The aim of this study was to test for association between MHC2TA gene polymorphisms and risk for restenosis after coronary stent placement in a group of Mexican patients.
MethodsThe MHC2TA-168A>G (rs3087456), 1614C>G (rs4774), and 2536G>A (rs2229320) single nucleotide polymorphisms were genotyped using 5′ exonuclease TaqMan genotyping assays in a group of 202 patients, who underwent coronary artery stenting. Basal and procedure coronary angiography were analyzed, looking for angiographic predictors of restenosis and follow-up angiography was performed to screen for binary restenosis.
ResultsThe results obtained in this study showed that the frequency of the three polymorphisms studied was similar in patients with and without restenosis. Univariate analysis showed that the use of drug-eluting stent (DES) reduces the risk of developing restenosis (p<0.001, OR=0.26). In contrast, the diameter<2.5mm of the stent and bifurcations increased the risk of developing restenosis (p=0.049, OR=1.74 and p=0.041, OR=1.8).
ConclusionThe present study suggests that the MHC2TA polymorphisms are not involved in the risk of developing restenosis after coronary stent placement.
El propósito de este estudio fue evaluar la asociación de los polimorfismos del gen MHC2TA y el riesgo de desarrollar reestenosis, después del implante de stent coronario en un grupo de pacientes mexicanos.
MétodosLos polimorfismos de un solo nucleótido MHC2TA-168A>G (rs3087456), 1614C>G (rs4774) y 2536G>A (rs2229320), se determinaron en un grupo de 202 pacientes tratados con stent coronario. Los polimorfismos fueron evaluados utilizando ensayos de genotipificacion TaqMan 5′ exonucleasa. El procedimiento basal y la búsqueda de predictores de reestenosis fueron analizados por medio de angiografía coronaria, y seguimiento angiográfico con el fin de detectar reestenosis binaria.
ResultadosLos resultados obtenidos en este estudio mostraron que la distribución génica de los tres polimorfismos estudiados fue muy similar, en pacientes con o sin reestenosis. Sin embargo, el análisis univariado mostró que el uso de los stent medicados reducen el riesgo de desarrollar reestenosis (p<0.001, OR=0.26). En contraste, con las bifurcaciones y el diámetro<2.5mm del stent que se incrementa el riesgo de desarrollar reestenosis (p=0.049, OR=1.74 y p=0.041, OR=1.8).
ConclusiónEl presente estudio sugiere que los polimorfismos del gen MHC2TA no están asociados con el riesgo de desarrollar reestenosis, después del implante de stent coronario.
In the 20th century, coronary artery disease (CAD) was the major cause of morbidity and mortality in the world, and World Health Organization (WHO) statistics demonstrate that this trend will continue well into the future.1 The invasive treatment strategies are coronary artery bypass grafting, percutaneous transluminal coronary angioplasty (PTCA), and intracoronary stent. However, after PTCA, restenosis occurs in about 30% to 32% of patients and after intracoronary stent placement in 12% to 32% of patients.2–5
The restenosis is the arterial wall's healing response to mechanical injury and comprises two main processes-neointimal hyperplasia (i.e., smooth muscle migration/proliferation, extracellular matrix deposition) and vessel remodeling.6 Immediately after coronary stenting, thrombus formation and acute inflammation occur, followed by neointimal hyperplasia.7–9
The major histocompatibility complex class II transactivator (MHC2TA) is considered a candidate gene in the inflammatory process regulation. The MHC2TA is a transcriptional co-activator, it is the key intermediate responsible for INF-γ inducible and is required for expression of MHC class II and other genes related to antigen presentation in antigen-presenting cells.10–13 On the other hand, the MHC2TA is a master regulator of MHC class II transcription and has an important role in the activation of several genes14–19 that play a role in the processes-neointimal hyperplasia and arterial remodeling after coronary stent placement. The MHC2TA gene is located in the 16p13 region.10 This gene presents three polymorphisms (-168 A>G, 1614 C>G, and 2536 G>A) that have been associated with low and abnormal expression of the MHC2TA in lupus erythomatosus, rheumatoid arthritis, multiple sclerosis, myocardial infarction, metabolic syndrome, and atherosclerosis.20–23
Considering the prominent role of the MHC2TA as regulator of several genes, this study was based on the assumption that the MHC2TA gene polymorphisms have a measurable influence in the arterial remodeling after coronary stent placement and contribute to or reduce the occurrence of restenosis. The objective of this study was to establish the role of the MHC2TA gene polymorphisms in the risk of developing restenosis after coronary stent placement in a group of Mexicans patients.
MethodsThe study included 202 Mexican mestizo patients with symptomatic coronary artery disease who underwent coronary stent implantation at our institutions and went to follow-up coronary angiography because of symptoms of ischemia documented in a myocardial perfusion imaging test. Basal and procedure coronary angiographies were analyzed for angiographic predictors of restenosis, and follow-up angiography was performed to screen for binary restenosis. Using a > 50% stenosis at follow-up (50% reduction in the luminal diameter of the stenosis compared with the coronary angiography findings immediately following angioplasty) as the criterion to define restenosis, we selected 79 patients with restenosis and 123 without restenosis. All subjects were ethnically matched, and we considered as Mexican Mestizo only those who for two generations, including their own, had been born in Mexico. A Mexican Mestizo is defined as someone born in Mexico who is descendant of the original autochthonous inhabitants of the region and of individuals, mainly Spaniards of Caucasian and/or black origin, who came to American during the 16th century. The Institutional Ethics and Research Committee approved the study, and all subjects signed informed consent.
DNA extractionGenomic DNA from whole blood containing EDTA was extracted by standard techniques.24
Determination of the MHC2TA genotypeThe MHC2TA-168 A>G (rs3087456), 1614 C>G (rs4774), and 2536 G>A (rs2229320) single nucleotide polymorphisms were genotyped using 5′ exonuclease TaqMan genotyping assays on an ABI Prism 7900 HT Fast Real time PCR System, according to the manufacturer's instructions (Applied Biosystems®, Foster City, CA, USA).
Statistical analysisGene frequencies of MHC2TA polymorphisms in the two patient categories were obtained by direct counting. Hardy-Weinberg equilibrium was evaluated by a chi-square test. Continuous variables are expressed as the mean±standard deviation; discrete variables are expressed as percentages. Differences in genotyping distribution were assessed by chi-square analysis of the relevant 2×2 contingency table or Fisher's exact test, as appropriate. Odds ratios (OR) with 95% confidence intervals (CI) were also calculated. Multiple logistic regressions were used to assess the association between the presence of a particular genotype and the presence of restenosis. We tested for independent association in multiple variable models (multiple logistic regression) of restenosis that included all the variables that were p<0.1 in the univariate analysis using forward stepwise (conditional) analysis. Clinical variables (gender, age, diabetes mellitus, hypertension, hypercholesterolemia, smoking habit, co-morbidities, prior acute myocardial infarction, prior percutaneous coronary angioplasty, prior coronary artery bypass graft, reason for intervention, and use of statins) were analyzed separately from angiographic characteristics (treated vessel, vessel diameter, type of lesion, lesion length, percentage of the lesion, chronic total occlusion, evidence of thrombus or calcification, ostial lesions, bifurcation lesions, bare metal stent (BMS), drug-eluting stent (DES) and diameter and length of stent). The analysis was performed by the SPSS 11 statistical package. Pairwise linkage disequilibrium (LD, D’) estimations between polymorphisms and haplotype reconstruction were performed with Haploview version 4:1 (Broad Institute of Massachusetts Institute of Technology and Harvard University, Cambridge, MA, USA).
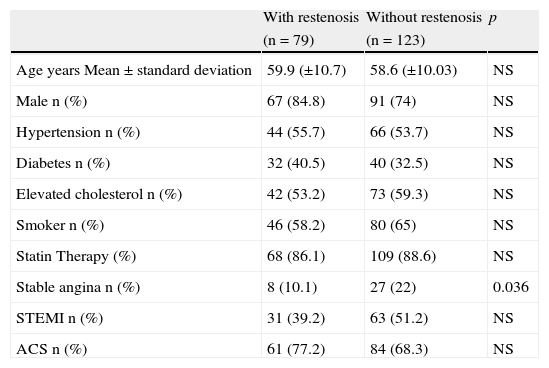
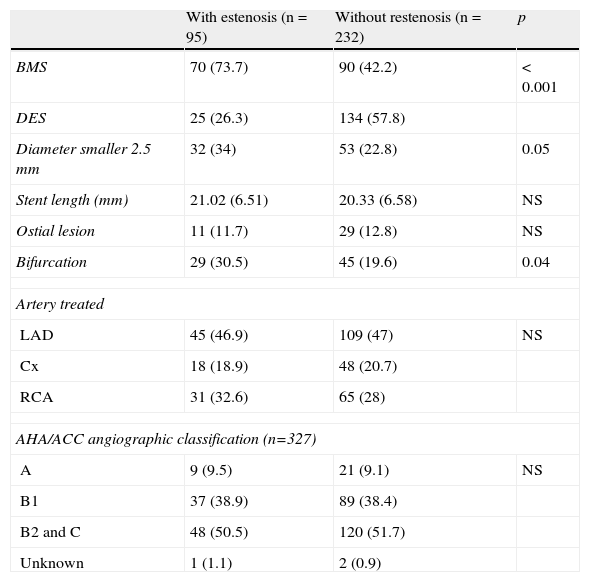
ResultsBaseline characteristic of the patients included in the study are shown in Table 1. No significant differences between patients with and without restenosis with regard to age, diabetes, hypertension, or smoking were observed. However, the presence of stable angina was a protective factor for restenosis (p=0.036). Table 2 shows the angiographic characteristics of the treated coronary lesions (n=327). We observed that the 95 lesions present in the patients that were treated with bare metal stent (BMS) implantation, the 73.7% develop more restenosis in contrast with the patients that were treated with drug-eluting stent (DES) (p<0.001). Restenosis was independent of the artery treated. In addition, stent with diameters smaller 2.5mm and bifurcations increased the risk of developing restenosis (p=0.05 and p=0.041, respectively). No differences were observed in the American Heart Association/American College of Cardiology (AHA/ACC) angiographic classification between patients with and without restenosis.
Baseline clinical characteristics of the studied individuals.
| With restenosis | Without restenosis | p | |
| (n=79) | (n=123) | ||
| Age years Mean±standard deviation | 59.9 (±10.7) | 58.6 (±10.03) | NS |
| Male n (%) | 67 (84.8) | 91 (74) | NS |
| Hypertension n (%) | 44 (55.7) | 66 (53.7) | NS |
| Diabetes n (%) | 32 (40.5) | 40 (32.5) | NS |
| Elevated cholesterol n (%) | 42 (53.2) | 73 (59.3) | NS |
| Smoker n (%) | 46 (58.2) | 80 (65) | NS |
| Statin Therapy (%) | 68 (86.1) | 109 (88.6) | NS |
| Stable angina n (%) | 8 (10.1) | 27 (22) | 0.036 |
| STEMI n (%) | 31 (39.2) | 63 (51.2) | NS |
| ACS n (%) | 61 (77.2) | 84 (68.3) | NS |
NS: not significant (data are proportions or mean±standard deviation); p: p value; STEMI: acute myocardial infarction; ACS: acute coronary syndrome; n: number.
Angiographic characteristics of the coronary lesion treated (n=327).
| With estenosis (n=95) | Without restenosis (n=232) | p | |
| BMS | 70 (73.7) | 90 (42.2) | < 0.001 |
| DES | 25 (26.3) | 134 (57.8) | |
| Diameter smaller 2.5 mm | 32 (34) | 53 (22.8) | 0.05 |
| Stent length (mm) | 21.02 (6.51) | 20.33 (6.58) | NS |
| Ostial lesion | 11 (11.7) | 29 (12.8) | NS |
| Bifurcation | 29 (30.5) | 45 (19.6) | 0.04 |
| Artery treated | |||
| LAD | 45 (46.9) | 109 (47) | NS |
| Cx | 18 (18.9) | 48 (20.7) | |
| RCA | 31 (32.6) | 65 (28) | |
| AHA/ACC angiographic classification (n=327) | |||
| A | 9 (9.5) | 21 (9.1) | NS |
| B1 | 37 (38.9) | 89 (38.4) | |
| B2 and C | 48 (50.5) | 120 (51.7) | |
| Unknown | 1 (1.1) | 2 (0.9) | |
BMS: bare-metal stent; DES: drug-eluting stent; LAD: left anterior descendent coronary artery; Cx: circumflex coronary artery; RCA: right coronary artery; AHA/ACC: American Heart Association/American College of Cardiology; NS: not significant; p: p value.
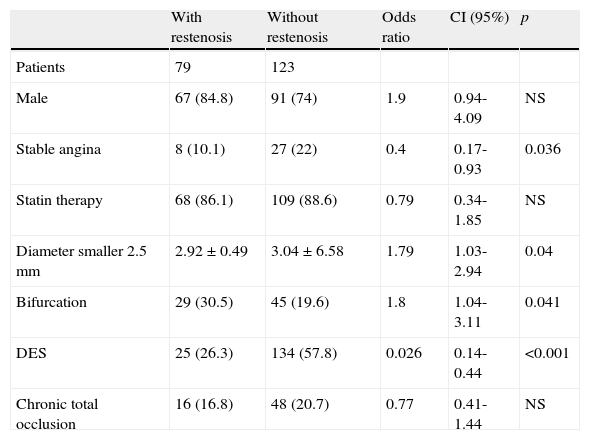
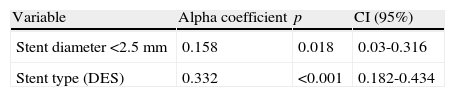
The observed and expected frequencies in the three polymorphic sites of the major histocompatibility complex class II transactivator gene (MHC2TA) were in Hardy-Weinberg equilibrium. The allele and genotype frequencies of MHC2TA gene at positions -168 A>G (rs3087456), 1614 C>G (rs4774), and 2536 G>A (rs2229320) were similar between patients with and without restenosis (data not shown). The univariate analysis in patients with and without restenosis is presented in Table 3. Patients with DES implantation showed a decrease risk of developing restenosis (p<0.001, OR=0.26). In addition, the diameter of the stent (≤ 2.5mm) and bifurcations increased the risk of developing restenosis (p=0.049, OR=1.74 and p=0.041, OR=1.8, respectively). However, none of the risk factors analyzed in the univariate analysis showed an interaction with the polymorphisms studied. After multivariate adjustment, the predictors of restenosis were the type of stent (BMS) and the diameter of the stent (≤ 2.5mm) (Table 4). On the other hand, the polymorphisms 1614 C>G (rs4774) and 2536 G>A (rs2229320) showed a strong linkage disequilibrium with a D’=0.87. However, the distribution of the different haplotypes was similar in patients with and without restenosis.
Univariate analysis (202 patients with 327 coronary lesions).
| With restenosis | Without restenosis | Odds ratio | CI (95%) | p | |
| Patients | 79 | 123 | |||
| Male | 67 (84.8) | 91 (74) | 1.9 | 0.94-4.09 | NS |
| Stable angina | 8 (10.1) | 27 (22) | 0.4 | 0.17-0.93 | 0.036 |
| Statin therapy | 68 (86.1) | 109 (88.6) | 0.79 | 0.34-1.85 | NS |
| Diameter smaller 2.5 mm | 2.92±0.49 | 3.04±6.58 | 1.79 | 1.03-2.94 | 0.04 |
| Bifurcation | 29 (30.5) | 45 (19.6) | 1.8 | 1.04-3.11 | 0.041 |
| DES | 25 (26.3) | 134 (57.8) | 0.026 | 0.14-0.44 | <0.001 |
| Chronic total occlusion | 16 (16.8) | 48 (20.7) | 0.77 | 0.41-1.44 | NS |
CI: confidence interval; DES: drug-eluting stent; NS: not significant (Only the most important variables are shown. Non-significant variables are not included but were analyzed); p: p value.
Multivariate analysis.
| Variable | Alpha coefficient | p | CI (95%) |
| Stent diameter <2.5 mm | 0.158 | 0.018 | 0.03-0.316 |
| Stent type (DES) | 0.332 | <0.001 | 0.182-0.434 |
CI: confidence interval; DES: drug-eluting stent; NS: not significant (Only the most important variables are shown. Non-significant variables are not included but were analyzed).
The pathogenesis of restenosis after PTCA and after stent implantation has an inflammatory component.3–5 Morphological analysis after coronary stenting demonstrates an early thrombus formation and acute inflammation, followed by neointimal hyperplasia after stenting.6–9 The severity of arterial injury during stent placement correlates with inflammatory processes that play an important role in the developing restenosis and remodeling neointimal.6 Recent studies provide evidence on the emerging role of MHC2TA gene as master regulator of MHC class II transcription and several genes, such as collagen,14 matrix metalloproteinase-9,15 IL-4,16 cathepsin E, IL-10 and TGF-β,17–19 play important roles in the arterial remodeling after coronary stent placement. Moreover, several studies associate this gene with several inflammatory diseases, such as systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, myocardial infarction, metabolic syndrome, and atherosclerosis.20–23 In the present work, we studied 202 Mexican Mestizo patients with symptomatic coronary artery disease who underwent coronary stent implantation. The patients were divided in seventy-nine with restenosis and one-hundred twenty-three without restenosis. The distribution of the studied polymorphisms was similar in patients with and without restenosis. The MHC2TA gene polymorphism association studies in different diseases and different populations are controversial with positive and negative results. Koizumi et al. reported no association of the three MHC2TA gene polymorphisms with systemic lupus erythematosus in Japanese patients,21 agreeing with our results. Yazdani-Biuki et al. studied the MHC2TA-168 A/G gene polymorphism in Australian patients with rheumatoid arthritis and no association was observed.25 In contrast, Swamberg et al. reported that the -168 G was significantly associated with increased risk of myocardial infarction and rheumatoid arthritis (OR=1.39 and OR=1.29, respectively) in a Caucasian population.22 However, they did not observe significant differences in the MHC2TA 1614 C>G and MHC2TA 2536 G>A polymorphisms distribution. On the other hand, Lindholm et al. studied two populations (Finland and Sweden) and reported the association of the -168 AG and GG genotypes with cardiovascular mortality after myocardial infarction, but only in the Finnish population.23
The results of the univariate analysis showed that bare-metal stent (BMS), bifurcations and the diameter of the stent (≤ 2.5mm) increased the risk of developing restenosis. These data confirm the previous results obtained by our group in a small group of patients.26–28 In addition, this analysis showed no association between the MHC2TA gene polymorphisms with both angiographic and clinical characteristic of the restenosis after coronary stent placement in a group of Mexican patients. Clinical and angiographic measures of restenosis were evaluated over six months after coronary stent placement.
ConclusionThe present study suggests that the MHC2TA gene polymorphisms are not a risk factor for restenosis after coronary stenting. Our data are preliminary and additional studies in a larger number of samples and in other populations could help to define the true role of this marker as risk factor for developing restenosis after coronary stenting.
FundingThis manuscript was supported with personal funding.
Conflict of interestThere are no conflicts of interest to disclose.
This work was supported in part by grants from the Consejo Nacional de Ciencia y Tecnología (50352-M/24147) and Fundación Gonzalo Río Arronte, Mexico City, Mexico. The authors are grateful to the study participants. Institutional Review Board approval was obtained for all sample collections.