The postoperative care of patients subjected to cardiac surgery frequently require a complete recovery with intravenous fluids, but crystalloid solutions like normal saline may increase the interstitial oedema, and it is also well known that fluid overload increases mortality.
ObjectiveTo compare the effect of 7.5% hypertonic saline (HS) with 0.9% normal saline (NS) on lactate clearance, as well as the haemodynamic response of patients during the first day after cardiovascular bypass surgery.
MethodsThe study included patients 18 years of age and older with coronary artery disease and/or heart valve disease, and who underwent bypass surgery and/or cardiac valve replacement and were randomly assigned to receive 4mL/kg of HS or NS intravenously for 30min once they were admitted to the ICU. Lactate, arterial blood gases, heart rate, central venous pressure, and pulmonary wedge pressure were measured at 0, 6, 12, and 24h after being admitted to the ICU. The analyses were carried out with an intention-to-treat principle.
ResultsOut of a total of 494 patients evaluated, 102 were included and assigned to the HS groups (51 patients) or NS (51 patients). The mean age of the participants was 59±14 years, and 59.8% were male. No statistically significant differences were observed between two groups in the lactate clearance, or in any of the secondary outcomes.
ConclusionsOur study failed to show a better lactate clearance in the group on hypertonic saline, and with no evidence of a higher incidence of adverse effects in that group.
El cuidado postoperatorio de pacientes sometidos a cirugía cardíaca requiere frecuentemente una reanimación completa con líquidos intravenosos, pero las soluciones cristaloides pueden incrementar el edema intersticial y la sobrecarga de líquidos incrementa la mortalidad.
ObjetivoComparar el efecto del salino hipertónico (SH) al 7.5% con respecto al salino normal (SN) del 0.9% en la depuración de lactato y la respuesta hemodinámica durante el primer día postoperatorio de pacientes con cirugía cardiovascular con circulación extracorpórea.
MétodosPacientes mayores de 18 años con cirugía de arterias coronarias o de enfermedad valvular cardíaca fueron aleatorizados a recibir 4ml/kg de SH o SN intravenosos en los primeros 30min de admisión a UCI. Se midieron los valores de lactato, estado ácido-base, frecuencia cardíaca, presión venosa central y presión en cuña pulmonar a las horas 0, 6, 12 y 24 después del ingreso a UCI. Se hizo un análisis con el principio de intención de tratar para un modelo de datos longitudinales.
ResultadosSe evaluaron 494 pacientes y se aleatorizaron 102 a los grupos de SH (n=51) o SN (n=51). El promedio de edad fue 59±14 años y el 59.8% fueron hombres. No se observó ninguna diferencia estadísticamente significativa entre los 2 grupos en la depuración de lactato o en cualquiera de los desenlaces secundarios.
ConclusionesNuestro estudio no mostró mejor depuración de lactato con el uso de una dosis de SH ni mayor frecuencia de efectos adversos en ese grupo.
The postoperative cardiovascular care of patients exposed to on-pump surgery frequently require a thorough reanimation with intravenous fluids during the reheating process in the intensive care unit (ICU), because the induced hypothermia within the surgery generates an important flow of liquids to the extracellular space.1,2 Since it also reduces plasma oncotic intravascular pressure and increases capillary permeability, the subsequent capillary leakage requires intravascular volume load to maintain preload.1,2
Crystalloid solutions like normal saline (NS), although widely used in the ICU,3 may increase the interstitial edema when they are administered in heart surgery, and also it is well known that fluid overload increases mortality.4–6 Colloid infusions are more expensive and have not proven to be a better option; indeed, some of them may produce severe adverse effects as allergic reactions and kidney failure.7 A third option is the use of hypertonic solutions, which produce high intravascular osmolarity and may show some benefit for patient reanimation improving the hemodynamic profile with a lower capillary leakage in the interstitium.2,8–11
There are physiological bases to believe that hypertonic saline (HS) may be an ideal option when reanimating patients in the postsurgical care of the heart-lung machine bypass, by increasing intravascular osmolarity and redistribute the liquids toward this compartment. Some studies show a decrease of dry weight and an increase of the diuresis of patients treated with HS, but up to this moment, there is no conclusive evidence that shows the superiority of hypertonic solutions over isotonic solutions regarding mortality or the length of hospital stay.8–10 On the other hand, it has been demonstrated that hyperlactatemia and lactic acidosis in acutely ill patients is related with higher morbidity, and that lactate depuration during the postoperative period may be used to evaluate the clinical response to reanimation in a cardiovascular postsurgical period.11,12 Therefore, it is expected that a successful reanimation reduces the concentration of lactate quickly, and this indicator may be useful in the evaluation of the effectiveness of HS compared to NS. Likewise, it is possible to indirectly approach the potential effect of HS on morbidity.
Consequently, we conducted a randomized clinical trial to estimate the effect of a 7.5% saline (HS) infusion compared with a 0.9% saline (NS) over lactate values, hemodynamic and metabolic variables in the first 24 post-surgical hours of patients undergoing heart-lung machine bypass.
MethodsDesign of the studyThis is a 1:1 parallel groups, randomized clinical trial in a single center, blinded for the ones in charge of the patients and the evaluations of the outcomes. The protocol was approved by the hospital ethics committee and it was registered in the Australian New Zealand Clinical Trials Registry (ACTRN12615000466549).
SettingHospital Universitario San Vicente Fundación (HUSVF) is a high-complexity hospital which is a reference center for the region and the country. It has 450 beds, 4 adult intensive care units, and the study was conducted in the cardiovascular ICU which has 6 available beds.
PatientsPatients were deemed eligible if they were 18 years older, if they were undergoing cardiac valve replacement surgery and/or coronary revascularization with heart-lung machine bypass and if they signed an informed consent. We excluded patients undergoing heart transplant or because they willingly withdrew from the research.
AllocationThe treatment assignment ratio was 1:1, fixed throughout the study. The allocation was defined by randomly permuted blocks of size 2, 4 and 6 generated by a random number generator (ralloc program, Stata co. 8.2, College Station, TX, USA). The allocation was coupled to a sequence of numbers between 001 and 102 corresponding to each solution of the study. This allocation was only known by the pharmaceutical mixing center, located outside the hospital and in charge of manufacturing and packing solutions to administer to participants. Masking was kept until the final recruitment of the study and until the start of the data analysis.
Study interventionsPatients were assigned to receive 4mL/kg of ideal body weight of either 0.9% saline (NS) or 7.5% saline (HS) administered by a central line for 30min upon being admitted to the ICU. Before heart surgery, the anesthesiologist placed a pulmonary artery catheter and hemodynamic variables were measured upon being admitted to the ICU and thereafter 30min, 4, 6, 12 and 24h.
Outcome measuresThe primary outcome was the rate of change of serum lactate in hours 0, 6, 12 and 24 following surgery. Secondary outcomes included heart rate, mean arterial blood pressure, central venous pressure, pulmonary wedge pressure, cardiac index, arterial pH, bicarbonate, hematocrit, base deficit, mixed venous oxygen saturation and oxygenation index (PaFi) into the same measurements time. There were also evaluated days of hospital and ICU stay, hours of invasive and non-invasive mechanical ventilation, balance of fluids administered and eliminated, requirement of vasoactive and inotropic drugs, transfusion requirements and ICU readmission. We also evaluated the following complications: acute kidney injury according to AKIN criteria, hypernatremia, osmotic demyelination, cerebral edema and cardiogenic pulmonary edema.
Statistical analysisWe determined that a sample of 88 patients had a 90% power to detect an absolute difference between groups of 16mg/dL of lactate levels as an average of the 24h after being admitted to the ICU, assuming a mean lactate level of 36mg/dL in the control group. 102 patients were included to compensate possible losses of information monitoring in the serial lactate values.
A database was created using EPI info 7 and the statistical analysis was conducted using MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium) and Stata 12 (Stata Corp., TX, USA). Data were expressed as proportions or medians with interquartile range (IQR) in accordance with the type of variable.
An efficacy analysis was carried out with the principle of intention-to-treat, the Mann–Whitney test was used for continuous variables and a chi-square test for categorical variables. For lactate measurements, and also for all repeated measurements, we developed an analysis with a generalized estimating equation (GEE) model, assuming an interchangeable correlation between repeated measurements. The intervention was included in the model as a dummy variable with two indicators in which the reference value was NS. The final result of the GEE model estimated the average of the difference in the change of the outcome variable between the two groups (HS or NS) during the first 24h at the ICU. Considering the presence of various outcomes on the analysis and the dependence between some of them (bicarbonate, base excess), only differences with a P value below 0.01 were considered significant.
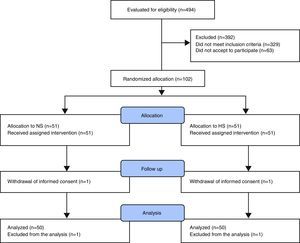
ResultsRandomized allocation and baseline characteristicsA total of 494 patients were admitted to the ICU from April 18, 2013 to January 20, 2015. Of these, 102 patients met the inclusion criteria and they were randomly allocated to HS (n=51) and NS (n=51), respectively. One patient from each group withdrew their informed consent immediately after the allocation and were removed from the analysis. There was no overlapping between the treatment groups or withdrawals for other causes (Fig. 1). Participants’ mean age was 59 years (IQR=22–84) and 59.7% (n=61) were men. Preoperative risk factors, baseline characteristics, surgery times and heart-lung machine bypass times were similar in both groups (Table 1).
Basal characteristics of the population upon being admitted at the ICU.
| Characteristic | HS Median (IQR) | NS Median (IQR) | P value |
|---|---|---|---|
| Age (years) | 63 (51–72) | 59 (50–69) | 0.237 |
| Male gender (%) | 74.5 | 64.7 | 0.274 |
| Heart rate (bpm) | 104 (90–114) | 99 (87–111) | 0.140 |
| Mean arterial blood pressure (mmHg) | 88 (78–100) | 83 (73–101) | 0.123 |
| Central venous pressure (mmHg) | 8 (5–11.5) | 9.5 (7.5–12) | 0.173 |
| Wedge pressure (mmHg) | 12 (9.5–15) | 12 (9–15) | 0.541 |
| Cardiac index (L/min/m2) | 3.1 (2.75–3.6) | 2.74 (2.2–3.5) | 0.432 |
| SaO2 (%) | 98.8 (96.4–99.8) | 97.8 (94.3–99.9) | 0.678 |
| Lactate (mg/dL) | 27.6 (19.7–47.6) | 28.1 (14.1–45.2) | 0.345 |
| pH | 7.35 (7.31–7.39) | 7.36 (7.31–7.38) | 0.784 |
| HCO3 (meq/L) | 20.6 (19–21.7) | 20.6 (19.1–22.3) | 0.856 |
| Base deficit (meq/L) | −4.6 (−6.1/−3.3) | −4.8 (−6.6/−2.8) | 0.213 |
| PAFI | 221 (160–305) | 221 (149.5–318.9) | 0.567 |
| Hematocrit (%) | 33 (30–40) | 32.2 (30.2–34.9) | 0.323 |
| Hemoglobin (g/dL) | 11.4 (10.4–12.5) | 11.1 (10.4–12.1) | 0.498 |
| SVmO2 (%) | 72.9 (65.6–77.2) | 71.2 (64.1–79.7) | 0.812 |
| Creatinine (mg/dL) | 1 (0.8–1.2) | 0.9 (0.8–1.3) | 0.649 |
| BUN (mg/dL) | 17.5 (14–28) | 18 (13–25) | 0.536 |
| Sodium (meq/L) | 137.8 (135–141) | 138.5 (136.4–140) | 0.251 |
| PT (s) | 13.4 (12.2–15.1) | 13.5 (12.7–15.6) | 0.742 |
| INR | 1.25 (1.16–1.43) | 1.23 (1.15–1.43) | 0.541 |
| PTT (s) | 31.2 (28.4–34.5) | 31.8 (28.4–35.4) | 0.691 |
| Platelets (×103mL−1) | 170 (134–247) | 192 (161–247) | 0.129 |
| Time on heart-lung machine (min) | 120 (103–147) | 126 (105–147) | 0.212 |
| Time of ischemia (min) | 109 (92–130) | 111 (93–125) | 0.311 |
| Euroscore | 4.41 (3.19–7.17) | 4.2 (2.27–6.1) | 0.232 |
SaO2, oxygen saturation; HCO3, bicarbonate; PAFI, arterial oxygen pressure and concentration of inspired oxygen relation; SVmO2, mixed venous oxygen saturation; BUN, blood urea nitrogen; PT, prothrombin time; INR, international normalized ratio; PTT, partial thromboplastin time; IQR, interquartile range; HS, hypertonic saline; NS, normal saline.
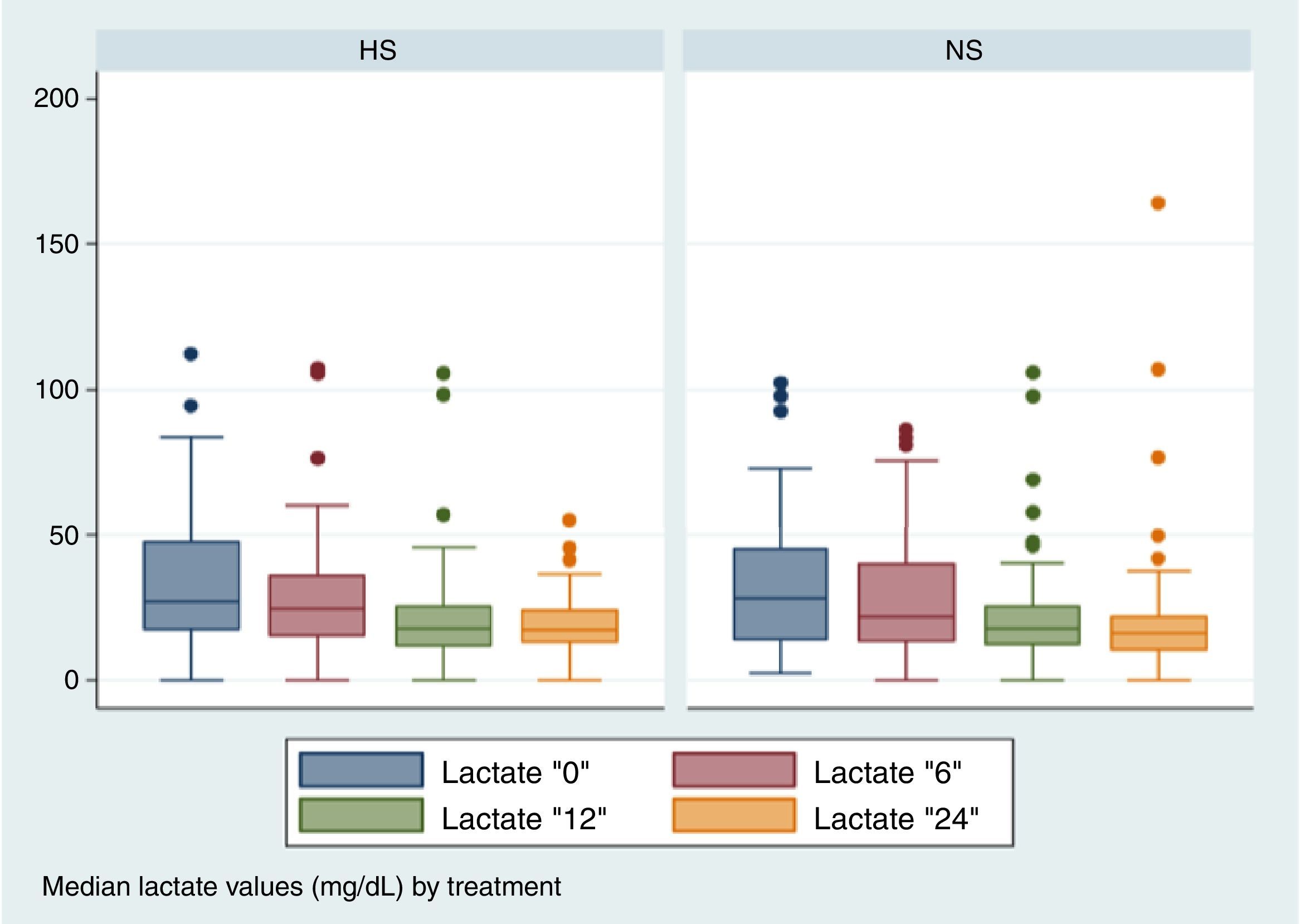
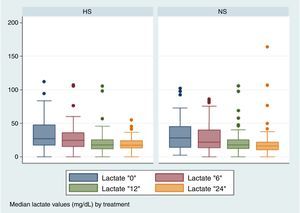
No significant differences were found between the two groups in the lactate depuration in the first 24h (Fig. 2), nor were they found in other repeated measurements (Table 2).
Estimation of the difference between groups in the change of metabolic and cardiovascular repeated measurements during the first 24h (GEE model).
| Variable | NS vs HS difference (24h) | 95% CI | P value |
|---|---|---|---|
| Lactate (mg/dL) | 0.91 | −4.73; 6.56 | 0.751 |
| Heart rate (bpm) | −0.04 | −9.45; 9.3 | 0.992 |
| Mean arterial blood pressure (mmHg) | −1.81 | −9.3; 5.6 | 0.636 |
| Central venous pressure (mmHg) | 0.38 | −0.94; 1.70 | 0.571 |
| Wedge pressure (mmHg) | 0.04 | −1.55; 1.6 | 0.955 |
| Cardiac index (L/min/m2) | −0.26 | −0.61; 0.08 | 0.145 |
| SaO2 (%) | 0.63 | −6.72; 8.00 | 0.865 |
| pH | −0.11 | −0.34; 0.11 | 0.337 |
| HCO3 (meq/L) | −0.25 | −1.28; 0.78 | 0.633 |
| Base deficit (meq/L) | −0.06 | −1.05; 0.91 | 0.889 |
| PAFI | 2.75 | −24.21; 29.72 | 0.841 |
| SVmO2 (%) | −2.40 | −6.61; 1.80 | 0.263 |
| Urine (mL) | −163.75 | −406.95; 79.44 | 0.187 |
SaO2, oxygen saturation; HCO3, bicarbonate; PAFI, arterial oxygen pressure and concentration of inspired oxygen relation; SVmO2, mixed venous oxygen saturation; NS, normal saline; HS, hypertonic saline.
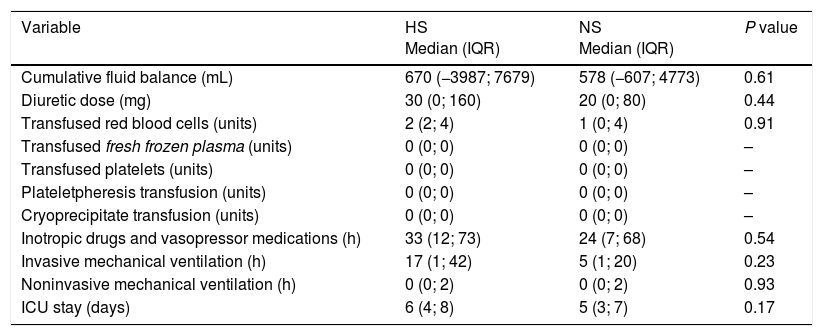
No statistically significant differences were observed in any secondary outcome (Table 3). 10 patients of each group had acute kidney injury stage I according to AKIN classification, and there were no reports of cases of hypernatremia, osmotic demyelination, cerebral edema, cardiogenic pulmonary edema or needed of renal replacement therapy. Furthermore, returning patients to the ICU was not necessary.
Comparison of secondary outcomes according with the treatment group.
| Variable | HS Median (IQR) | NS Median (IQR) | P value |
|---|---|---|---|
| Cumulative fluid balance (mL) | 670 (−3987; 7679) | 578 (−607; 4773) | 0.61 |
| Diuretic dose (mg) | 30 (0; 160) | 20 (0; 80) | 0.44 |
| Transfused red blood cells (units) | 2 (2; 4) | 1 (0; 4) | 0.91 |
| Transfused fresh frozen plasma (units) | 0 (0; 0) | 0 (0; 0) | – |
| Transfused platelets (units) | 0 (0; 0) | 0 (0; 0) | – |
| Plateletpheresis transfusion (units) | 0 (0; 0) | 0 (0; 0) | – |
| Cryoprecipitate transfusion (units) | 0 (0; 0) | 0 (0; 0) | – |
| Inotropic drugs and vasopressor medications (h) | 33 (12; 73) | 24 (7; 68) | 0.54 |
| Invasive mechanical ventilation (h) | 17 (1; 42) | 5 (1; 20) | 0.23 |
| Noninvasive mechanical ventilation (h) | 0 (0; 2) | 0 (0; 2) | 0.93 |
| ICU stay (days) | 6 (4; 8) | 5 (3; 7) | 0.17 |
IQR, interquartile range; HS, hypertonic saline; ICU, intensive care unit; NS, normal saline.
Our study showed that in adults taken to myocardial bypass or valve replacement and undergoing an on-pump surgery, the use of 4cc/kg of 7.5% saline bolus upon being admitted to the ICU, compared with a similar volume of 0.9% saline, did not show statistically significant differences in the lactate depuration in the first 24h or in any other secondary outcome; however, is not an objective of our study to evaluate the benefits of 7.5% saline in the lactate depuration of persistently elevated lactate after the first 24h post-surgery, that would be an objective of another study.
A heart-lung machine bypass has inconveniences for a patient, who is submitted to sharp temperature changes and to the effects of anesthetic drugs as well as of systemic anticoagulants, heparin, and its posterior reversal with protamine. Furthermore, the blood is moved through a surface free of vascular endothelium in a non-pulsatile flow excluding lungs and heart of the circulation; thus, eliminating the immune, filter and metabolic functions that they normally perform.1,2 This predisposes patients to complications as embolism, coagulation disorders, hemodilution, hemolysis and immunity alterations; all related to a neuroendocrine response that activates a systemic inflammatory response that mainly affects the endothelium. The final condition provokes an increase in capillary permeability and sustained vasodilation, with the subsequent filtration of intravascular volume toward the interstice, a decrease of the preload and drop of cardiac output.1
Then, the replacement of blood volume becomes a priority in the postsurgical handling of heart-lung machine bypass. This is so especially during the warming stage, in which blood losses that were concealed by vasoconstriction produced during the induced hypothermia manifest themselves by the secondary vasodilatation when warming the patient's body. From a mathematical point of view, replacing losses seems simple for it only supposed an increase of intravenous fluid contribution. Nevertheless, these patients are vasoplegic as a consequence of proinflammatory cytokines; in addition to this, they increase capillary permeability so most of the intravascular content filters toward the interstice generating edemas. All this process deteriorates cardiovascular, respiratory and kidney functions, which translates into an increase in the requirement of vasopressor and inotropic medications, as well as the increase of times of mechanical ventilation and hospital stay.3,4
In intravenous fluid management, it should be administered enough volume to maintain preload and to avoid overload and excess in positive water balance.10 Therefore, it is reasonable to think that those volume expanders that improve preload at the lowest doses possible to remain in the intravascular space without filtering into the interstice offer a better hemodynamic profile. The options available at this moment in a clinical context are hemocomponents, crystalloids and colloids.1,2,5,10 The latter seem the least suitable candidates because of their cost and safety profile.13
The use of hypertonic solutions to manage cardiovascular postoperative care is reasonable, its intravenous administration creates an osmotic gradient in the cell membrane which facilitates the flow of fluids from intracellular space to extracellular space, eliminating excess of fluids through the kidneys and additionally, causing direct kidney vasodilatation and an increase of the glomerular filtration rate.5–8 The foregoing physiological concepts set the bases for research in animal models and in humans. In a study conducted on pigs exposed to transient myocardial ischemia, they observed that HS has an inodilator effect to increase compliance and myocardial contractility, simultaneously, reducing vascular resistances.14 Moreover, in a study on rodents, they observed that it has an immunomodulatory effect to reduce the sticking of polymorphonuclear neutrophils to the endothelium and reduce vascular leaking in the interstice in reanimation during hemorrhagic shock.15
Studies on the use of HS in a postsurgical heart-lung machine bypass conducted up to date on human beings have had small sample sizes or have only evaluated physiological outcomes. A clinical trial allocated patients in the postoperative care of myocardial revascularization to NS, HS or synthetic colloids. There were 16 patients in each group, and they found that the effect of HS on plasma volume was brief, but it stimulated the excretion of excess fluids accumulated during a heart-lung machine bypass.9 The foregoing was corroborated in another study of the same group where they found higher diuresis and less weight on the first postoperative day in patients treated with HS.10 In our study, we did not find any difference regarding blood volume or diuresis depending on the type of crystalloid received. It is noticeable that in our study we did not identify a significant heart rate increase using HS, which was an effect described in the literature with this therapy.7 This finding is directly related to the lack of effect on postoperative lactate levels that has been identified as a prognostic marker in patients’ results in heart surgery.12–15 Our results agree with a recent systematic revision, which concluded that there were not enough data to define if the hypertonic solutions were better than isotonic solutions in the reanimation of trauma, burned or postsurgical patients.7
As limitations, our study was conducted in a single center, which could affect the applicability of its results. Likewise, the study intervention was limited to a sole bolus that in average represented 250cc of fluid. Therefore, we do not know the effects of repeated doses or HS titers in accordance with certain goals.
ConclusionsOur study was not able to demonstrate better lactate depuration using a dose of 7.5% hypertonic saline compared with a 0.9% saline bolus. Since we did not detect any adverse effects of the HS intervention, it would be justified to conduct a complementary study to determine if a dynamically complete reanimation with repeated doses of HS could be more effective than NS in the postoperative reanimation of heart surgery with a heart-lung machine bypass.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe study was partially funded by the “Sustainability Strategy 2013–2014 Clinical Epidemiology Academic Group”, University of Antioquia.
Conflicts of interestThe authors have no conflicts of interest to declare. The design and conduction of the research, as well as the decision to publish, are entirely author-driven and independent of the institution that provided the solutions for the trial.
“Corporación de Fomento Asistencial del Hospital Universitario San Vicente de Paúl” (CORPAUL) (San Vicente de Paul University Hospital Corporation for the Promotion of Care) provided the labeled solutions for this study.