Fragmentation of QRS complex (QRSf) is an easily evaluable, non-invasive electrocardiographic parameter that represents depolarisation anomalies and has been associated with several adverse outcomes, such as sudden death, fibrosis, arrhythmic burden, and a worse prognosis in different conditions, including coronary artery disease (CAD). The case is presented of a 69-year old male referred due to symptoms of chronic stable angina. His electrocardiogram showed sinus rhythm, absence of Q waves, but the presence of QRSf in the inferior leads and V4–V6. A Tc-99 myocardial perfusion SPECT scan revealed a fixed perfusion defect in the inferolateral region, corresponding to the finding of QRSf. QRSf is an easily valuable electrocardiographic marker with relative sensitivity, but poor specificity. Its routine clinical application could contribute to an increase in the suspicion of coronary artery disease.
ConclusionThe presence of fragmented QRS represents distortion of signal conduction and depolarisation, which is related to myocardial scar or myocardial fibrosis.
La fragmentación del QRS (fQRS) es un parámetro electrocardiográfico fácilmente evaluable que representa anomalías de despolarización y que se ha asociado a varios resultados adversos como muerte súbita, fibrosis, carga arrítmica y peor pronóstico en diferentes afecciones, incluyendo la enfermedad arterial coronaria (EAC). Se presenta el caso de un hombre de 69 años referido para estudio por síntomas compatibles con angina de esfuerzo. El electrocardiograma mostró ritmo sinusal, sin ondas Q, pero con fQRS en la cara inferior y en V4-V6. Un SPECT cardiaco con Tecnecio-99 demostró fijo inferior e inferolateral, correspondiente al territorio electrocardiográfico de fQRS. La fQRS es un marcador electrocardiográfico fácilmente valorable, relativamente sensible, aunque poco específico, el cual puede contribuir en la práctica clínica a aumentar la probabilidad de sospecha de una enfermedad arterial coronaria.
Fragmentation of QRS (QRSf) complex is an easily evaluated non-invasive electrocardiographic parameter that has been previously associated with myocardial scar, ischemia or fibrosis. Herein we present a case of a patient with chronic angina and QRSf.
CaseA 69-year-old man is referred to a community cardiologist with symptoms consistent with stable Canadian Cardiovascular Society (CCS) class II/III angina that he has experienced for the previous 12 months without changes in severity and frequency. His cardiac risk factors were dyslipidemia, diabetes mellitus and hypertension. He had no history of myocardial infarction or congestive heart failure.
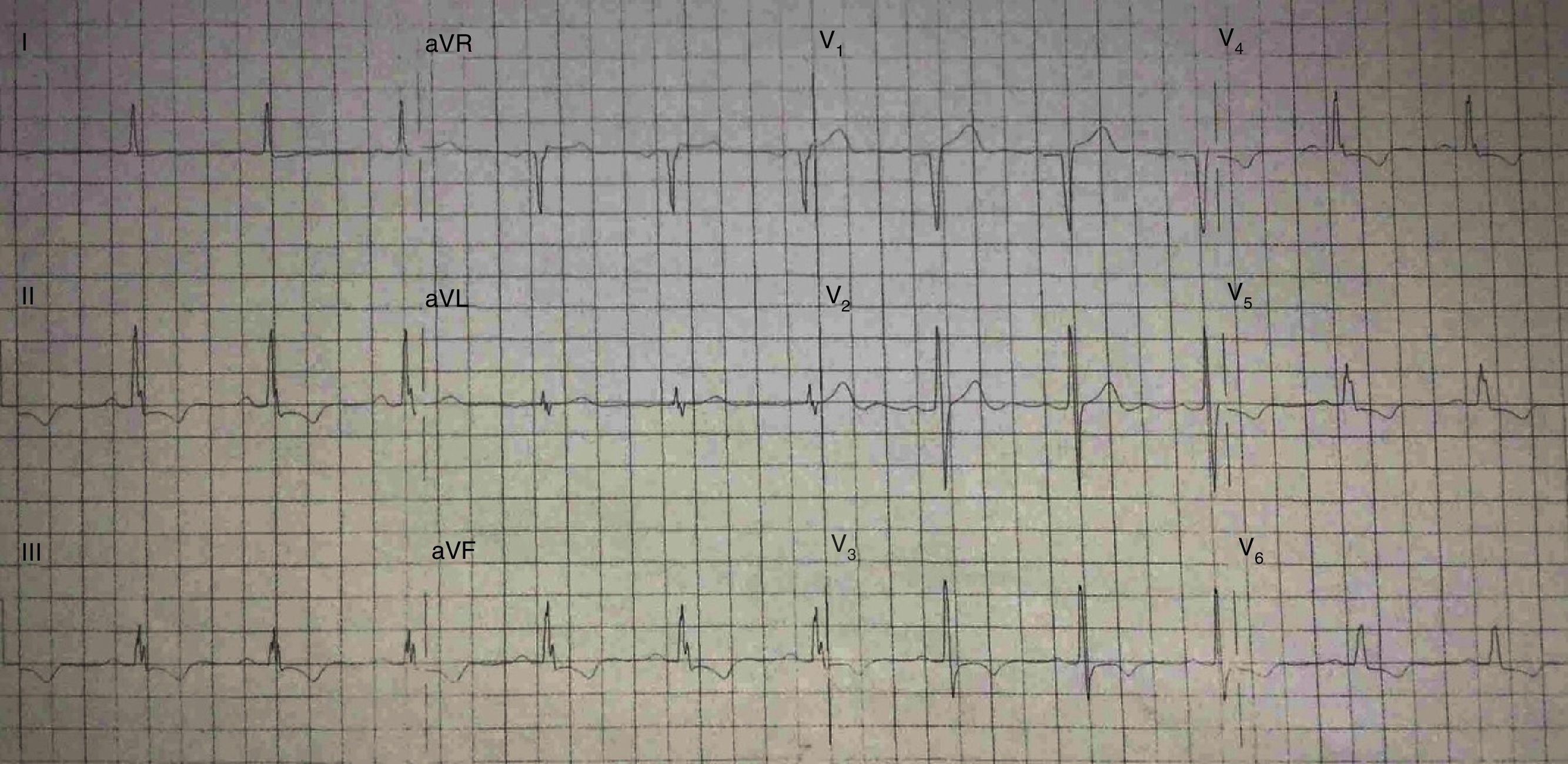
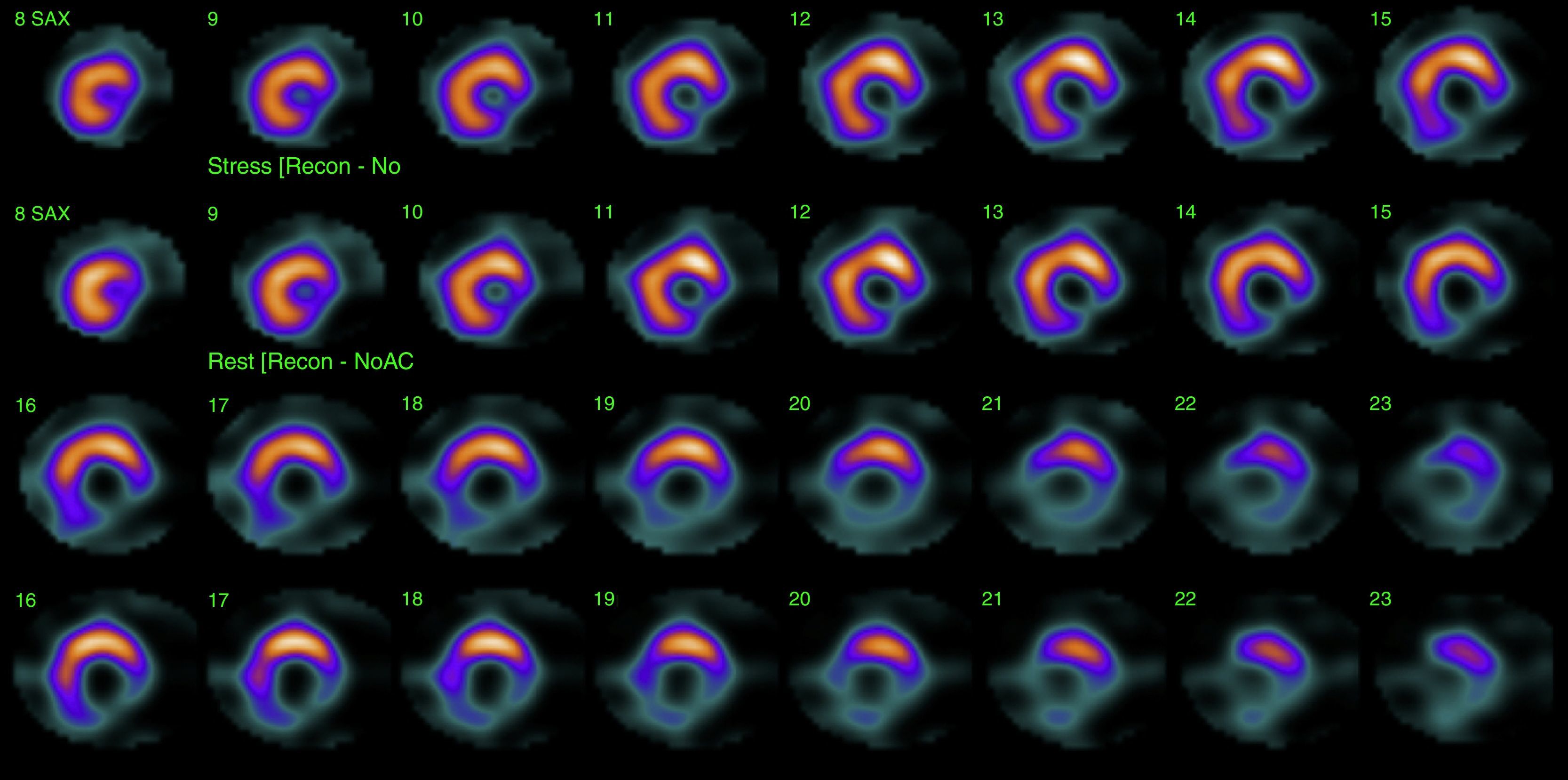
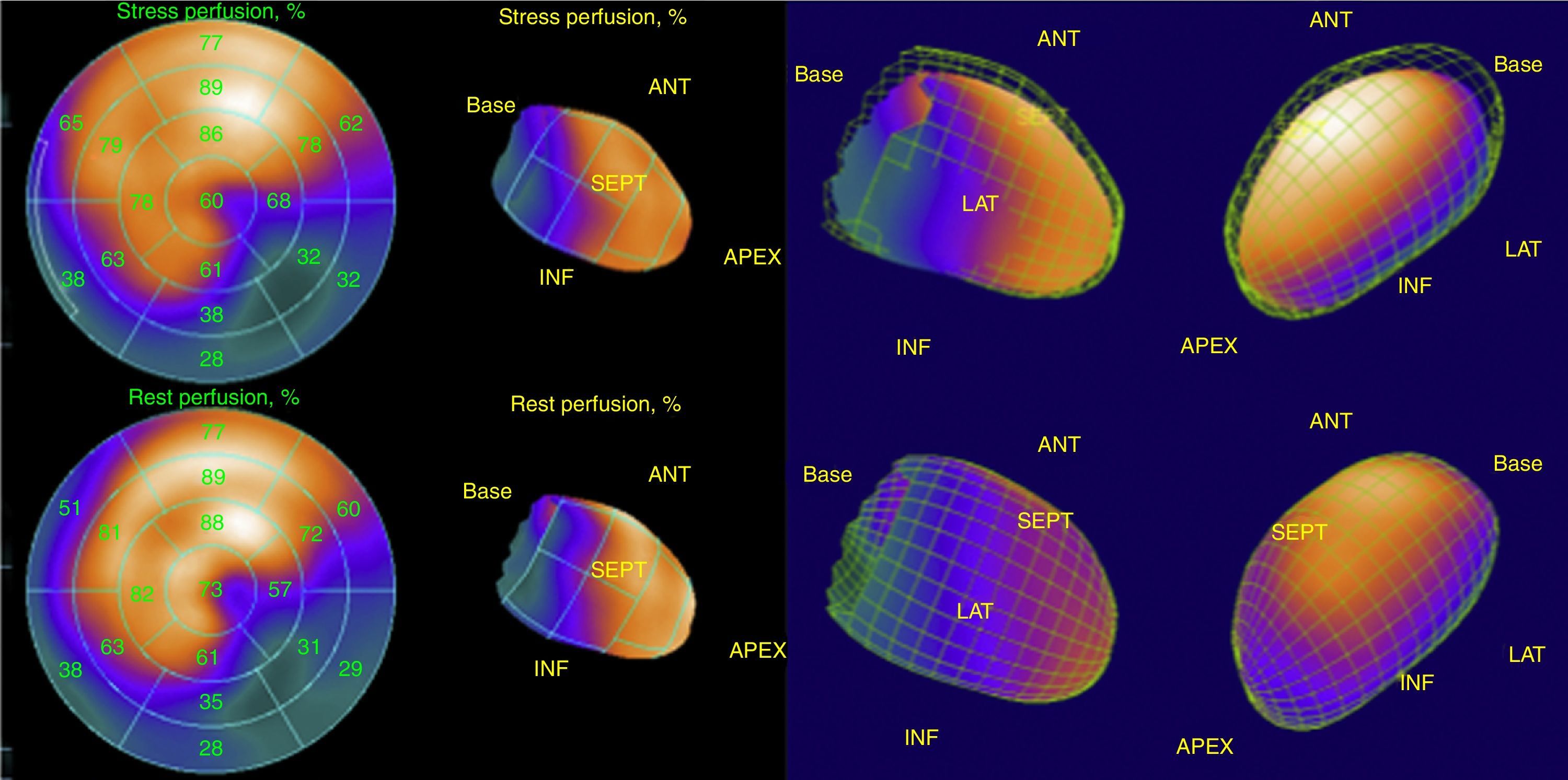
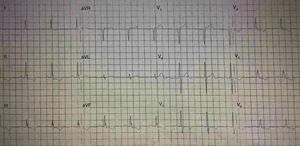
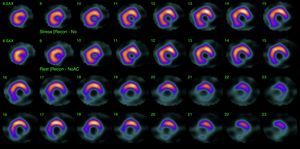
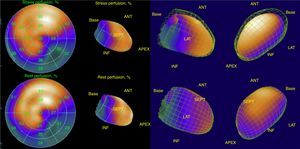
His current medications included aspirin 81mg daily, atorvastatin 20mg daily, metoprolol 50mg BID and metformin 850mg BID. His resting heart rate was 64beats/min, his blood pressure was 140/70mmHg, and his body mass index was 30kg/m2; otherwise, his examination findings are within normal limits. His baseline electrocardiogram demonstrated incomplete left bundle branch block; subendocardial lesion and fragmentation of QRS in inferior (II, III, aVF) and lateral leads V4–V6 (Fig. 1). Echocardiogram reported left ventricular ejection fraction preserved with inferior hypokinesia in three segments. Given that his baseline electrocardiogram was abnormal and his symptoms persisted, a Technetium—99 m—sestamibi SPECT exercise protocol was performed. He achieved a target heart rate of 85% and the exercise was stopped because of limiting chest pain. The myocardial perfusion imaging revealed mid to basal fixed inferior and inferolateral defect (Fig. 2), decreased ejection fraction (46%), and moderate hypokinetic inferior wall with transient ischemic dilatation of left ventricle (Fig. 3). Angiography was performed, revealed three-vessel disease, chronic total occlusion in the distal segment of the right coronary artery, SYNTAX score=22.
QRSf has been defined as the presence of an additional R wave (R′) or notching in the nadir of the S wave, or the presence of >1 R′ in 2 contiguous leads, corresponding to a major coronary artery territory on the resting 12-lead ECG.1 Criteria for defining QRSf in wide complexes has also been published: various RSR patterns with or without a Q wave, more than 2 R waves (R′) or more than 2 notches in the R wave, or more than 2 notches in the downstroke or upstroke of the S wave, in two contiguous leads corresponding to a major coronary artery territory.1 The presence of QRSf has been associated with alteration of myocardial activation due to myocardial scar and fibrosis due to a myriad of causes such as coronary artery disease, congenital heart disease, hypertrophic myocardiopathy, arrhythmogenic right ventricular dysplasia and Chagas’ disease.2 Several studies have evaluated the significance of QRSf in CAD, where it seems to be an important non-invasive marker of high risk, as described elsewhere.3 QRSf has been shown to be associated with previous myocardial scars. Initial studies reported higher sensitivity of QRSf than the presence of a Q-wave for detecting myocardial scar and suggested that the QRSf could be a good electrocardiographic predictor of cardiac events among patients with coronary artery disease. Of note, it has also been demonstrated that there is no significant relationship between the number of Q waves and the number of scar segments on PET in patients with Q-wave MI, and a single regional infarction identified by PET was more common in the non Q-wave MI than in the Q-wave MI patients.4
Not only that, but the Q wave may regress or even disappear over time in as many as 25–63% of patients with a history of a Q-wave MI by ECG.3,5 There is no established ECG sign for a remote non-Q-wave MI. This limits the ability to detect the presence of a myocardial scar by ECG in as many as two thirds of patients with a documented Q-wave or non-Q-wave MI.6 The presence of fibrosis in the region of a myocardial scar is associated with alteration in QRS morphology, leading to a terminal conduction delay or a fragmentation of QRS complexes on the 12-lead ECG,7 which could be used as a marker that rise suspicion for past myocardial infarction. QRSf has also been associated with motion abnormalities: when present in inferior leads, it has been associated with inferior wall motion abnormalities.7
QRSf has been linked with overall prognosis. The presence of QRSf was associated with higher all-cause mortality (34% vs 26% in patients without QRSf) and cardiac event rate defined as MI, cardiac death and need for revascularization (50% vs 28% in patients without QRSf.8,9 The association between the (number) of leads with QRSf and the risk of cardiac death or hospitalization for heart failure has also been assessed in patients with prior MI. The number of leads with fQRS complex was a significant predictor of the cardiac death or heart failure hospitalization: more than 3 leads with fQRS complex was the most useful predictor of adverse outcomes.9
Significance of the appearance of QRSf in the patients with acute coronary syndrome has been investigated. In a cohort of 896 patients with acute coronary syndrome, 2 groups were identified: 337 patients with MI (both STEMI and NSTEMI), and a control group of 445 patients with unstable angina. Fragmented QRS developed in 224 patients with MI and 17 with unstable angina (51% vs 3.7%; p<0.001) and new Q waves developed in 122 (28%) with STEMI, 76 (23%) with NSTEMI, and 2 (0.4%) with unstable angina.10
Myocardial single photon emission tomography (SPECT) stress studies have previously demonstrated the ability to identify regional perfusion abnormalities from a prior myocardial scar. Cardiac SPECT studies are currently routinely performed to identify the extent of ischemia or the presence of a previous MI and the size of the infarct.11 Therefore, clinicians have to depend on various noninvasive and invasive studies, such as echocardiography, nuclear imaging, or cardiac catheterization, to confirm the presence of obstructive CAD.
Finally, QRSf has also been investigated among patients with ischemic and non-ischemic cardiomyopathy suggesting that this ECG parameter may affect prognosis and risk of sudden cardiac death, risk of implantable cardioverter-defibrillator therapy and response to cardiac resynchronization therapy.2 It is important to emphasize that, as the scarring processes might be patchy or diffuse in non-ischemic cardiomyopathies, the presence of QRS aids in the initial suspicion for the condition but lacks the capability of identifying which specific heart regions are affected by the scarring process.
ConclusionsPresence of QRSf represents distortion of signal conduction and depolarization process within ventricles, which is related to myocardial scar or myocardial fibrosis. The meaning and value of this ECG marker seems to be different in different populations and conditions, with fairly good sensitivity but low specificity for ischemic heart disease and previous myocardial infarction. Its incorporation in daily clinical practice adds an inexpensive, easily valuable marker that might contribute to raise the probability of a clinically suspected coronary artery disease.
FundingWe did not receive any type of financing.
Conflict of interestNone declared.