Healthy volunteers participate in phase I clinical trials mainly in search of an economic compensation; their economic instability may constitute a vulnerability factor for anxiety/depression.
ObjectivesTo select suitable and rapid screening tests for anxiety and depression in healthy volunteers, to know their socioeconomic situation and to identify the main reason for their participation.
MethodsCross-sectional study, under a nonparametric statistical analysis and ROC curve analysis. Goldberg's Anxiety and Depression Scale (GADS) (fast test) and the Beck Depression (BDI-II) and Anxiety (BAI) Inventories (standard tests) were applied to all participants.
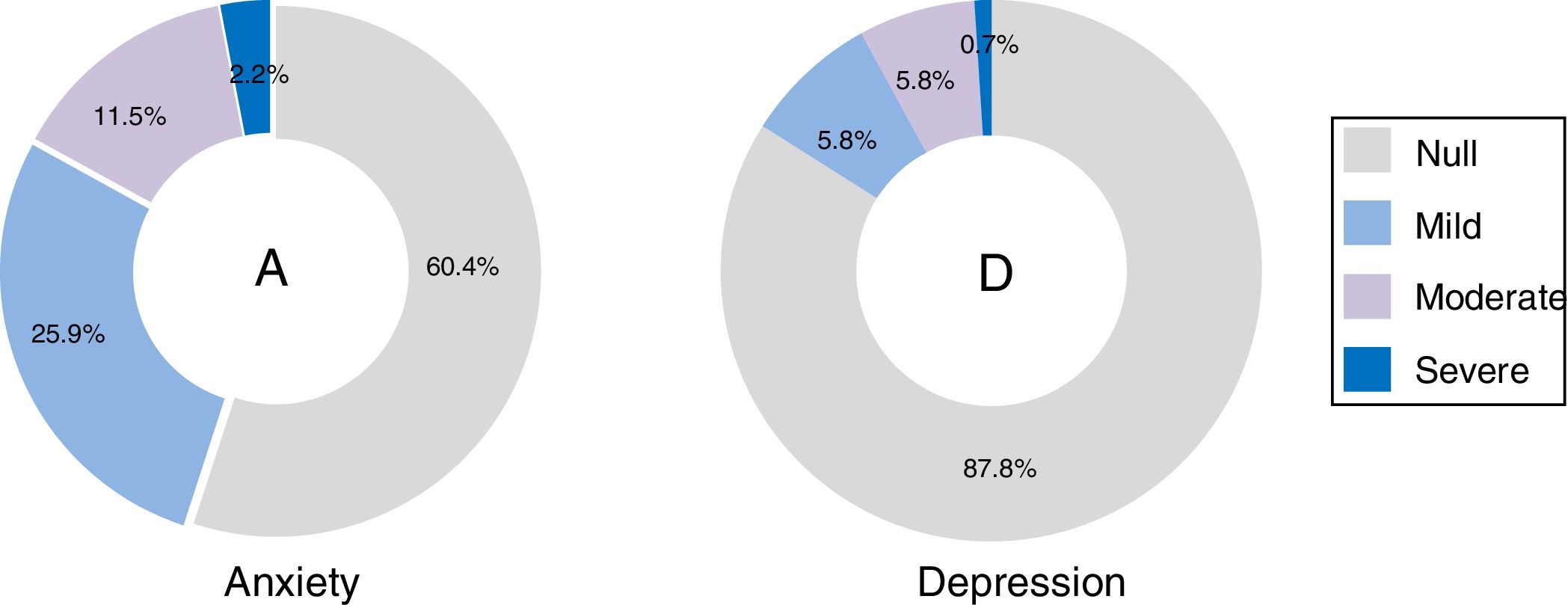
ResultsOne hundred and thirty-nine potential candidates were recruited; the average age was 32 years (SD = 8.8); 53.9% were students, 43.9% employers and 2.2% were unemployed; 85.6% had university studies, 13.7% secondary education and .7% primary studies. GADS, BDI-II and BAI results were: 24.5% volunteered with anxiety and 15.8% with depression (GADS); anxiety levels (BAI): 60.4% had null, 25.9% mild, 11.5% moderate and 2.2% severe; depression (BDI-II): 87.8% had null, 5.8% mild, 5.8% moderate and .7% severe. Socioeconomic characteristics: 48.2% low stratum, 43.2% medium, 5.7% medium high and 2.8% high. Motivations: 46.1% for economic compensation, 35.9% contribution to science, 14.4% for curiosity and 3.6% access to health.
ConclusionsGADS shows insufficient capacity to discriminate between anxiety/depression and the use of BAI and BDI-II is suggested; anxiety and depression levels were higher in healthy volunteers than the prevalence in the general population but lower when compared to university population; employment status was mostly composed of university students with low socioeconomic characteristics and a high economic motivation.
Introducción El motivo principal para participar como voluntario sano en un ensayo clínico fase I responde generalmente a aspectos de compensación económica, por tanto, su inestabilidad económica podría configurar un factor de vulnerabilidad a la ansiedad/depresión. Consecuentemente, sería conveniente utilizar test para valorar su estado mental.
ObjetivosEvaluar los niveles de ansiedad y depresión mediante test óptimos y confiables, conocer las características socioeconómicas y los motivos de participación de voluntarios sanos en ensayos clínicos en fase I.
MétodosEstudio transversal, bajo un análisis estadístico no paramétrico y metodología de análisis de curvas ROC.
ResultadosSe encuestaron a 139 candidatos con media de edad de 32 años (DE: 8.8); el 53.9% estudiantes, el 43.9% trabajadores y el 2.2% desempleados; formación: el 85.6% universitaria, el 13.7% secundaria y el 0.7% primaria. Se seleccionó la Escala de Ansiedad-Depresión de Goldberg (EADG), el Inventario de Depresión (BDI-II) y el Inventario de Ansiedad de Beck (BAI); registrando: el 24.5% ansiedad y el 15.8% depresión (EADG); ansiedad (BAI): el 60.4% nula, el 25.9% leve, el 11.5% moderada y el 2.2% severa; depresión (BDI-II): el 87.8% nula, el 5.8% leve, el 5.8% moderada y el 0.7% severa. Las características socioeconómicas: el 48.2% estrato bajo, el 43.2% medio, el 5.7% medio alto y el 2.8% alto. Motivaciones: el 46.1% económicas, el 35.9% contribución a la ciencia, el 14.4% curiosidad y el 3.6% por acceso a salud.
ConclusionesLos niveles de ansiedad y depresión encontrados superan la prevalencia en la población general, pero son inferiores a la población universitaria; EADG muestra insuficiente capacidad para distinguir entre ansiedad y depresión, se sugiere el uso de BAI y BDI-II; predominan características socioeconómicas bajas y motivaciones de participación económicas.
A clinical trial is a planned, controlled and monitored research executed with humans, where randomization and blinding are essential to assess the efficiency and safety of potential therapeutic molecules (Lazcano, Salazar, Gutiérrez, & Ángeles, 2004). This means that before approving a new drug a clinical trial must show its quality, safety and efficacy as a therapeutic agent. Clinical trials testing new drugs can be classified in different phases: I, II, III and IV.
In relation to phase I, these clinical trials are considered the first link of clinical research because it is in these circumstances where the first administration of the therapeutic molecule in a human occurs. Therefore, it is essential to monitor and evaluate the safety of the new drug and the transcendental aspects like pharmacokinetics and pharmacodynamics (Nieto, 2017).
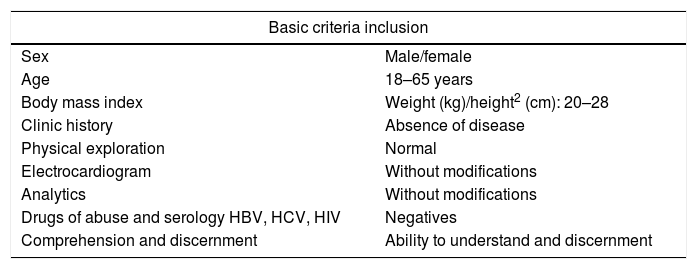
Overall, it is performed in small groups of healthy volunteers that provide critical information and make it possible to identify administration guidelines in order to continue with the subsequent phases. A healthy volunteer in a phase I clinical trial does not expect therapeutic benefits from his/her participation. The objective is to serve the common good, through the useful knowledge regarding safety (Idoipe & Idoate, 2002). So, in order to be considered candidates, they should be healthy people with an optimal Body Mass Index, age range≤65 years, with a minimum risk of suffering from physical and mental disorders and able to understand and give informed consent (Tables 1 and 2).
General inclusion criteria for healthy volunteers in clinical trials phase I (Valera, 2016).
| Basic criteria inclusion | |
|---|---|
| Sex | Male/female |
| Age | 18–65 years |
| Body mass index | Weight (kg)/height2 (cm): 20–28 |
| Clinic history | Absence of disease |
| Physical exploration | Normal |
| Electrocardiogram | Without modifications |
| Analytics | Without modifications |
| Drugs of abuse and serology HBV, HCV, HIV | Negatives |
| Comprehension and discernment | Ability to understand and discernment |
General exclusion criteria for healthy volunteers in clinical trials phase I (Valera, 2016).
| Basic exclusion criteria |
|---|
| Record of disease. |
| Allergies to the active substance/excipient of the medication under study.History of adverse reactions to similar drugs.Smoking patients.Alcohol and drug abuse.Parallel or recent participation in another clinical trial (<3 months).Difficulty to collaborate with the requirements of the study.Absence of informed consent.Other conditions that may result in increased risk orCommitment to the interpretation of results. |
Participation in an ordinary phase I trial is characterized by a constant monitoring regime in which it is possible to generate states of anxiety in volunteers. Moreover, when they can be considered “experimental subjects”. On the other hand, these volunteers may have certain employment conditions, which can affect their anxiety and depression levels.
In this sense, we find it relevant to understand anxiety as a diffuse and unpleasant feeling, capable of mimicking physical and behavioral sensations. It has been found that in a sample with more than 51,500 individuals from 21 countries there is a 10% prevalence of anxiety (Alonso et al., 2018). Furthermore, in some cases the need for treatment is not perceived by the Health System nor the patient. In fact, only 41.3% of the people with anxiety perceive the need for treatment (Alonso et al., 2018).
Moreover, the comorbidity between anxiety and depression are widely known. According to data from the World Health Organization (WHO), the prevalence of depression in the general population ranges between 3% and 5%. Yet when we associate anxiety, this mixed form rises the percentage up to 8% (MSCBS, 2014), interfering significantly with the quality of life of individuals.
In general, for healthy volunteers to participate in a clinical trial, a confirmation diagnosis is not required at the selection visit. It is also not necessary to apply different assessment batteries. However, it would be convenient to evaluate the anxiety and depression states through a simple questionnaire in order to check if the events are more likely to be associated with the studied medication or are influenced by the baseline mental state of the volunteer.
AimsThe main purpose of this study is to evaluate the percentage and states of anxiety, depression or both in candidates to be healthy volunteers for phase I clinical trials. Secondary objectives are to select optimal and reliable tests to evaluate anxiety and depression as well as to know their socioeconomic levels and identify their main reasons to participate in these clinical trials.
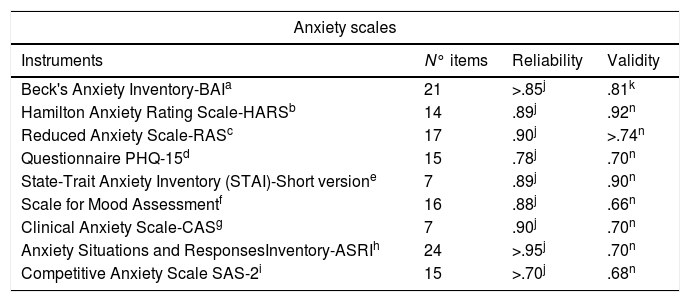
Materials and methodsA cross-sectional study was performed involving potential candidates to participate in a phase I clinical trial. In the first stage, a literature search was conducted to select psychological tests for the screening of anxiety/depression using PubMed, PsicoINFO, Psycodoc, BiblioPRO, Cibersam, Dialnet and Redalyc. Subsequently, the scales were compared and selected based on: levels of reliability and validation as adapted to the Spanish population (Tables 3 and 4).
Instruments used in Spain to evaluate Anxiety.
| Anxiety scales | |||
|---|---|---|---|
| Instruments | N° items | Reliability | Validity |
| Beck's Anxiety Inventory-BAIa | 21 | >.85j | .81k |
| Hamilton Anxiety Rating Scale-HARSb | 14 | .89j | .92n |
| Reduced Anxiety Scale-RASc | 17 | .90j | >.74n |
| Questionnaire PHQ-15d | 15 | .78j | .70n |
| State-Trait Anxiety Inventory (STAI)-Short versione | 7 | .89j | .90n |
| Scale for Mood Assessmentf | 16 | .88j | .66n |
| Clinical Anxiety Scale-CASg | 7 | .90j | .70n |
| Anxiety Situations and ResponsesInventory-ASRIh | 24 | >.95j | .70n |
| Competitive Anxiety Scale SAS-2i | 15 | >.70j | .68n |
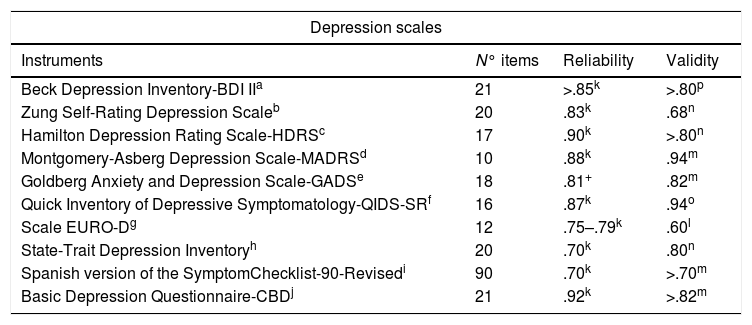
Instruments used in Spain to evaluate Depression.
| Depression scales | |||
|---|---|---|---|
| Instruments | N° items | Reliability | Validity |
| Beck Depression Inventory-BDI IIa | 21 | >.85k | >.80p |
| Zung Self-Rating Depression Scaleb | 20 | .83k | .68n |
| Hamilton Depression Rating Scale-HDRSc | 17 | .90k | >.80n |
| Montgomery-Asberg Depression Scale-MADRSd | 10 | .88k | .94m |
| Goldberg Anxiety and Depression Scale-GADSe | 18 | .81+ | .82m |
| Quick Inventory of Depressive Symptomatology-QIDS-SRf | 16 | .87k | .94o |
| Scale EURO-Dg | 12 | .75–.79k | .60l |
| State-Trait Depression Inventoryh | 20 | .70k | .80n |
| Spanish version of the SymptomChecklist-90-Revisedi | 90 | .70k | >.70m |
| Basic Depression Questionnaire-CBDj | 21 | .92k | >.82m |
Data was collected for the following variables: age, sex and number of past participations in clinical trials. Apart from that, Educational level, Employment status and Socioeconomic characteristics (monthly income) were registered based on Canary-Cardiovascular Project's Questionnaire considering the Socioeconomic Classification System Reference Guide according to the General Media Study. This suggests a monthly income distribution of six sections: <745 €; 745–1312€; 1313–1602€; 1603–2145€; 2452–3005€ and >3005€. Therefore, a numeric code was assigned to each participant in the study to guarantee the confidentiality of their information.
Selection of scalesFor the selection of the tests, scales with high validity and reliability rates for screening were considered. Spanish tests were selected in relation to reliability, validity and the number of items included. In addition, tests that exceed the criteria value of .70 established by Nunnally are considered acceptable instruments in the field of R&D in Psychology (Nunnally, 1978).
Hence, among the most frequently used to analyze anxiety are: The Goldberg Anxiety and Depression Scale (GADS), the Hamilton Anxiety Rating Scale (HARS), the Beck Anxiety Inventory (BAI) and the Clinical Anxiety Scale (CAS) (Estella, Rodriguez, & Castro, 2008). Similarly, for the assessment of depression: The Beck Depression Inventory (BDI-II), the Hamilton Depression Rating Scale (HDRS) and the Montgomery-Asberg Depression Scale (MADRS).
When comparing the psychometric properties of the instruments, concerning the reliability of the anxiety scales, the following results were found: GADS: .81, HARS: .89, BAI: ≥.85 and CAS: .90; CAS barely overpasses the instruments of its type. Nonetheless, not only HARS but also BAI show a high significance, over .85. On the other side, in the case of depression: BDI-II: .85, HDRS: .90 and MADRS: .88; the values of the three scales show proximity, yet HDRS outdoes it slightly.
Either way, both types of instruments reflect a greater consistency and stability in their results. Concerning the internal validity of the instruments, we follow the next distribution: GADS: ≥.82, HARS: .92, BAI: .81 and CAS: .70. This suggests the superiority on HARS, followed by BAI. On the other hand, BDI-II: ≥.80, HDRS: ≥.80 and MARDS: .94. Here MARDS outdoes, yet HDRS and BDI-II stay put.
In addition, considering the characteristics and the nature of the study, it was thought convenient to analyze the quantity of items that conform each scale. In this sense, the distribution of GADS items is: 18 items (9 for the Depression subscale and 9 for the Anxiety subscale), HARS: 14 items, BAI: 21 items and CAS: 7 items; and for Depression: BDI-II: 21 items, HDRS: 21 items and MADRS: 10 items.
For the selection of the scales, it was considered that the instruments with higher indexes of validity and reliability should be taken into a significant exploratory analysis. According to the criteria exposed, the Hamilton Anxiety Rating Scale (HARS) and Hamilton Depression Rating Scale (HDRS) were considered as optimal and suitable tools. Nonetheless, the use of both scales requires a previous psychological interview.
In other words, they are designed to be completed at the end of a semi-structured interview and the participant can then calculate the results. This particularity could generate a burden on the volunteers, more than the clinical essay itself. For this reason, it is considered acceptable to use the Beck Depression Inventory (BDI-II) and the Beck Anxiety Inventory (BAI). Both are preferred due to their self-admissible condition, elevated psychometric properties and for their high use by the Spanish psychologists in clinical practice.
The Beck Anxiety Inventory (BAI) is a self-administered scale, developed by Beck and Steer in 1988. Later, it was adapted to a Spanish context by Sanz and collaborators in 2011 (Copmadrid, 2013a), incorporating an instrument with rigorous psychometric guarantees for Spain. It considers an index of reliability of .85 and a validation of .81; as well as a sensitivity and specificity of ≥.70 (Sanz, 2014).
The Beck Depression Inventory (BDI-II) also has high validation and acceptance rates in Spain. Its original version was developed in 1961 and the self-administered version was adapted by Sanz and Vasquez in 2011 (Copmadrid, 2013b), having a reliability of ≥.85 and a validation of ≥.80, as well as a sensitivity and specificity of ≥.70 (Sanz & Vasquez, 1998). This type of evaluation is widely used given its capacity to detect the presence of depressive symptoms and to value its intensity using the configuration of its items, which describe the most frequent symptoms in clinical practice.
Additionally, Goldberg Anxiety-Depression Scale (GADS) is widely used in primary care because of its simplicity and practicality given its 83.1% sensitivity and 81.3% specificity (Cibersam, 2019). It is conformed by 19 items which are divided equally in 2 groups: a subscale of Anxiety (items from 1 to 9) and a subscale of Depression (items from 10 to 18). In either way, the first 8 items act as a precondition to determine if the rest of the items should be answered.
Statistical aspectsThe data was analyzed using the PSPP® 1.2.0 program, using non-parametric tests (Chi square) and descriptive statistics were used to find significant differences (p<.05). However, to determine the predictive validity of GADS in the screening of the mental state of the healthy volunteers, the methodology of the analysis of the ROC curves was used (VassarStats: Website for Statistical Computation). BAI and BDI-II were used as a reference, considering the cut-off points established for each scale (GADS-AS=≥4, GADS DS=≥2, BAI=≥8 and BDI=≥14) in which scores above the cut-off level are considered positive. The GADS's sensitivity and specificity values were determined for each break-point.
Sample sizeA sample size of 139 participants was calculated with a 95% confidence and a precision±10 percent units, a population percentage considered to be around 10%, using the GRANMO® calculator. In this sense, volunteer candidates have been recruited in the Clinical Research Unit in Clinical Trials of the Hospital del Mar Medical Research Institute (IMIM) in Barcelona.
Ethical aspectsThe study was approved by Ethics Committee – Parc de Salut Mar (CEIm-PSMar). All the participants recruited signed the informed consent.
This study was performed following Good Clinical Practices and Helsinki Declaration. The participation in this study was voluntary, confidential and without any intervention, beyond what was answered to the questionnaires. This, according to the Organic Law 3/2018 of 5th of December on the Protection of Personal Data and the Guarantee of Digital Rights and the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27th of April 2016 on data protection.
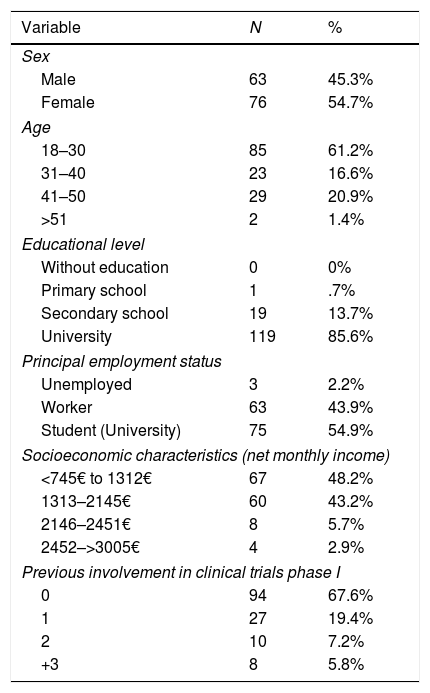
ResultsOne hundred and thirty-nine candidates to become volunteers were interviewed: 45.3% women and 54.7% men. There was an average age of 32 years (S.D. 8.8). Regarding their employment status: 53.9% were university students, 43.9% were employers and 2.2% unemployed. Concerning their education level: .7% had a basic primary education; 13.7%, secondary education and 85.6%, university training (Table 5).
Description of the study sample.
| Variable | N | % |
|---|---|---|
| Sex | ||
| Male | 63 | 45.3% |
| Female | 76 | 54.7% |
| Age | ||
| 18–30 | 85 | 61.2% |
| 31–40 | 23 | 16.6% |
| 41–50 | 29 | 20.9% |
| >51 | 2 | 1.4% |
| Educational level | ||
| Without education | 0 | 0% |
| Primary school | 1 | .7% |
| Secondary school | 19 | 13.7% |
| University | 119 | 85.6% |
| Principal employment status | ||
| Unemployed | 3 | 2.2% |
| Worker | 63 | 43.9% |
| Student (University) | 75 | 54.9% |
| Socioeconomic characteristics (net monthly income) | ||
| <745€ to 1312€ | 67 | 48.2% |
| 1313–2145€ | 60 | 43.2% |
| 2146–2451€ | 8 | 5.7% |
| 2452–>3005€ | 4 | 2.9% |
| Previous involvement in clinical trials phase I | ||
| 0 | 94 | 67.6% |
| 1 | 27 | 19.4% |
| 2 | 10 | 7.2% |
| +3 | 8 | 5.8% |
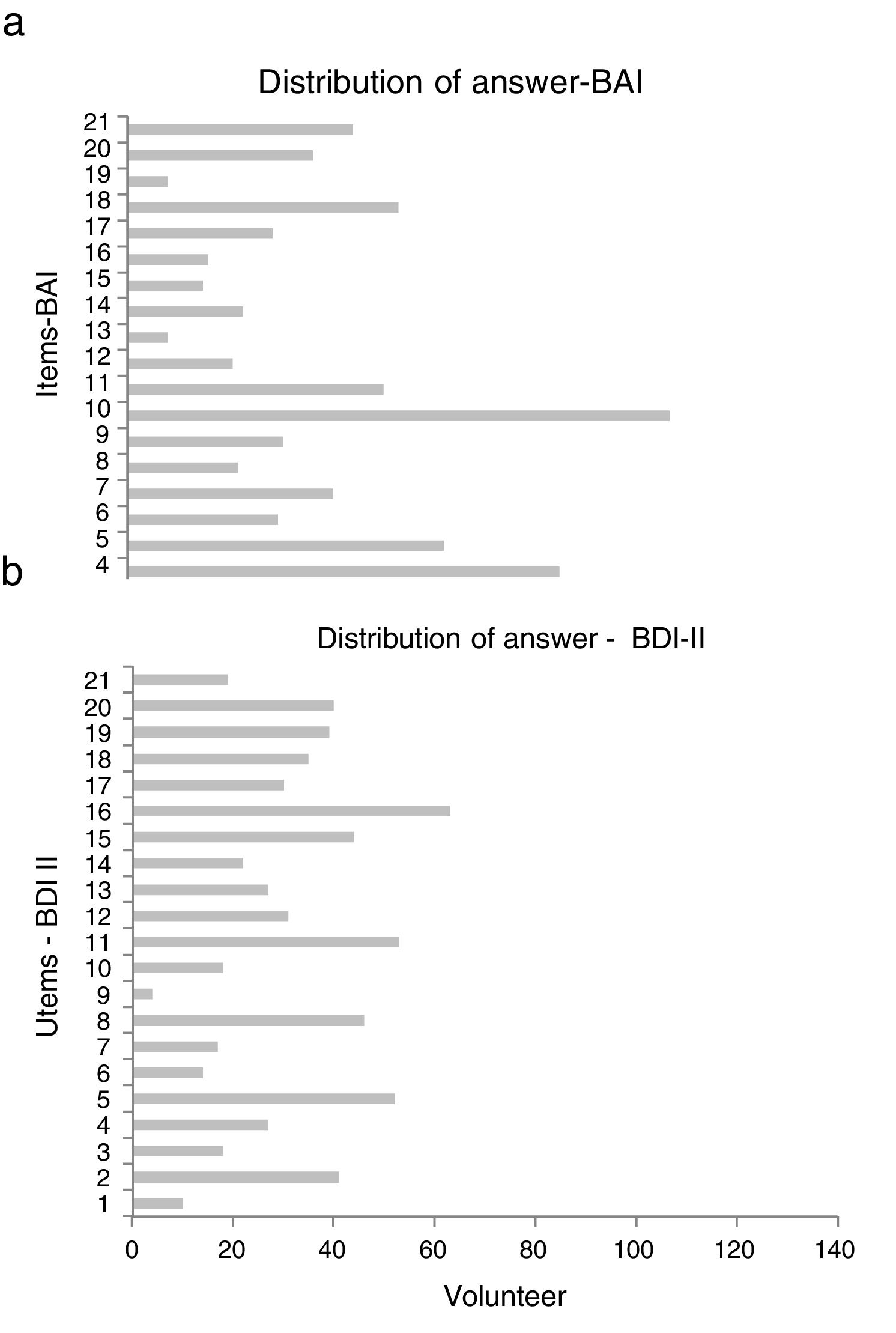
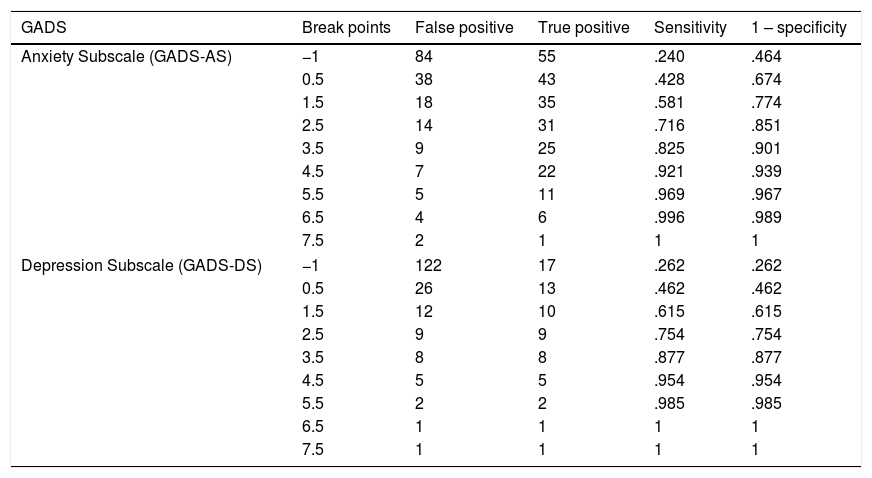
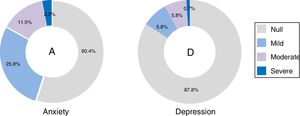
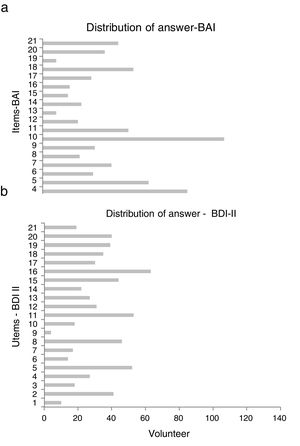
The scales with GADS reflect the presence of 24.5% (n=34) of anxiety and 15.8% (n=22) of depression. The BAI test showed 60.4% (n=84) null, 25.9% (n=36) mild, 11.5% (n=16) moderate and 2.2% (n=3) severe levels of anxiety; and with BDI II, the depression levels were: 87.8% (n=122) null, 5.8% (n=8) mild, 5.8% (n=8) moderate and .7% (n=1) severe (Fig. 1). The most frequent items selected from the BAI, had the following distribution: n° 10 (n=110), n° 4 (n=86) and n° 5 (n=63) (Fig. 2a); and from the BDI-II: n° 16 (n=63), n°11 (n=53), n° 5 (n=52) and n° 8 (n=46) (Fig. 2b). With regard to the psychological tests, significant differences were found between BAI vs GADS-AS (p<.001), BDI-II vs GADS-DS (p<.001). Moreover, the positive predictive value of GADS was 72% when it was considered that the global positive results are more than 3. When the test was divided in two subscales, it raised up to 74% for anxiety subscale and for depression was 45%. However, the area under the curve of GADS-AS is .371 and for GADS-DS it is .231 (Table 6).
Coordinates of the curve for cases with and without anxiety/depression of GADS-AS and GADS-DS.
| GADS | Break points | False positive | True positive | Sensitivity | 1 – specificity |
|---|---|---|---|---|---|
| Anxiety Subscale (GADS-AS) | −1 | 84 | 55 | .240 | .464 |
| 0.5 | 38 | 43 | .428 | .674 | |
| 1.5 | 18 | 35 | .581 | .774 | |
| 2.5 | 14 | 31 | .716 | .851 | |
| 3.5 | 9 | 25 | .825 | .901 | |
| 4.5 | 7 | 22 | .921 | .939 | |
| 5.5 | 5 | 11 | .969 | .967 | |
| 6.5 | 4 | 6 | .996 | .989 | |
| 7.5 | 2 | 1 | 1 | 1 | |
| Depression Subscale (GADS-DS) | −1 | 122 | 17 | .262 | .262 |
| 0.5 | 26 | 13 | .462 | .462 | |
| 1.5 | 12 | 10 | .615 | .615 | |
| 2.5 | 9 | 9 | .754 | .754 | |
| 3.5 | 8 | 8 | .877 | .877 | |
| 4.5 | 5 | 5 | .954 | .954 | |
| 5.5 | 2 | 2 | .985 | .985 | |
| 6.5 | 1 | 1 | 1 | 1 | |
| 7.5 | 1 | 1 | 1 | 1 | |
Likewise, the socioeconomic characteristics (about net monthly income) have the following stratum distribution: 48.2% (n=67) low (<745€ to 1312€ net monthly income); 43.2% (n=60) middle (1343€ to 2145€); 5.7% (n=8) middle high (2146€ to 2451€) and 2.9% (n=4) high (2452€ to >3005€). About the experience in previous clinical trials, 67.6% (n=94) answered they had no previous experience, 19.4% (n=27) participated in 1 occasion, 7.2% (n=10) up to 2 occasions and 5.8% (n=8) more than 3.
The reasons to participate in a phase I clinical trial varied widely: 46.1% (n=64) for the economic compensation, 35.9% (n=50) for the contribution to the advancement of science, 14.4% (n=20) for curiosity and 3.6% (n=5) to get access to health services. By contrast, subgroups according to the working conditions showed that a 47.5% did it for the contribution to science (n=29), 32.8% (n=20) had economic motivations, 14.8% (n=9) curiosity and 4.9% (n=3) access to health. In the students groups: 54.7% (n=41) for economic compensation, 28% (n=21) for the contribution to science, 14.7% for curiosity (n=11) and 2.7% (n=2) for access to health; and in the group of unemployed they responded entirely (n=3) for economic reasons.
Finally, significant relationships were found between: net monthly income with reasons to participate (p<.001) and also with depression levels: GADS (p=.020). This was also found in: Experience in previous clinical trials with reasons to participate (p=.022) and with depression levels BDI II (p=.030). No significant differences were found between the reasons to participate with sex (p=.459), age (p=.193) or employment status (p=.840).
DiscussionThe characteristics of the sample could be explained by the closeness between the clinical trials unit with the medical school, which hosts a representative quantity of participants belonging to the unit's database.
Regarding the psychological tests, there are meaningful relationships between them. The BAI, BDI-II and GADS can be applied in the screening for anxiety and depression states in healthy volunteers in phase I clinical trials. BAI and BDI-II reveal their capacity to asses anxiety and depression levels, respectively. GADS has shown the ability to evaluate the presence or absence of these states. In any case, their choice will be subject to the interests of the researcher. At the same time, it is necessary to mention that the higher the anxiety levels are, the higher the depression levels. This contrasts with the data from WHO, which indicates that the presence of depression may increase when mixed forms of anxiety are included (MSCBS, 2014). Thus, the prevalence of anxiety and depression in this study is higher than the prevalence of anxiety in the general population, which is around 10% (Alonso et al., 2018) and for depression up to 8.56% (Cardila et al., 2015). However, those are lower than values in university populations, where it can reach 47.1% for anxiety and 55.6% for depression (Balanza, Morales, & Guerrero, 2009).
There is a statistically significant relationship between the psychometric tests. Nevertheless, GADS does not discriminate between anxiety and depression in this population. This can be explained because their subscales are configured as a whole and not as independent units in the screening, especially in terms of depression. Optimal values of sensitivity and specificity are found in literature, but the analysis of the ROC curves is not published. The difficulties in discriminating anxiety and depression states could be explained due to the small number of items (GADS-AS=9 and GADS-DS=9), even more considering the preconditions that are established to answer the rest of their items (GADS-AS=≥2 and GADS DS=≥1). From another point of view, BAI and BDI-II being autonomous instruments allow specific screening to identify the presence of anxiety and depression, respectively. Also, their items offer the possibility to assess their levels, allowing to discriminate categories according to the degree of intensity. Although BAI and BDI are reference screening tests, it would be advisable to replicate the analysis with the diagnosis of a specialist.
About the employment status, taking into consideration the prevalence rates of Spanish mental health, it is shown that anxiety states doubles for the jobless (9.4%) rather than the employed subjects (4.4%) and similarly goes for depression (7.9% vs 3.1%) (MSCBS, 2017). In this sense, the relation between the unequal net monthly income with the reasons to participate, suggest that economic motivations tend to be more frequent the lower the healthy volunteer's income goes.
However, the data supporting the association among being voluntary and belonging to a low socioeconomic level remains unclear. In this sense, it can be mentioned that the study conducted by Halpern, Karlawish, Casarett, Berlin, and Asch (2004), whose objective was to assess the influence of monetary compensation on the volunteer's participation simulating the supposed development of a clinical trial (n=126) with an antihypertensive pseudo-medicament placebo-controlled, offered the following compensation for participation: $100, $1000 and $2000 dollars. The results showed a positive interaction between patients with high economic levels and payment influence regarding the indicators of willingness to participate, concluding that participants with better socio-economic conditions were more likely to say that the amount of financial compensation was relevant for their decision to participate (37%) compared with the overall sample (20%).
About the motivations to participate, the significant relationship between previous experiences in other clinical trials and the reasons to participate, showed that the volunteers with one or more previous experiences tend to have predominantly economic motivations. These values can be found in studies carried in national contexts, as the qualitative research also conducted in Barcelona (Bajén-Lázaro, n.d.); where a healthy volunteer was interviewed about his experience in three phase I clinical trials, stating that the main reason for his participation was the economic compensation.
Likewise, another study was carried out in the same city, with medical students (n=250) and inexperienced volunteers (n=80). It found that 39.7% of university students expressed that they would never participate in clinical trials, 24.7% would do it for scientific interest, 32.2% for scientific interest plus compensation and just 4.2% only for economic compensation. On the contrary, the mean reason in the experienced volunteers was 90% for compensation and just 6.3% for curiosity (Bigorra & Baños, 1990). These findings are close to the values obtained in this study, which was mostly formed by university students associated with scientific interest like motivation, while participants with more than one clinical trial experience pursue economic compensation mainly. As a matter of fact, percentages of curiosity as reasons for participation were around 14.4%, so it is recommended to evaluate risk behaviors in volunteers.
Nevertheless, the values found concerning the order of motivations to participate in clinical trials differ from other studies. In the USA, a study which aimed to identify reasons and barriers for participating in clinical trials through a survey in 236 participants (potential volunteers, volunteers and a sample of university students) showed the following distribution about the main reasons of their participation: economic compensation, access to health services and contribution to science (Cunny & Miller, 1994). In the case of Canada, 41 volunteers were recruited and through semi-structured interviews, it was found that the main motivations of their participation were the access to health services that came with their participation (Townsend & Cox, 2013). In Australia's case, 36 volunteers were included in a study (8 individuals belonging to indigenous population and 28 non-indigenous), so through thematic analysis, they found qualitative differences between the members about the reasons for their participation as volunteers. In this sense, indigenous partakers said that their main reason was to benefit their community through their participation, while non-indigenous partakers prioritized personal interests rather than helping others (Guillemin et al., 2015).
The motivations to participate in phase I clinical trial as a healthy volunteer have consistently shown the following reasons: the economic compensation, the contribution to the advancement of science and the access to health services. Although, the order changes with respect to the place and context where it is conducted. This would respond to the special characteristics of each space, where the impulse to participate in a phase I clinical trial is determined by the social realities that happen in each context. Consequently, access to health services in countries with shortcomings in its system and the expectation around a Clinical Trials Unit, could be overlapped by the economic compensation itself, or scenarios where the monetary compensation transcends any risk associated with their participation, as well as altruistic situations where healthy individuals participate as volunteers to benefit their collective. So, economic compensation plays a relevant role in the real moment to recruit healthy volunteers but depends critically on the volunteer's immediate context.
The relationship between previous experience and depression levels (BDI-II) shows that the higher the number of previous participations in clinical trials, the lower the depression levels. Even though no statistical significance was found (p=.549), the same thing happens with anxiety levels. Nevertheless, this decrease does not reach a total remission; there are still moderate and severe levels of anxiety and depression states despite the experience. This corresponds to the feelings of worthlessness and the perception of the volunteers who may come to perceive themselves as “experimental subjects” during the development of clinical trials. This can be related to the economic situation of the participants, generating certain affective vulnerability.
Furthermore, when comparing the frequent response rate of the BDI-II, which particularly include the following items: n° 16 “changes in sleep habits”, n° 11 “agitation”, n° 5 “guilt feelings” and n° 8 “self-criticism”, it suggests a degree of emotional engagement that a clinical trial may involve for the healthy volunteers. Likewise, comparing the BAI's frequent responses, the following items are included: n° 10 “nervous”, n° 4 “inability to relax” and n° 5 “fear that the worst can happen”. Thus, regardless of the conditions of phase I clinical trials, emotions of anxiety are generated in healthy volunteers.
Finally, the limitations found were the impossibility of conducting a longitudinal study, where levels of anxiety and depression can be assessed at the beginning and end of clinical trials. Therefore, it is suggested to carry out a prospective and follow-up study to contrast the results presented.
ConclusionsA presence of 24.5% of anxiety and 15.8% of depression was found mainly from mild to moderate level (37.4% anxiety/11.6% depression), which are higher than the prevalence in the general population but lower than the prevalence in the university population. GADS showed the insufficient capacity to discriminate between anxiety and depression, so the use of BAI and BDI-II is suggested to assess the psychological state of healthy volunteers in the interviews of a clinical trial.
The employment status was mostly composed by university students predominantly with socioeconomic low characteristics, which have mainly economic motivations for participation, followed by contribution to science, curiosity and access to health.
Future studies should try to analyze if the nature of the clinical trials and their processes can generate a reactive scenario for anxiety states and, in a lesser sense, for depression.
Conflict of interestsThe authors have no conflicts of interest to disclose.
This study was made possible through the collaboration of scientific and technical staff at the Barcelona Biomedical Research Park and especially at the Clinical trials unit to the Hospital del Mar Medical Research Institute.