Biological aerosols play a vital role in the interactions between the atmosphere, biosphere, climate and public health and fungal spores are a component with allergic importance.
We constructed a database in Castile & Leon (Spain) and carry out molecular-level component-resolved diagnosis to complete the air quality study carried out since 2006 by our aerobiological network (RACYL) to aid clinical diagnosis and treatment.
MethodsWe reviewed a database of 19,774 patients (adults and children) with allergic respiratory disease treated in our unit during the last 12 years. We also made a component-resolved diagnosis of the molecules involved in the pathology in a randomly selected population of 150 patients.
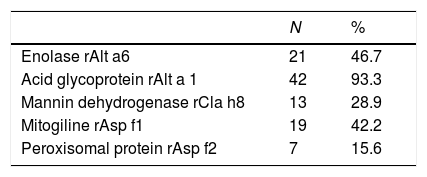
ResultsThe dimeric glycoprotein Alt a1 from Alternaria is the most prevalent and most useful allergen in the diagnosis of patients with allergy to fungi in our area (94.4%), followed by enolase Alt a 6 (Alternaria), ribonuclease Asp f 1 of Aspergillus and mannitol dehydrogenase from Cla h 8 (Cladosporium).
ConclusionsOur results have helped determine which spore molecules are most-closely associated with allergies. Molecular analysis will be useful to determine more accurate and useful immunotherapy in these patients.
Airborne bacteria, fungal spores, pollen and other bioparticles are essential for the reproduction and propagation of organisms through diverse ecosystems, and may cause or worsen diseases in humans, animals and plants. Their interaction is implicated in serious pathologies such as asthma, stroke, ischemic heart disease and cancer.1 Fungi may cause respiratory allergic pathology in both the upper and lower airways in sensitive people, and other allergic disorders such as urticaria, dermatitis, pneumonitis and alveolitis.
Recent studies have shown the prevalence of fungal allergy to be higher than previously thought and that fungi, as a cause of bronchial asthma and other respiratory diseases, may have been underestimated.9 Fungal spores are found in the air at levels much higher than those of pollens and, in many cases, are smaller than pollen grains, and may therefore reach the lower respiratory tract more easily and produce asthma.10 The prevalence of allergic respiratory disease due to fungi varies greatly between studies, especially with respect to the population studied. A 1997 multicenter European study by the Aerobiology Subcommittee of the European Academy of Allergy and Clinical Immunology (EAACI) found that 9.5% of patients with suspected respiratory allergy were sensitized to Alternaria and/or Cladosporium, with the highest prevalence being found in Spain (20%).2 Fungi are considered to be the third most-frequent cause of allergic respiratory disease, after mites and pollens.2,3
The most frequent fungi that induce type I allergy are Ascomycetes (Alternaria, Aspergillus, Bipolaris, Candida, Cladosporium, Epicoccum and Phoma), Basidiomycetes (Calvatia, Coprinus, Ganoderma, Pleurotus and Psilocybe) and some Zygomycetes. Fungal spores are ubiquitous: atmospheric levels worldwide range between 240 and 106spores/m3, and may be 100–1000-fold that of pollen grains (Burge 1989) and in many cases are also seasonal. The main causes of allergy in Castile & Leon, Spain, are Alternaria, Aspergillus, Cladosporium and Penicillium, with Cladosporium being more frequent in the north and Alternaria in the central area, where cereal crops abound.4Alternaria spore aerosols, which are very abundant in the atmosphere of Castile & Leon, are associated with the harvesting of cereals, winemaking and the recurrence of severe asthma.3–8
Despite their importance as aeroallergens, mold allergy may be difficult to diagnose, resulting in less research and publications. This is probably because the commercial diagnostic extracts available are not very effective, with low allergenic activity and high batch-to-batch variability. Most fungal fractions inhaled are spores, although some are fragments of mycelia, which are not usually tested by prick tests. Unlike most other etiological agents, it is not clear whether the original source of sensitization to fungi is mycelia, spores, or their metabolites, and therefore it is not clear how extracts with antigenic activity should be produced, or which are the most suitable methods for their standardization. In addition, there is great antigenic variability in fungal strains.5–7 For these reasons, there are difficulties in the diagnosis of, and immune therapy for, hypersensitivity to fungi.11
The objective of this study was to generate a database in Castile & Leon to aid the clinical diagnosis and subsequent treatment of allergy. The air quality study of pollens has been carried out since 2006 (RECYL) and enables the evaluation of reliable data by any parties interested in environmental biological control. In addition, the study also aims to carried out a molecular analysis with recombinant and native allergens (CRD) can be of great help to specify the diagnosis of hypersensitivity and decide on a more adequate immunotherapy.12,13
Material and methodsTarget populationDatabase: 19,774 patients treated at the Allergy Unit, University Hospital Río Hortega de Valladolid (HURH).
We made an observational cross-sectional retrospective study in patients (adults and children) diagnosed with rhinitis and/or asthma between 2006 and 2017 selected from the database. New cases appearing between January and May 2018 were studied prospectively. Patients with allergic symptoms after eating edible fungi and mushrooms were also included. The protocol was evaluated by the HURH Clinical Research Ethics Committee.
All persons aged >6 months with dyspnea due to variable airflow obstruction without other demonstrable cause or episodes of cough during three consecutive nights and sleep disorders were defined as asthmatic (ISAAC Criteria). Rhinitis was defined according to ARIA criteria. Fungal sensitization was defined as: (a) ≥1 positive skin tests to an environmental fungus (Alternaria, Cladosporium, Penicillium, Aspergillus, Candida or Mucor, (b) a positive CAP (IgE) >0.35IU/mL to these allergens, or (c) positive specific provocation. Fungal asthma was defined by prick, specific IgE and spirometric criteria. The results of previous tests to Alternaria were chosen as a measurable environmental parameter, as Alternaria is the leading cause of allergy in our region. It was considered that all patients included were exposed to similar levels of pollens and other environmental factors due to the analysis of levels of environment quality compiled weekly by the Directorate of Public Health, Ministry of Health, Castile & Leon (SACYL).
We also made a component-resolved diagnosis of the molecules involved in the pathology in a randomly selected population of 150 patients.
Patients sensitized to spores and patients sensitized to grass pollen by IgE-mediated mechanisms (prick and positive specific IgE to spores) were randomly selected from the database for comparison. A control group (evaluated in the same period) of 50 healthy non-smoking blood donors who had never attended the allergy unit was randomly selected by the SACYL Blood Donor Unit.
A clinical epidemiological survey was carried out to evaluate the characteristics and origin of hypersensitivity, the possible appearance of severe asthma, the potential involvement of organs and systems and the treatment required.
After signed informed consent was obtained, conventional allergic analysis (prick, IgE and bronchial challenge if necessary) and molecular analysis using component resolved diagnosis (CRD) was made using ISAC® microarrays to environmental molecules, including allergenic fungal molecules in the three groups:
- 1.
Patients with asthma and/or allergic rhinitis due to fungi attended between January 2017 and May 2018 (50).
- 2.
Patients allergic to grass pollen in the same period (50).
- 3.
Healthy blood donors (50).
Sample size calculation: Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral contrast, 48 subjects in each group were required to detect a minimum between-group difference of eight, assuming there were three groups and a standard deviation of 10. A loss of 20% was estimated.
In vivo testsSkin tests: Conventional prick tests were used for commercialized allergens and proteins purified from native and recombinant fungal spores (DIATER). Prick tests were made in accordance with EAACI criteria (papule diameter ≥3mm). Each allergen was tested in duplicate.
Allergenic extracts: Standard battery of aeroallergens and foods that included pollens (grasses, trees, weeds and flowers), mites (Dermatophagoides and storage), fungi (Alternaria, Cladosporium, Aspergillus, Penicillium and Candida), animal antigens and common foods: wheat, barley, rye, egg, milk, legumes, nuts, fish, seafood, anisakis, and profilins (ALK-Abelló, Madrid, Spain).
In vitro testsMolecular analysis using CRD: The following fungal allergenic molecules were quantified:
rAlt a 1: Alternaria acid glycoprotein
rAlt a 6: Enolase (associated with hypersensitivity to edible mushrooms)
rAsp f1: Aspergillus mitogiline
rAsp f2: Peroxisomal protein
r Cla h8: Mannitol dehydrogenase of Cladosporium
In addition, the response to 112 other proteins of different origins and the response due to cross-reactivity with other plant allergens (profilins, LTPs, poltazines, etc.) were evaluated.
Statistical analysisThe groups were compared simultaneously. The Pearson chi-square test was used to analyze associations between study variables. When the number of cells with expected values <5 was >20%, Fisher's exact test or the likelihood ratio test were used. The Student's t test was used for independent samples in the comparison of mean values and when the number of groups to be compared was greater, ANOVA was used. Non-parametric tests (Mann–Whitney U test for two groups or the Kruskal–Wallis H test for more than two groups) were used as required. In the multivariate analysis, a multinomial logistic regression model was adjusted. The statistical analysis was made using SPSS version 15.0.
ResultsAerobiological and clinical data obtained from the database of 19,744 patients:
Alternaria spores are detected in the atmosphere of Valladolid (as in other areas in Castile & Leon) throughout the year at varying levels. The maximum levels are found in July and August, but practically never exceed 1000 spores/m3. The main conidia emission season, including 95% of the total spores counted annually, lasts from the beginning of June until the end of October.
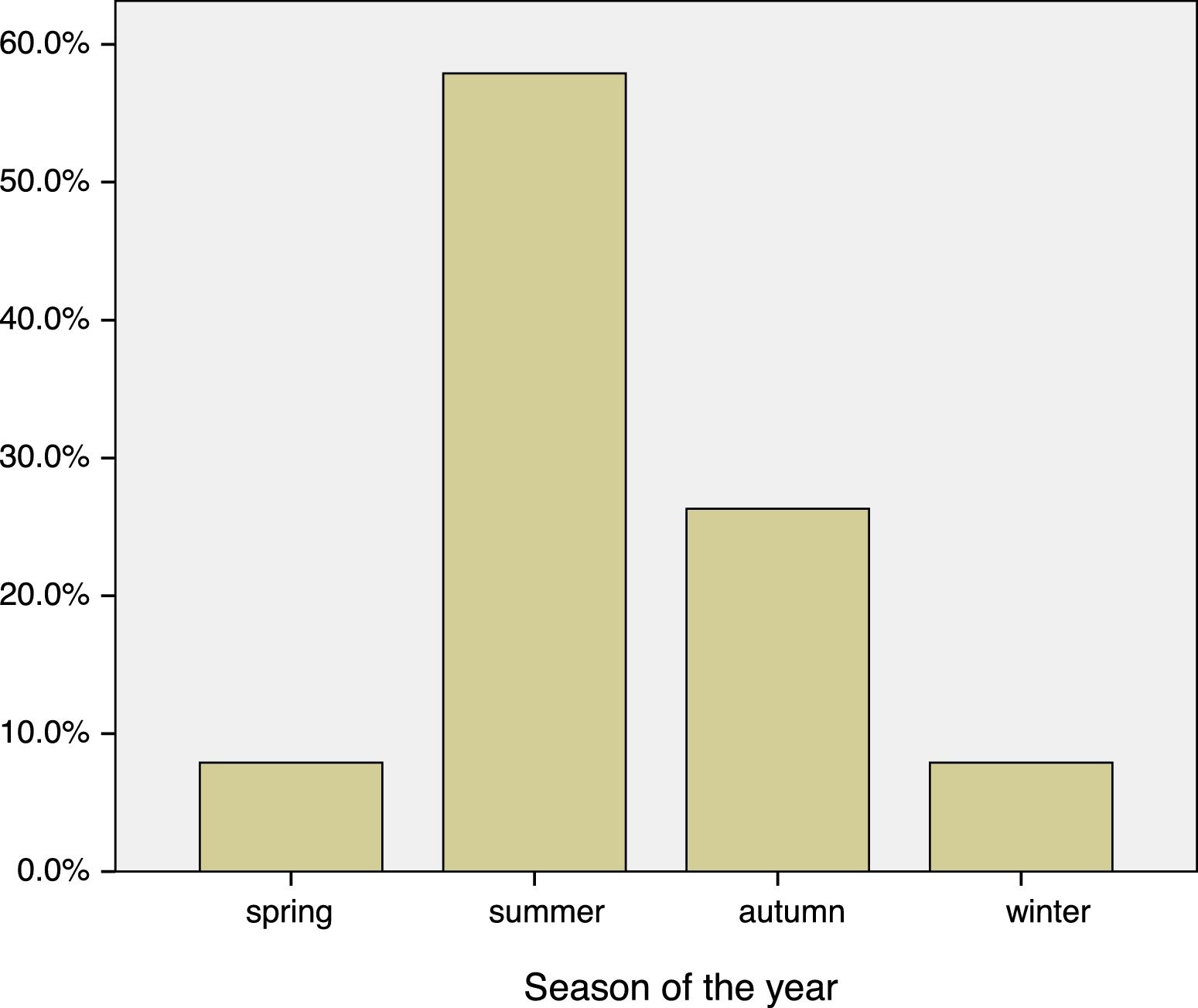
Most patients allergic to fungi in our database have perennial symptoms, although the peak of symptoms occurred during the summer months, coinciding with the highest concentrations of spores in the atmosphere. In Valladolid and province, the maximum level occurred in July, August and September, overlapping with sensitization to grass pollens.
Of the 19,774 patients in our database, 15% were children under 15 years (mean age 12.05±2.66), and all of them were diagnosed with allergic asthma by prick test and positive specific IgE. From adults, 1979 (32.84%) were sensitized to pollens, 561 (9.31%) to mites and 701 (11.63%) to fungi. Of the fungal species studied, Alternaria (387, 61.04%), Aspergillus (115, 18.13%) and Penicillium (46, 7.25%) caused the greatest levels of sensitization. Candida (29 patients) and Mucor (one monosensitized patient) were less implicated.
The most common pathologies were rhinitis and asthma together (269); rhinoconjunctivitis (203); and asthma alone (143). Other, less frequent allergic pathologies were urticaria (50), conjunctivitis22 atopic dermatitis,13 and one case of anaphylaxis due to food intake contaminated by Penicillium, one case of a child who ingested Pleurotus and one woman who ate mushrooms and shitake.
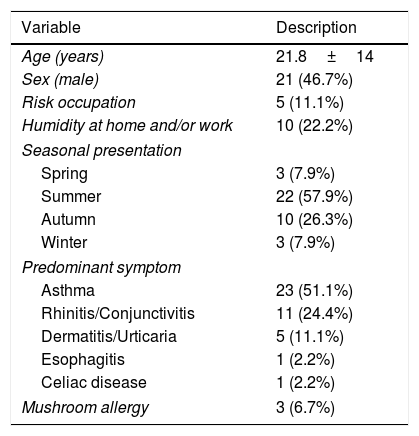
Molecular studyCRD showed no positive molecular response in the control group and only two patients with pollen allergy were positive for Alt a 1. These two patients presented symptoms mainly in May during the pollen season. Of the 50 patients allergic to fungi, 45 completed the study (five were lost to transfer out): of these 21 were male and 24 female, with a mean age of 21.8±14 years, without significant between-sex differences. The predominant clinical manifestation was asthma (59.3%), followed by predominantly seasonal rhinoconjunctivitis. The clinical and epidemiological characteristics are shown in Table 1.
Clinical and epidemiological characteristics of 45 patients allergic to fungi who underwent molecular analysis.
| Variable | Description |
|---|---|
| Age (years) | 21.8±14 |
| Sex (male) | 21 (46.7%) |
| Risk occupation | 5 (11.1%) |
| Humidity at home and/or work | 10 (22.2%) |
| Seasonal presentation | |
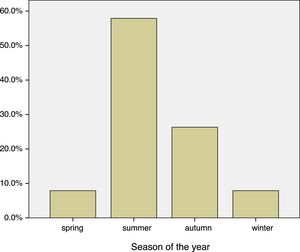
| Spring | 3 (7.9%) |
| Summer | 22 (57.9%) |
| Autumn | 10 (26.3%) |
| Winter | 3 (7.9%) |
| Predominant symptom | |
| Asthma | 23 (51.1%) |
| Rhinitis/Conjunctivitis | 11 (24.4%) |
| Dermatitis/Urticaria | 5 (11.1%) |
| Esophagitis | 1 (2.2%) |
| Celiac disease | 1 (2.2%) |
| Mushroom allergy | 3 (6.7%) |
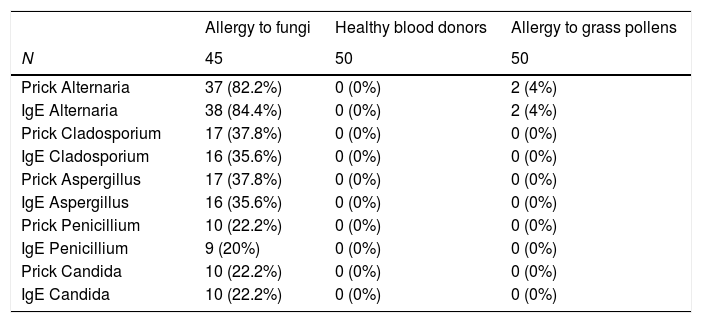
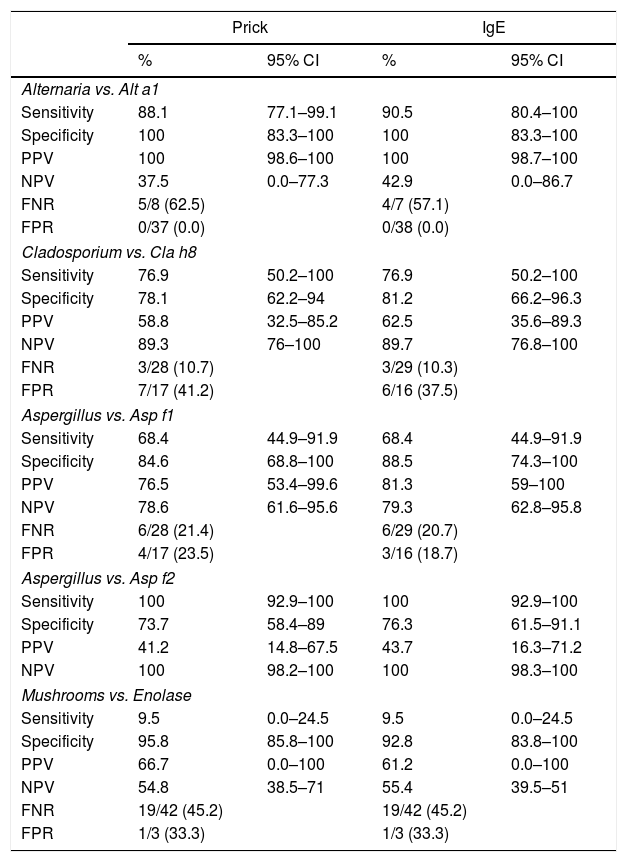
The results of the traditional allergy tests for fungi (prick, IgE) are shown in Table 2.
Traditional allergic tests. Sensitization to fungi in the three groups studies.
| Allergy to fungi | Healthy blood donors | Allergy to grass pollens | |
|---|---|---|---|
| N | 45 | 50 | 50 |
| Prick Alternaria | 37 (82.2%) | 0 (0%) | 2 (4%) |
| IgE Alternaria | 38 (84.4%) | 0 (0%) | 2 (4%) |
| Prick Cladosporium | 17 (37.8%) | 0 (0%) | 0 (0%) |
| IgE Cladosporium | 16 (35.6%) | 0 (0%) | 0 (0%) |
| Prick Aspergillus | 17 (37.8%) | 0 (0%) | 0 (0%) |
| IgE Aspergillus | 16 (35.6%) | 0 (0%) | 0 (0%) |
| Prick Penicillium | 10 (22.2%) | 0 (0%) | 0 (0%) |
| IgE Penicillium | 9 (20%) | 0 (0%) | 0 (0%) |
| Prick Candida | 10 (22.2%) | 0 (0%) | 0 (0%) |
| IgE Candida | 10 (22.2%) | 0 (0%) | 0 (0%) |
The positive results of the molecular tests for fungi are shown in Table 3. There were no significant differences between positive and negative results with respect to age or sex, except for age in the case of maninol-dehydrogenase (negative, 23.8±15.9 years, positive, 16. 8±5.6 years, p=0.036). Occupational risk occupations (wine industry, agriculture and livestock) were associated with peroxisomal protein positivity (positive, three cases, 42.9%, negative, two cases, 5.3%, p=0.021). There were no associations with humidity, the season or the predominant symptoms, except for maninol-dehydrogenase, which was weakly associated with asthma (positive, 10 cases, 76.9%, negative, 13 cases, 43.3%, p=0.043).
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), false positive rate (FPR) and false negative rate (FNR) of the molecular tests vs. the skin prick and IgE tests for the corresponding fungi are shown in Table 4.
Comparison of conventional allergy tests and component-resolved diagnosis in patients with allergy to fungi.
| Prick | IgE | |||
|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |
| Alternaria vs. Alt a1 | ||||
| Sensitivity | 88.1 | 77.1–99.1 | 90.5 | 80.4–100 |
| Specificity | 100 | 83.3–100 | 100 | 83.3–100 |
| PPV | 100 | 98.6–100 | 100 | 98.7–100 |
| NPV | 37.5 | 0.0–77.3 | 42.9 | 0.0–86.7 |
| FNR | 5/8 (62.5) | 4/7 (57.1) | ||
| FPR | 0/37 (0.0) | 0/38 (0.0) | ||
| Cladosporium vs. Cla h8 | ||||
| Sensitivity | 76.9 | 50.2–100 | 76.9 | 50.2–100 |
| Specificity | 78.1 | 62.2–94 | 81.2 | 66.2–96.3 |
| PPV | 58.8 | 32.5–85.2 | 62.5 | 35.6–89.3 |
| NPV | 89.3 | 76–100 | 89.7 | 76.8–100 |
| FNR | 3/28 (10.7) | 3/29 (10.3) | ||
| FPR | 7/17 (41.2) | 6/16 (37.5) | ||
| Aspergillus vs. Asp f1 | ||||
| Sensitivity | 68.4 | 44.9–91.9 | 68.4 | 44.9–91.9 |
| Specificity | 84.6 | 68.8–100 | 88.5 | 74.3–100 |
| PPV | 76.5 | 53.4–99.6 | 81.3 | 59–100 |
| NPV | 78.6 | 61.6–95.6 | 79.3 | 62.8–95.8 |
| FNR | 6/28 (21.4) | 6/29 (20.7) | ||
| FPR | 4/17 (23.5) | 3/16 (18.7) | ||
| Aspergillus vs. Asp f2 | ||||
| Sensitivity | 100 | 92.9–100 | 100 | 92.9–100 |
| Specificity | 73.7 | 58.4–89 | 76.3 | 61.5–91.1 |
| PPV | 41.2 | 14.8–67.5 | 43.7 | 16.3–71.2 |
| NPV | 100 | 98.2–100 | 100 | 98.3–100 |
| Mushrooms vs. Enolase | ||||
| Sensitivity | 9.5 | 0.0–24.5 | 9.5 | 0.0–24.5 |
| Specificity | 95.8 | 85.8–100 | 92.8 | 83.8–100 |
| PPV | 66.7 | 0.0–100 | 61.2 | 0.0–100 |
| NPV | 54.8 | 38.5–71 | 55.4 | 39.5–51 |
| FNR | 19/42 (45.2) | 19/42 (45.2) | ||
| FPR | 1/3 (33.3) | 1/3 (33.3) | ||
CI: confidence interval. PPV: positive predictive value. NPV: negative predictive value. FNR: false negative rate. FPR: false positive rate.
Asthma was associated with the season in patients with fungal allergy (Fig. 1), and was significantly higher in Summer–Autumn (21 cases, 65.6%) than in Winter–Spring (one case, 16.7%), p=0.03.
Acid glycoprotein Alt a 1 of Alternaria was the most prevalent allergen (94.4%), followed by enolase of Alternaria, Aspergillus mitoglicine Aspf1 and mannitol dehydrogenase Cla h8 of C Cladosporium.
DiscussionAllergy to environmental spores is a cause of severe rhinitis and asthma, but responds well to specific immunotherapy.10–12 The tools traditionally used in the etiological diagnosis of allergy are usually allergenic extracts obtained from homogenates of the supposed natural allergenic sources.2 These extracts are a mosaic of varied composition and include molecules of various natures (proteins, carbohydrates, lipids, etc.) that allow detection of the biological source of sensitization but not its molecular cause, in this case the causal allergens of the allergic phenomena. Frequently, there is no cutaneous reactivity to usual fungal extracts in patients with an obvious clinical manifestation of mold allergy or patients clearly reactive to fungal extracts who do not respond satisfactorily to immunotherapy. The lack of rigor of the results obtained depends on factors such as the population group studied, the extracts used and the species used.5,14
After the publication of a position paper (Raulf et al., 2014)15 on the connection between allergy, aerobiology and air pollution by an EAACI panel of experts, more information is being requested about some airborne fungal spores in Europe that are “causing or amplifying” the allergic responses at some times of the year. The interaction between various biotic and abiotic components is implicated in other serious pathologies such as asthma, stroke, ischemic heart disease and cancer (Reinmuth-Selzle et al., 2017).16
The predominantly summer symptoms cause the spore symptoms to overlap those caused by grass pollen, which is the major cause of allergy in Europe,20–23 although spores contribute to the severity of the symptoms that make it a major public health risk.2 Different indices have been applied to quantify atmospheric allergenicity, especially in urban areas, since the content of particles in the atmosphere has important implications for the quality of life,1 and the dynamics of our ecosystems and environment, hence the need to effectively measure airborne particles.4 One way would be to specify the measurement of the molecules most-closely related to the most serious clinical symptoms.17–19
Alternaria alternata is an important source of aeroallergens and is a saprophyte of the leaves of numerous cultivated species and a secondary parasite of cruciferous vegetables and grasses. We could not positively correlate its levels with the humidity of the home, the proximity of rivers or lakes, temperature, hours of sun, wind speed. Rain and humidity inversely correlated,5 suggesting that sporulation depends on dry weather. Our area is mainly cereal growing, with low rainfall, and this entails an increase in the sporulation of this fungus and its abundance in the atmosphere. In particular, acid glycoprotein Alt a 1 is an allergenic component that indicates primary sensitization and is a risk factor for the development of asthma in children and adults.
In the molecular CRD study, Alternaria and Aspergillus were the spores most-closely associated with clinical symptoms, which were predominantly asthmatic. The most profitable allergens for diagnosis were the acid glycoprotein of Alternaria (Alt a 1), followed by superoxide dismutase of Aspergillus (Asp f 6), although mitoglicine (Asp f 1) had greater specificity. In the case of allergy to edible mushrooms, enolase (Alt a 6) was the most frequently detected.
Asp f 1 is responsible for many allergic diseases, the most severe of which is allergic bronchopulmonary aspergillosis. Another molecule detected, Cla h 8 (mannitol dehydrogenase) is a component that indicates primary sensitization and may cause allergic symptoms in almost all climatic zones due to its ubiquity.14
Studies with some extracts of Aspergillus fumigatus and Fusarium culmorum have shown that these allergenic extracts are unstable, which can also affect the diagnostic sensitivity.12
Currently, 22 recombinant allergens of A. fumigatus have been obtained and some studies highlight a different allergen sensitization pattern in patients with allergic bronchopulmonary aspergillosis compared with patients with other types of allergic disease due to Aspergillus.
Atmospheric pollutants, including CO2, can modify the sporulation of Alternaria and the amount of allergenic proteins it produces (Wolf et al., 2010).22Alternaria spores cause severe respiratory pathology and, once diagnosed, precision immunotherapy with recombinant allergens achieves a very favorable clinical response (Horst et al., 1990,25 Bousquet et al., 1998,11 Tabar et al., 2000,24 Prieto et al.26)
The validity and viability of our study could have been compromised by the fact that it would only detect possible causal allergens in patients with an atopic background and is not applicable to respiratory pathology due to other causes. We were also unable to determine whether the response obtained to a fungal epitope would have clinical value because subclinical sensitization is reported in allergic patients. Studying the clinical evolution of patients receiving specific immunotherapy for at least three years after detection of the causal molecule would clarify the utility of CRD in these patients.
In conclusion, in patients with positive skin tests and IgE response to one of the most prevalent spore molecules, bronchial provocation, currently considered the diagnostic gold standard, may not be indicated. Only measurement of IgE to Alt a 1, rAsp f1, Asp f6 and Cla h8 may be preferable.
FundingNone.
Conflicts of interestsThe authors report no conflict of interest.