To present the current in-hospital outcomes and mid-term survival of acute type A aortic dissection (AAAD) surgery performed by a group of dedicated high-volume thoracic aortic surgeons in a University Hospital in Argentina.
MethodsA retrospective analysis of prospectively collected data over a 6-year period (2011–2016) was performed on a consecutive series of 53 adult patients who underwent emergency cardiac surgery for AAAD in the Buenos Aires University Hospital in Argentina.
ResultsA mean of 8.8 AAAD repairs were performed yearly during the 6-year period. In-hospital mortality was 17%, and was statistically equivalent to the expected operative mortality rate of 21% (EuroSCORE II) (observed-to-expected mortality ratio 0.81; p=0.620). New neurological deficit appeared postoperatively in 6% of cases, and the observed major postoperative morbidity rate was 42%. All-cause death cumulative survival probability was 0.711 (SE 0.074), with a mean follow-up period of 49.2 (SE 5.0) months. Cumulative survival probability for in-hospital survivors was 0.903 (SE 0.053), with a mean follow-up period of 62.5 (SE 3.6) months.
ConclusionAlthough the present results do not reach international standards, AAAD surgery in our institution was associated with an acceptable mortality risk and satisfactory mid-term survival compared with previous local studies. In addition to in-hospital mortality, the incidence of new permanent neurological deficit after surgery must be considered the most devastating complication to avoid. Patient-focused care in referral aortic centers with surgery performed by specialized teams should be encouraged in order to improve surgical outcomes in acute aortic dissection surgery in Argentina.
Presentar los resultados hospitalarios actuales y la supervivencia a mediano plazo de la cirugía de la disección aguda aórtica tipo A (DAAA) realizada por un grupo de cirujanos de alto volumen de cirugías en un Hospital Universitario de Argentina.
MétodoSe realizó un análisis retrospectivo de datos recolectados en forma prospectiva durante 6 años (2011-2016) de una serie de 53 adultos sometidos a cirugía de emergencia por DAAA en un Hospital Universitario de Buenos Aires, Argentina.
ResultadosDurante 6 años se operaron en promedio 8.8 DAAA por año. La mortalidad hospitalaria fue del 17% y estadísticamente equivalente a una tasa de mortalidad esperada del 21% por el EuroSCORE II (razón de mortalidad observada/esperada 0.81; p=0.620). El déficit neurológico postoperatorio apareció en el 6% de los casos, y la tasa de morbilidad mayor fue del 42%. La probabilidad acumulada de supervivencia fue de 0.711 (EE 0.074), con un promedio de seguimiento de 49.2 (EE 5.0) meses. La supervivencia acumulada descartando la mortalidad operatoria fue de 0.903 (EE 0.053), con un promedio de seguimiento de 62.5 (EE 3.6) meses.
ConclusionesAunque estos resultados no alcanzan los estándares internacionales, la cirugía de la DAAA en nuestra institución estuvo asociada a un riesgo aceptable de mortalidad y una supervivencia satisfactoria a mediano plazo comparadas con estudios previos locales. Además de la mortalidad, la incidencia de daño neurológico permanente después de la cirugía debe considerarse la complicación más devastadora a evitar.
At present, in-hospital mortality of patients undergoing surgery for acute type A aortic dissection (AAAD) varies between 5% and 20% according to different centers.1–10 However, the same mortality rate reported in the past decade in Argentina ranged between 28.2% and 31.0%.11–13Though it was expected that improvements in surgical and perfusion techniques in the present decade might influence favorably on local outcomes, a recent Argentine registry reported an operative mortality of 57% in 62 patients undergoing surgery for AAAD between 2011 and 2016.14 Since AAAD is an emergency condition with excessive mortality if surgery is delayed,1 poor local outcomes could be justified by the average time delay between diagnosis and surgery, usually ranging from 20 to 24h.13,14 Other preoperative factors have been described to predict mortality on surgically treated AAAD patients, including older age (odds ratio (OR)=1.12), comatose state (OR=3.50), shock/tamponade (OR=3.74), cardiopulmonary resuscitation (OR=3.75), myocardial (OR=5.48) or neurological ischemia (OR=6.64), a higher number of malperfused organs (OR for two organs=2.44; OR for more than two organs=3.39), and longer operating times.1,2,10 The incidence of these prognostic variables may also depend on the time delay to surgery. Furthermore, these factors may be not only associated to mortality, but also to the occurrence of new permanent neurological deficit, which is considered to be the main major complication after AAAD surgery.6
Some recent research has focused on the impact of surgical experience on the outcome of AAAD surgery.6 This study demonstrated that patients operated by experienced aortic team surgeons had better outcomes, and the advantages of these aortic teams seemed to depend on better cannulation and perfusion management, and perhaps, on shorter operating times. Moreover, a Japanese study exploring the learning curve of aortic dissection surgery by means of risk-adjusted cumulative sum analysis revealed that no excess deaths took place beginning with the seventh case and thereafter.15 From the perspective of these studies, the implementation of multidisciplinary thoracic aortic surgery programs to standardize and centralize AAAD treatment should help to improve local outcomes.16
The purpose of this study was to present the current in-hospital results and mid-term survival of AAAD surgery performed by a group of dedicated high-volume thoracic aortic surgeons at a University Hospital in Argentina.
MethodsA retrospective analysis of prospectively collected data (ambispective design) over a 6-year period (2011–2016) was performed on a consecutive series of 53 adult patients who underwent emergent cardiac surgery for AAAD at the Buenos Aires University Hospital in Argentina and its associated clinics. Data consisting of 45 variables including demographics, history, physical findings, imaging studies, surgical procedures and clinical outcomes were recorded on a standardized form. The diagnosis of aortic dissection was based on clinical data and on the results of imaging studies (chest X-ray, aortogram, computed tomography, magnetic resonance imaging and/or transthoracic or transesophageal echocardiography). Patients with diagnosis of aortic dissection lasting not more than two weeks were included in the study, and individuals with chronic or traumatic aortic dissection were excluded. Major morbidity, especially new neurological deficit, operative mortality and mid-term survival were assessed. The EuroSCORE II was used to calculate the expected operative mortality rate, and the observed-to-expected mortality ratio was determined. Postoperative follow-up was conducted by telephone interviews or personal examination to assess mid-term outcomes. In this case, the endpoint was all-cause mid-term mortality.
Elective and non-elective thoracic aortic operations were performed primarily by two principal surgeons (R.A.B. and M.R.). Over the past 6 years, 127 open proximal thoracic aortic operations (ascending aorta, aortic root, and/or aortic arch replacement) were carried out over a total of 2158 cardiac surgeries.
All operations were performed by median sternotomy with invasive hemodynamic monitoring, and retrograde perfusion via the left or right femoral artery. Antegrade perfusion via the right axillary artery was used when the femoral artery was unsuitable for cannulation. Preoperative hydrocortisone (1g intravenously) was administered to all patients for pharmacological neuroprotection. The standard operation procedure involved aortic valve resuspension with supracoronary ascending aorta replacement, and eventually, hemi-arch replacement. Aortic root replacement, or ascending aorta plus aortic valve replacement were performed selectively for aortic root aneurysm, intrinsic aortic valve pathology not amenable to repair, or extensive destruction of the aortic root intima by the dissection process. In case of extensive destruction of the intima at the distal end of the ascending aorta, or in order to perform hemi-arch repair, the open distal anastomosis was completed under a period of deep hypothermic circulatory arrest. In these cases, patients were cooled until the nasopharyngeal temperature was below18°C.
This study was approved by the institutional review board, and the need for individual patient consent was waived. To perform statistical analysis, continuous variables were expressed as mean and standard deviation (SD) or standard error (SE) and categorical variables as number and percent with no decimal place, since sample size was less than 100. The Kolmogorov–Smirnov goodness-of-fit test was used to analyze normal distributions. Univariate comparison of dichotomous variables was performed using the χ2 test and odds ratio (OR) with the associated 95% confidence interval (95% CI). Two-tailed Fisher's exact test was used when cell expected values were below 5. Observed-to-expected mortality ratio was calculated and evaluated using the χ2 test. Time-related survival probability was assessed with the Kaplan–Meier method. Statistical analyses were performed using SPSS Statistics for Windows, Version 17.0. Chicago, SPSS Inc. A two-tailed p value ≤0.05 was considered statistically significant.
ResultsBetween January 2011 and December 2016, 53 patients underwent surgical repair for AAAD at our institution. Mean age was 65 (SE 1.79) years, and 66% (n=35) were men.
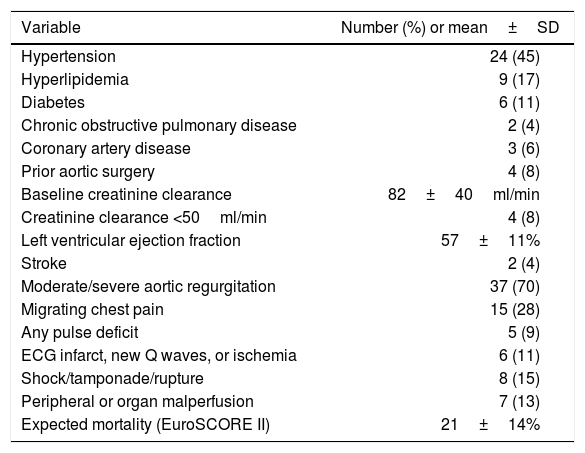
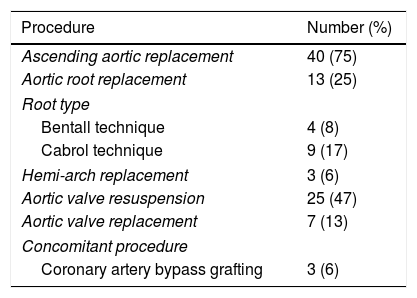
Baseline cohort characteristics are shown in (Table 1), and operative procedures are presented in (Table 2). During the 6-year period an average of 8.8 AAAD repairs were performed yearly. Mean extracorporeal circulation time was 86 (SE 3.57)min, and mean aortic cross-clamp time was 48 (SE 2.06)min. Twenty-three percent of patients (n=12) underwent repair with open distal anastomoses and hypothermic circulatory arrest, with mean circulatory arrest time of 12 (SE 0.87)min.
Baseline population characteristics (n=53).
| Variable | Number (%) or mean±SD |
|---|---|
| Hypertension | 24 (45) |
| Hyperlipidemia | 9 (17) |
| Diabetes | 6 (11) |
| Chronic obstructive pulmonary disease | 2 (4) |
| Coronary artery disease | 3 (6) |
| Prior aortic surgery | 4 (8) |
| Baseline creatinine clearance | 82±40ml/min |
| Creatinine clearance <50ml/min | 4 (8) |
| Left ventricular ejection fraction | 57±11% |
| Stroke | 2 (4) |
| Moderate/severe aortic regurgitation | 37 (70) |
| Migrating chest pain | 15 (28) |
| Any pulse deficit | 5 (9) |
| ECG infarct, new Q waves, or ischemia | 6 (11) |
| Shock/tamponade/rupture | 8 (15) |
| Peripheral or organ malperfusion | 7 (13) |
| Expected mortality (EuroSCORE II) | 21±14% |
SD: standard deviation.
Operative procedures for surgical treatment of acute type A aortic.
| Procedure | Number (%) |
|---|---|
| Ascending aortic replacement | 40 (75) |
| Aortic root replacement | 13 (25) |
| Root type | |
| Bentall technique | 4 (8) |
| Cabrol technique | 9 (17) |
| Hemi-arch replacement | 3 (6) |
| Aortic valve resuspension | 25 (47) |
| Aortic valve replacement | 7 (13) |
| Concomitant procedure | |
| Coronary artery bypass grafting | 3 (6) |
Aortic dissection (n=53).
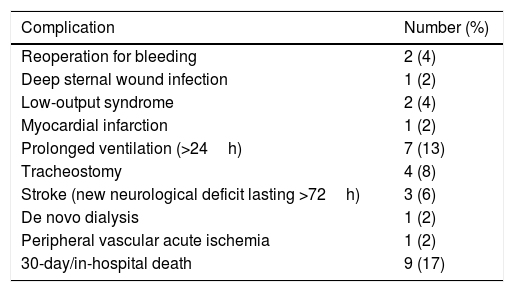
Overall in-hospital mortality was 17% (n=9) and was statistically equivalent to the expected operative mortality rate of 21% (observed-to-expected mortality ratio 0.81; p=0.620). Two patients (4%) had a stroke prior to surgery and new neurological deficit appeared postoperatively in 6% of cases (n=3). The observed major postoperative morbidity rate was 42% (n=22) (Table 3). In patients with AAAD complicated with rupture or malperfusion (n=15), in-hospital mortality was 33% (n=5); while in non-complicated dissections (n=38) mortality was 11% (n=4) (OR=4.3, 95% CI 0.96–18.9, p=0.097). In all the cases of complicated AAAD, deaths occurred within 24h postoperatively. Causes of in-hospital death in complicated dissections included one myocardial infarction, one ventricular arrhythmia, one new aortic rupture, and two low cardiac output/shock cases. Causes of death for non-complicated dissections occurred until the 7th postoperative day and included one stroke, one mesenteric ischemia, one late cardiac tamponade, and two low cardiac output/shock cases.
Major postoperative complications after surgery for acute type A.
| Complication | Number (%) |
|---|---|
| Reoperation for bleeding | 2 (4) |
| Deep sternal wound infection | 1 (2) |
| Low-output syndrome | 2 (4) |
| Myocardial infarction | 1 (2) |
| Prolonged ventilation (>24h) | 7 (13) |
| Tracheostomy | 4 (8) |
| Stroke (new neurological deficit lasting >72h) | 3 (6) |
| De novo dialysis | 1 (2) |
| Peripheral vascular acute ischemia | 1 (2) |
| 30-day/in-hospital death | 9 (17) |
Aortic dissection (n=53).
Mid-term outcomes were obtained for 36 of the 44 patients (82%) who survived surgery. All-cause death cumulative survival probability was 0.711 (SE 0.074), with a mean follow-up period of 49.2 (SE 5.0) months. Cumulative survival probability for in-hospital survivors was 0.903 (SE 0.053), with a mean follow-up period of 62.5 (SE 3.6) months.
DiscussionDespite improved surgical strategies for treatment of AAAD, in-hospital mortality remains high, and an important number of patients who survive often suffer from disabling neurological sequelae. Though in the present series of surgeries performed by a specialized aortic team, in-hospital mortality rate was significantly lower than previously reported data from Argentina further improvements are needed in the local setting to reach international standards. Furthermore, our results proved to be significantly worse when comparing complicated versus non-complicated AAAD. In this sense, in-hospital mortality was reported to be higher in patients with malperfusion than in those without it (30.5% versus 6.2%).17,18 Also, the International Registry of Acute Aortic Dissection (IRAD) identified previous aortic valve replacement, limb ischemia, hypotension and shock/tamponade as independent predictors of death, and these unstable patients showed an in-hospital mortality of up to 31.4% versus 16.7% for stable patients.19,20 Seventy two percent of our patients were diagnosed as stable patients; then, considering the IRAD outcomes as a standard, our population would have an expected mortality of about 10%, which is significantly lower than our observed in-hospital mortality (17%). In a previous local study,12 27% of AAAD patients presented limb/mesenteric ischemia or shock; however, mortality rate for these complicated cases was not reported. The local RADAR13 registry found 18% of AAAD patients presenting peripheral ischemia or shock, while in-hospital mortality reached 66% in patients with hypotension or shock/tamponade versus 28% for non-complicated cases. In the recent study of D’ Imperio et al.14 in Argentina, the high mortality rate observed can be explained by the fact that about half of AAAD patients presented with ischemic visceral involvement of at least one organ.
It is true that gradual outcome improvements should be solved by simultaneously targeting AAAD problems in each pre-hospital and in-hospital stage, particularly avoiding a delayed diagnosis and treatment17,21; but also, better trained aortic surgical teams will offer the best opportunity to patients. Outcomes of proximal aortic operations are improved when performed by high-volume centers and high-volume surgeons.22 Knipp et al.23 defined high-volume centers as those that performed more than 2.5 AAAD surgical repairs per year; however, in an updated study, the lowest operative mortality rate was achieved in centers that performed more than 13 aortic dissection repairs per year (although both type A and type B repairs were included in this analysis).24 Surgeon-specific procedural volume was also related with outcomes. Andersen et al.16 demonstrated that surgeon-specific mortality rates ranged from 20% to 67% for surgeons with average annual procedural volumes of 2.0 AAAD repairs/year; 8.3% for surgeons with an average of 4.0 repairs/year, and 1.7% for those performing 9.7 repairs/year. In our series, mortality rate was 17%, with an average of 4.4 repairs per year per surgeon. Although this mortality rate is still high by international standards, it was significantly lower than previously published results in Argentina. Implementation of a multidisciplinary thoracic aortic surgery program to standardize and centralize care of patients undergoing AAAD repair may be a potential solution for this challenging disease in the local setting.
According to international research, the 5-year survival rate after AAAD surgery ranges between 55% and 85%,10,16 in agreement with our findings. However, two other local Argentine studies reported mid-term survival only for in-hospital survivors, thus generating an inclusion bias. Indeed, reported survival in these studies ranged between 73% and 80%, versus 91.3% in the present series when in-hospital mortality was excluded.
New permanent neurological deficit is regarded as a main major morbidity outcome, and its avoidance should be considered a valuable goal to achieve.6 Postoperative severe neurological complications (coma, brain infarction, hemiparesis/plegia, paraparesis/plegia) after AAAD surgery appears in 2.7–25.4% of patients,6,9,25–28 negatively impacting on in-hospital and long-term survival (OR=14).28 In our series, 6% of patients presented new neurological major injury. Currently, the optimal brain protection strategy to use during AAAD surgery is controversial. Adjunctive antegrade cerebral perfusion via the right axillary artery has been recommended as the preferred cerebral protection strategy in circulatory arrest cases,16,28 but there is no consensus for other situations.29 Information given by neuromonitoring could help the surgical team to proceed in time to prevent neurological damage. For instance, electroencephalographic monitoring, near-infrared spectroscopy, and transcranial Doppler are non-invasive methods that can detect the occurrence of embolism and significant decrease in regional cerebral oxygen saturation and blood flow, events that are associated with worse neurological outcome.30 Furthermore, oxygen desaturation in the jugular bulb is a risk factor for postoperative neurocognitive dysfunction.31
In short, the timing and expediency of surgery, the standardization of the operative technique, the use of cerebral protection strategies, and the surgeon's expertise based on high-volume training should be considered the major areas of improvements in AAAD treatment.
Some limitations of this study should be acknowledged. First, reduction in operative mortality when compared with other local centers may be partially due to operation on lower-risk patients. Nevertheless, mean age of patients undergoing surgery in previous Argentine reports ranged from 52 to 63 years11–14; while the current series averaged 65 years. Second, this series was not compared with own historical controls. Finally, a selection bias may further exist with regard to patients who died before surgery.
ConclusionIn conclusion, though the present results are far from ideal international outcomes, AAAD at our institution was associated with current acceptable operative mortality risk and satisfactory mid-term survival compared with previous local studies. Nevertheless, mortality rate in complicated AAAD was three times higher than in patients presenting without shock or malperfusion. In addition to in-hospital mortality, the incidence of new permanent neurological deficit after surgery must be considered the most devastating complication to avoid. Future measures to reduce neurological injury should include neuromonitoring and adjunctive antegrade cerebral perfusion via the right axillary artery during circulatory arrest with deep hypothermia. Patient-centered care in referral aortic centers with surgery performed by specialized teams should be encouraged to improve surgical outcomes in acute aortic dissection surgery in Argentina.
FundingNo endorsement of any kind was received to conduct this study/article.
Conflict of interestThe authors declare no conflicts of interest.