pmol/L vs. 345.5 (215)pmol/L (p<0.01) as well as at 6h: 550 (599)pmol/L vs. 249 (237)pmol/L (p<0.05) (Fig. 2).
- •
It was also obtained statistically significant differences in the concentration of myeloperoxidase at time 0 among patients with ACS: 505 (413)pmol/L and patients with heart disease different of coronary artery disease (CAD), 392 (119.5)pmol/L (p<0.05) as well at 6h: 550 (599)pmol/L vs. 318 (90)pmol/L (p<0.05) (Fig. 2).
- •
Also, we obtained statistically significant differences when we compare the concentrations of myeloperoxidase in patients with ACS with those with stable angina at 6h: 550 (599)pmol/L vs. 345 (242)pmol/L (p<0.05) (Fig. 2) and at 0h the differences did not reach statistical significance: 505 (413)pmol/L vs. 396 (346)pmol/L (p=0.098).
Zhang et al.11 were the first to describe the correlation between serum MPO levels, leukocyte MPO levels and the presence of coronary artery disease.
In our study significant increases in MPO levels were observed in acute coronary syndrome patients compared to patients without ACS (p<0.001).
It confirms earlier reports indicating markedly elevated plasma concentrations of MPO within 2h of symptoms onset in patients with myocardial infarction12 or in patients with ACS presenting within 3–12h of their last episode of chest pain.13
MPO is linked to both inflammation and oxidative stress by its location in leukocytes and its role in catalyzing the formation of oxidizing agents. It seems to contribute directly to the pathogenesis of ACS. Oxidative stress participates in all stages of cardiovascular disease, from lipoprotein modification to plaque rupture, and biomarkers of oxidative stress predict development of coronary artery disease (CAD).14
Several lines of evidence strongly suggest also the existence of a link between MPO and CAD. Genetic studies of subjects with either total or subtotal MPO deficiency15 or of subjects with reduced expression of MPO due to a promoter polymorphism16,17 seem to be less likely to develop CAD or its clinical manifestations such as non-fatal myocardial infarction or cardiac death.
Elevated MPO in our study was associated with the presence of acute coronary syndrome with an area under ROC curve of 0.755 (CI 0.644–0.866; p<0.001) at time 0 and even better values of the area under the curve ROC at 6h 0.824 (CI 0.715–933; p<0.001).
Our results show that the concentration of myeloperoxidase at 6h allows us to discriminate better between ACS and non-ACS patients (best area under the ROC curve) and between ACS patients and patients with stable angina.
Like Samsamshariat et al.18 we find plasma MPO levels higher at time 0 in SCA patients: 505 (413)pmol/L than in patients with stable angina 396 (346)pmol/L and muscular pain patients 345.5 (215)pmol/L as well as at 6h.
MPO progressively increases with the increase in clinical severity of CAD from stable CAD to non-ST segment elevation acute coronary syndrome.
The higher concentration of MPO found in unstable CAD patients as compared with stable CAD patients may be related to plaque instability and neutrophil activation that have been demonstrated by some studies.19,20
This also highlights that the elevation of MPO can facilitate the diagnosis of ACS, having a greater interest in acute conditions, such as NSTEMI or unstable CAD.
Another important conclusion of our study is that the concentration of both MPO at 0h and at 6h allows differentiation between SCA patients and patients with any heart disease different from coronary artery disease, results that agree with those obtained by Sawicki et al.21
However, in a recent report Eggers et al.22 argued that MPO provides no clinically relevant information in unselected patients with chest pain presenting within 8h.
They use also a fully automated chemiluminescent microparticle immunoassay but do not find significant differences in median MPO concentrations between patients with unstable angina or myocardial infarction and those other with heart disease or non-cardiac disease were found.
Our study was performed like as the study performed by Sawicki et al.21 in one emergency department, whereas the study of Eggers et al.22 was performed in three different medical centers, which might have influenced the selection of chest pain patients. On the other hand, several recent clinical studies have shown that elevated MPO level correlates with angiographic severity of CAD and predicts adverse events in patients with acute coronary syndromes.23–25
In the study by Baldus et al.,13 myeloperoxidase remained an independent and powerful predictor of cardiac risk at both 30 days and follow-up.
In conclusion, although we have a small number of patients (n=83) we can say that myeloperoxidase clarifies the pathophysiology of ACS and also enables a better understanding of ACS helping the physician to differentiate between ACS patients and non-ACS patients.
FundingNo endorsement of any kind received to conduct this study/article.
Conflict of interestThe authors declare no conflict of interest.