The identification of children who will have persistent asthma has become a focus of recent research. The aim of this study was to assess whether impulse oscillometry (IOS) has a diagnostic value to predict modified API (asthma predictive index) in pre-schoolers with recurrent wheezing.
MethodsPre-school children aged 3–6 years with recurrent wheezing were enrolled. The study population was divided into two groups based on mAPI criteria. Lung function was assessed by IOS.
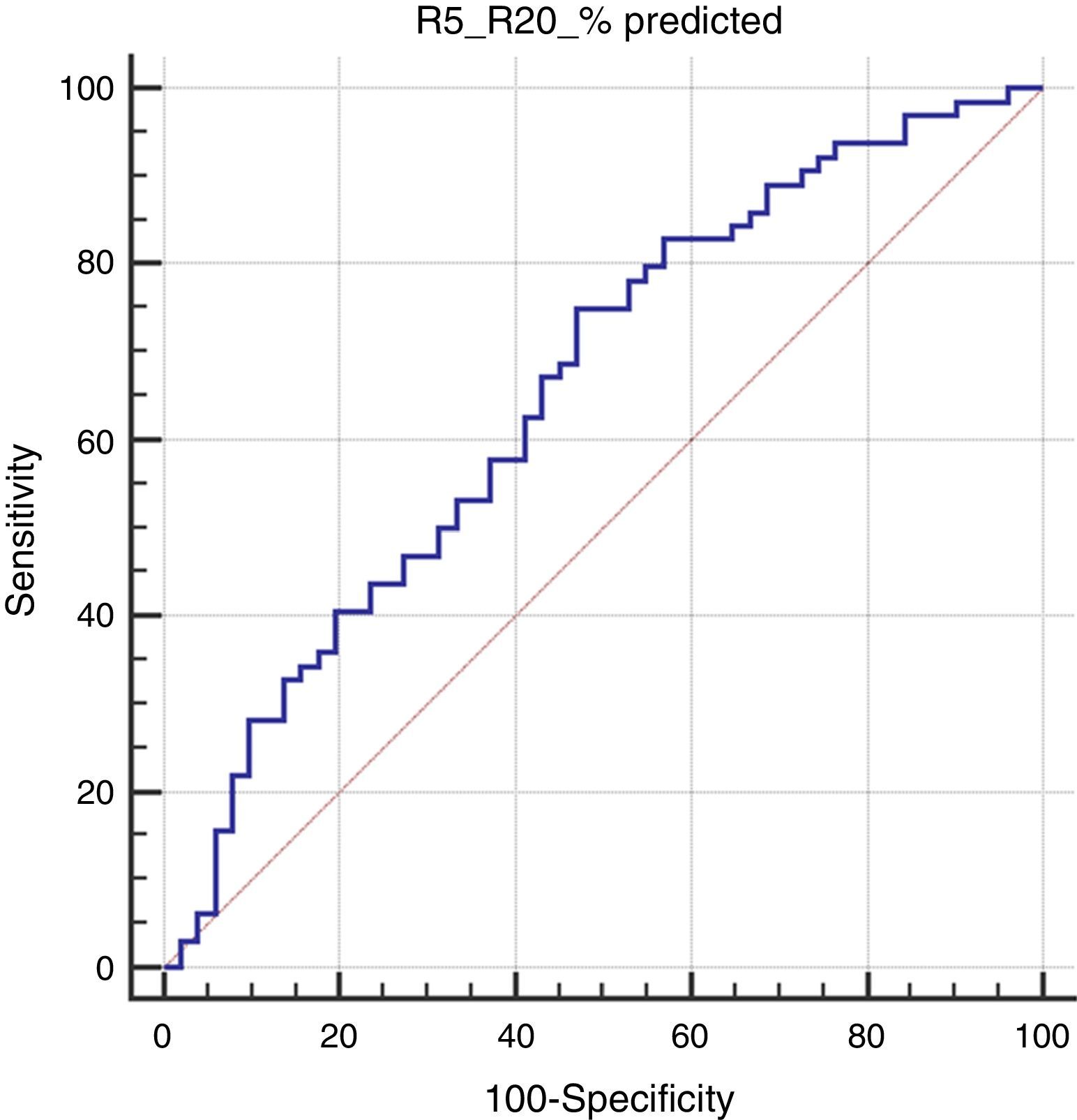
Results115 children were assessed; 75 (65.2%) of them were male. The median age was 39 months (min: 36, max: 68 months). 64 (55.6%) of the children were mAPI positive. The R5-R20% levels of children with positive mAPI were significantly higher compared to negative mAPI. Also, R5-R20% levels of children with parental asthma and R20% pred and resonant frequency (Fres) levels of children with inhalant sensitization were higher than those without. No significant differences were found in IOS indices between groups based on the presence of atopic dermatitis, food sensitization, eosinophilia, inhaled corticosteroid usage or wheezing without colds. R5-R20% and total IgE values were found to be significantly related to positive mAPI (aOR: 1.40, p=0.022 and aOR: 1.02, p=0.001, respectively). In the ROC analysis, R5-R20% levels >14.4 had a sensitivity of 75% and specificity of 53% for predicting a positive mAPI (p=0.003).
ConclusionIOS may help clinicians to identify the pre-school wheezers with a high risk of asthma.
Recurrent wheezing is a common problem in pre-school children. Approximately 40% of children wheeze in their first year of life. However, only 30% of pre-schoolers with recurrent wheezing still have asthma at the age of six years.1,2 Despite the fact that asthma usually starts in the early life years during which the substantial alterations in lung structure develop, the actual pathopyhsiology of infant lung function in the progression of asthma is still limited.3 Asthma is reported to be the most prevalent chronic respiratory disease in childhood and early detection of the children at potential risk in this crucial time may affect the natural course of the disease.4,5
The identification of pre-school children who will have persistent asthma has been the focus of interest.6 The asthma predictive index (API), which is the most widely used model was originally developed in the Tucson cohort study.7 A positive API requires recurrent episodes of wheezing during the first three years of life and one of two major criteria (physician-diagnosed eczema or parental asthma) or two of three minor criteria (physician-diagnosed allergic rhinitis, wheezing without colds or peripheral eosinophilia ≥4%). A positive API by the age of three years was associated with a 77% chance of active astma from ages 6 to 13, whereas a negative API at the age of three years had less than a 3% chance of having active asthma during the school years.4 The API was modified by the incorporation of aeroallergen sensitization as a major criterion and food sensitization as a minor criterion, replacing physician-diagnosed allergic rhinitis.7
The assessment of respiratory mechanics in children with recurrent wheezing may provide an objective information about the progression and the need for and response to interventions.3,8 However, detection of airway dysfunction in young children remains a challenge for clinicians because of the lack of methods easily applied in clinical care. Impulse oscillometry (IOS) can be easily done even in children who are unable to perform forced expiratory maneuvers.9 It is a simple, non-invasive method that requires minimal patient cooperation. This method uses sound waves to measure respiratory mechanics and has the potential to evaluate airflow limitation during tidal breathing. It can be used in various chronic respiratory diseases in order to identify the underlying airway dysfunction more accurately.10,11
To date, there is limited data available on impulse oscillometry in children with recurrent wheezing.12,13 The aim of the present study was to assess the diagnostic value of IOS to predict mAPI positivity in preschool children with recurrent wheezing.
MethodsStudy populationThe study included pre-school children aged three to six years who had attended to the department of Pediatric Allergy and Immunology of Mersin University with a history of recurrent wheezing between January 2014 and December 2016. Recurrent wheezing was defined as having more than three wheezing episodes confirmed by a doctor during the last one year. Children who were able to perform impulse oscillometry according to ATS/ERS recommendations were eligible for this study. Those children with prematurity, low weight for gestational age, airway malformations, cardiac, neurological diseases, chronic respiratory diseases such as cystic fibrosis, ciliary dyskinesia and bronchopulmonary dysplasia were excluded. Children who were experiencing an acute wheezing attack were not enrolled in the study.
A positive modified API requires four or more episodes of wheezing with at least one confirmed by a physician and one of three major criteria (physician-diagnosed eczema, parental asthma, aeroallergen sensitization) or two of three minor criteria (food sensitization, wheezing without colds or peripheral eosinophilia ≥4%).7 The wheezy children were divided into two groups; 1) Group with positive API; if they had one major or two minor criteria and 2) Group with negative API; if they did not meet these criteria.
The study was approved by the ethics committee of the Mersin University.
Data collectionMedical records of the patients were reviewed to determine demographic characteristics, history of wheezing, atopy (food and inhalant sensitization determined by specific IgE levels and skin prick tests), atopic dermatitis and parental asthma. Serum total IgE levels, peripheral eosinophil counts, usage of inhaled corticosteroids and leukotriene receptor antagonists (LTRA) were also obtained from medical records.
Impulse oscillometryImpulse oscillometry (IOS) was performed in compliance with the European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines by MasterScreen IOS system (Jaeger Co., Germany).14 IOS was performed during spontaneous breathing. The output pressure and flow signals were analyzed for 30s in the frequency range of 5–20Hz for their amplitude and phase differences to determine the resistance (R) and reactance (X) values. The IOS parameters obtained at the end of the application were resistances (R5, 20) at 5–20Hz, R5-R20 (resistance at 5Hz minus resistance at 20Hz), reactance at 5Hz (X5), resonant frequency (Fres, the frequency where the X value is zero), and area of the reactance curve (AX, integral of X values from 5Hz to Fres).
Statistical analysisThe Shapiro–Wilk test was performed to test the suitability of the normal distribution of the numerical data. Descriptive analyses were presented using median or mean±SD based on normal distribution or not. Unadjusted comparisons were made using the Independent samples t-test or Mann–Whitney U test for continuous endpoints and the Chi-Square test for categorical endpoints. In order to match the subgroups of pre-school wheezers for sample size equilibrium according to the clinical risk factors, the propensity score was used. A multivariate logistic regression model was used to identify independent predictors of API. Odds ratios with their 95% confidence intervals were estimated. For the variables with large measurement units, a regression coefficient was calculated by multiplying 10 in order to examine the increase in risk when there is a change of 10 units instead of 1 unit.15 Roc curve analysis was performed to determine the cut-off value for positive API. A p value of less than 0.05 was considered statistically significant.
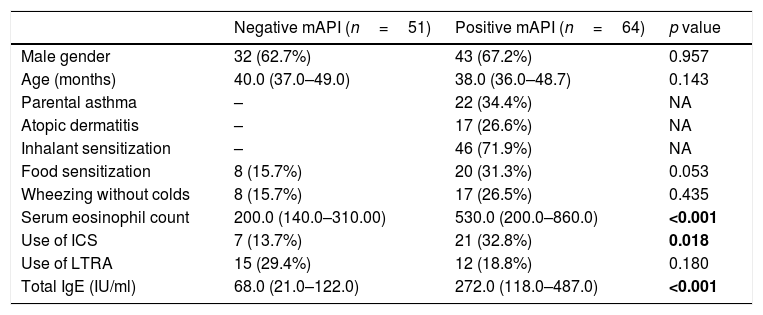
ResultsStudy populationWe evaluated 115 children admitted to the outpatient clinics of Pediatric Allergy and Immunology with recurrent wheezing. The median age of the subjects was 39 months (min: 36, max: 68 months) and 75 (65.2%) were male. Twenty-two (19.1%) of the subjects had parental asthma, 17 (14.8%) of them had atopic dermatitis. Among all subjects, 28 (24.3%) had food sensitization and 46 (40.0%) had inhalant sensitization. A history of wheezing without colds was present in 25 (20.1%) children. Twenty-eight (24.3%) children were using inhaled corticosteroids (ICS) and twenty-seven (23.5%) children were using leukotriene receptor antagonists (LTRAs) as prophylactic treatment at enrollment. A history of maternal smoking was present in seven (6.1%) children. Of the 115 preschoolers with recurrent wheezing, the modified asthma predictive index was positive (mAPI) in 64 (55.6%). Demographic characteristics of children with positive and negative mAPI are presented in Table 1.
Demographic characteristics of children with positive and negative modified asthma predictive index.
| Negative mAPI (n=51) | Positive mAPI (n=64) | p value | |
|---|---|---|---|
| Male gender | 32 (62.7%) | 43 (67.2%) | 0.957 |
| Age (months) | 40.0 (37.0–49.0) | 38.0 (36.0–48.7) | 0.143 |
| Parental asthma | – | 22 (34.4%) | NA |
| Atopic dermatitis | – | 17 (26.6%) | NA |
| Inhalant sensitization | – | 46 (71.9%) | NA |
| Food sensitization | 8 (15.7%) | 20 (31.3%) | 0.053 |
| Wheezing without colds | 8 (15.7%) | 17 (26.5%) | 0.435 |
| Serum eosinophil count | 200.0 (140.0–310.00) | 530.0 (200.0–860.0) | <0.001 |
| Use of ICS | 7 (13.7%) | 21 (32.8%) | 0.018 |
| Use of LTRA | 15 (29.4%) | 12 (18.8%) | 0.180 |
| Total IgE (IU/ml) | 68.0 (21.0–122.0) | 272.0 (118.0–487.0) | <0.001 |
p values in bold are statistically significant.
mAPI: modified asthma predictive index; ICS: inhaled corticosteroids; LTRA: leukotriene receptor antagonist; NA: not applicable.
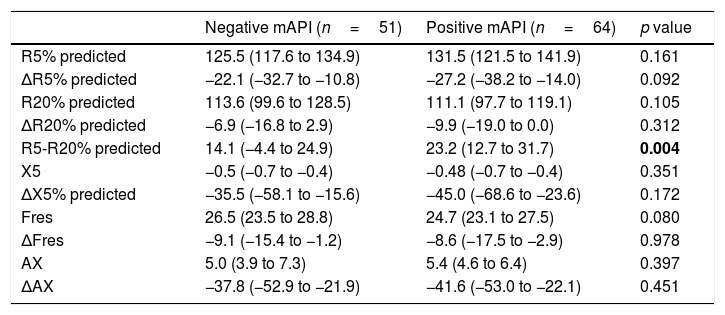
When we compared the groups with positive (n=64) and negative (n=51) mAPI, we found that only R5-R20% levels were significantly (p=0.004) higher in the positive mAPI group (Table 2). There was no significant difference between groups in terms of other impulse oscillometry (IOS) indices.
Impulse oscillometry parameters of children with positive and negative modified asthma predictive index.
| Negative mAPI (n=51) | Positive mAPI (n=64) | p value | |
|---|---|---|---|
| R5% predicted | 125.5 (117.6 to 134.9) | 131.5 (121.5 to 141.9) | 0.161 |
| ΔR5% predicted | −22.1 (−32.7 to −10.8) | −27.2 (−38.2 to −14.0) | 0.092 |
| R20% predicted | 113.6 (99.6 to 128.5) | 111.1 (97.7 to 119.1) | 0.105 |
| ΔR20% predicted | −6.9 (−16.8 to 2.9) | −9.9 (−19.0 to 0.0) | 0.312 |
| R5-R20% predicted | 14.1 (−4.4 to 24.9) | 23.2 (12.7 to 31.7) | 0.004 |
| X5 | −0.5 (−0.7 to −0.4) | −0.48 (−0.7 to −0.4) | 0.351 |
| ΔX5% predicted | −35.5 (−58.1 to −15.6) | −45.0 (−68.6 to −23.6) | 0.172 |
| Fres | 26.5 (23.5 to 28.8) | 24.7 (23.1 to 27.5) | 0.080 |
| ΔFres | −9.1 (−15.4 to −1.2) | −8.6 (−17.5 to −2.9) | 0.978 |
| AX | 5.0 (3.9 to 7.3) | 5.4 (4.6 to 6.4) | 0.397 |
| ΔAX | −37.8 (−52.9 to −21.9) | −41.6 (−53.0 to −22.1) | 0.451 |
p values in bold are statistically significant.
mAPI: modified asthma predictive index.
When we compared the children with and without parental asthma, we found that R5-R20% (p<0.001) levels were significantly higher in the group with parental asthma (data not shown). No significant difference was found in IOS indices between groups based on the presence of atopic dermatitis, food sensitization, eosinophilia, usage of ICS or wheezing without colds.
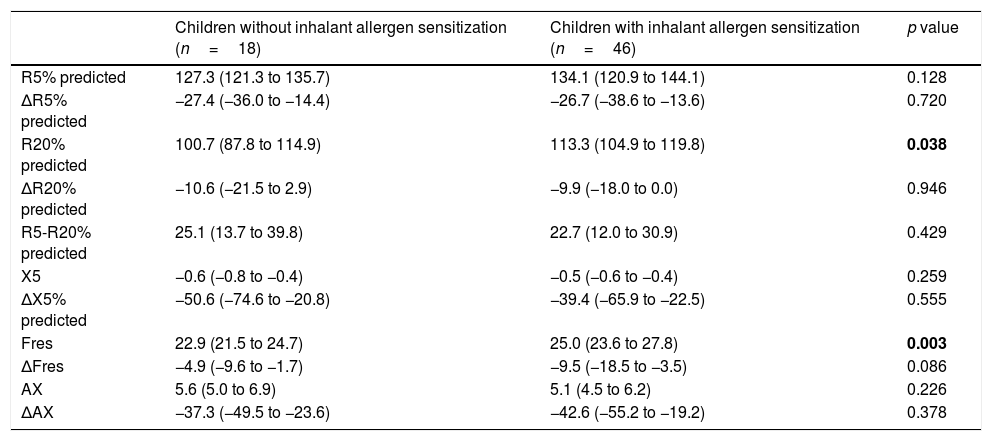
When we compared the children with and without inhalant allergen sensitization, we found that R20%pred (p=0.038), and Fres (p=0.003) levels were significantly higher in the group with inhalant allergen sensitization. No significant difference was found between these groups in terms of other IOS indices. (Table 3).
Impulse oscillometry parameters of children with and without inhalant allergen sensitization.
| Children without inhalant allergen sensitization (n=18) | Children with inhalant allergen sensitization (n=46) | p value | |
|---|---|---|---|
| R5% predicted | 127.3 (121.3 to 135.7) | 134.1 (120.9 to 144.1) | 0.128 |
| ΔR5% predicted | −27.4 (−36.0 to −14.4) | −26.7 (−38.6 to −13.6) | 0.720 |
| R20% predicted | 100.7 (87.8 to 114.9) | 113.3 (104.9 to 119.8) | 0.038 |
| ΔR20% predicted | −10.6 (−21.5 to 2.9) | −9.9 (−18.0 to 0.0) | 0.946 |
| R5-R20% predicted | 25.1 (13.7 to 39.8) | 22.7 (12.0 to 30.9) | 0.429 |
| X5 | −0.6 (−0.8 to −0.4) | −0.5 (−0.6 to −0.4) | 0.259 |
| ΔX5% predicted | −50.6 (−74.6 to −20.8) | −39.4 (−65.9 to −22.5) | 0.555 |
| Fres | 22.9 (21.5 to 24.7) | 25.0 (23.6 to 27.8) | 0.003 |
| ΔFres | −4.9 (−9.6 to −1.7) | −9.5 (−18.5 to −3.5) | 0.086 |
| AX | 5.6 (5.0 to 6.9) | 5.1 (4.5 to 6.2) | 0.226 |
| ΔAX | −37.3 (−49.5 to −23.6) | −42.6 (−55.2 to −19.2) | 0.378 |
p values in bold are statistically significant.
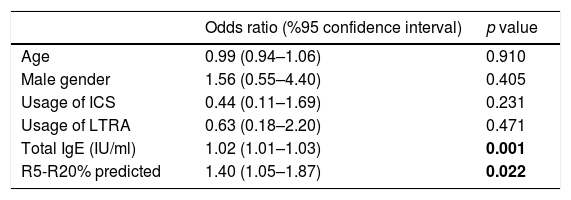
In the logistic regression analysis, R5-R20%pred and total IgE levels were found to be significantly related to positive mAPI in pre-school children with recurrent wheezing, independent of age, gender and use of ICS or LTRA prophylaxis (R5-R20%; OR: 1.40, 95% CI: 1.05–1.87, p=0.022 and total IgE; OR: 1.02, 95% CI: 1.01–1.03, p=0.001) (Table 4). Each 10% increase in (R5-R20)% levels predict a 1.40 fold increase in the risk of having positive mAPI.
Logistic regression analysis of factors that are associated with a positive modified asthma predictive index in pre-schoolers with recurrent wheezing.
| Odds ratio (%95 confidence interval) | p value | |
|---|---|---|
| Age | 0.99 (0.94–1.06) | 0.910 |
| Male gender | 1.56 (0.55–4.40) | 0.405 |
| Usage of ICS | 0.44 (0.11–1.69) | 0.231 |
| Usage of LTRA | 0.63 (0.18–2.20) | 0.471 |
| Total IgE (IU/ml) | 1.02 (1.01–1.03) | 0.001 |
| R5-R20% predicted | 1.40 (1.05–1.87) | 0.022 |
p values in bold are statistically significant.
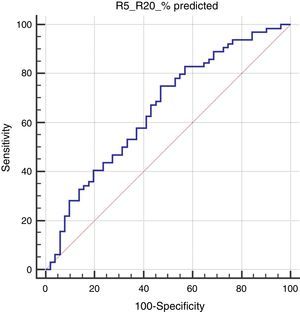
In the Roc analysis, (R5-R20)% pred level higher than 14.4% was found to be significantly related to the risk of positive mAPI (AUC: 0.656, p=0.003, sensitivity: 75% and specificity: 53%) (Fig. 1).
DiscussionWheezing is a common symptom in early life and a significant number of pre-school children will continue to wheeze in later childhood.1 In order to assess the predictive risk factors for early wheezers who will experience asthma in school years, several models were developed. One of the most utilized model was derived from the Tucson study; an asthma predictive index (API). Children with positive API were seven times more likely to have active asthma during the school years and also it has a high negative predictive value when API is negative.7 On the other hand, the modified API is reported to be useful in detecting children who are more likely to respond to ICS.16 In a population of children at high risk, the positive mAPI was reported to have increased future asthma probability.17 The identification of the children at risk for persistent asthma may lead to effective prevention and therapeutic strategies.5
The aim of this study was to assess the utility of IOS to predict the mAPI positivity in pre-schoolers with recurrent wheezing. We found that R5-R20% pred levels in IOS were significantly higher in children with positive mAPI compared to negative mAPI. While R5-R20% pred levels of children with parental asthma were higher than those without, R20%pred and resonant frequency (Fres) levels differed between children with and without inhalant sensitization. No significant difference was found in IOS indices between groups based on the presence of atopic dermatitis, food sensitization, eosinophilia, ICS usage or wheezing without colds. R5-R20% and total IgE values were found to be significantly related to the risk of positive mAPI in logistic regression analysis. In addition, a level of R5-R20% pred>14.4% was found to be significantly related to the risk of positive mAPI in ROC analysis.
Early onset of asthma has been associated with reduced lung function that continues into later childhood and adulthood.18–20 Besides the clinical risk factors, the assessment of lung function in early life may contribute to detection of children at high risk for future asthma. Compared with healthy controls, airway function is found to be reduced in young children with recurrent wheeze, particularly those at risk for subsequent asthma.21 It provides further evidence for the association between clinical risk factors and impaired lung function in early life. However, the association between wheezing phenotypes and early airway function still remains unclear.
The relation of API to the respiratory mechanics has not been fully studied. A recent study revealed that among recurrent wheezing infants and young children, those having a positive API have a significantly lower lung function as measured by VmaxFRC at an early age.22 Another study among infants with recurrent wheezing showed that those with a positive clinical index for asthma had significantly reduced FVC and FEF25-75 than those without such risk factors. The reduction in FVC accompanied by reduced flows in wheezy children with high risk may reflect small airway obstruction.21 It has also been reported that tidal breathing indices such as the ratio of time taken to reach peak tidal expiratory flow to total expiratory time (tPTEF:tE) may be associated with subsequent wheezing or asthma in later life.23,24 IOS has been introduced as an alternative technique to assess lung function. It is one of the most feasible methods to measure the respiratory mechanics in young children because it is easy to perform and non-invasive.9 A study on 325 children aged four years showed that those with persistent wheeze phenotype had significantly larger baseline resistance at 4Hz measured by force oscillation technique than children with early transient wheeze.25 Lezana et al. found no difference in basal lung function and post-bronchodilator response to salbutamol by IOS between API positive and negative preschoolers. However, API positive pre-schoolers under ICS treatment had significantly higher central basal airway resistance (R20Hz) and higher post bronchodilator response than those without ICS. They speculated that the former had more severe disease that makes their pediatricians more prone to prescribe ICS.26 We found that R5-R20%, a measure of frequency dependency in IOS, was significantly higher in children with positive mAPI compared to negative mAPI suggesting the involvement of small airway dysfunction to a great extent in recurrent wheezers with high risk for asthma. In addition, it may be considered that small airway disease does not benefit from ICS treatment in recurrent wheezers because there was no significant difference in IOS indices between those with and without ICS prophylaxis in the present study.
Previous studies have shown that various early life factors were associated with lung function impairment in childhood, including sex, low birth weight, maternal smoking, family history of asthma, exposure to sensitizing allergens and the presence of atopy.3,27 Consistent with this data, a previous study suggested that a family history of asthma contributes to elevated levels of airway responsiveness at an early age.28 Children at high risk (both parents atopic) for asthma has been reported to have a higher specific airway resistance (sRaw) than children at low risk.29 When we evaluated the subgroups based on the clinical risk factors, R5-R20% levels of children with parental asthma were significantly higher than those without. In this regard, parental asthma seems to be a major risk factor for early impairment of lung function.
Atopy is a risk factor for persistent wheeze.30 Even in the absence of respiratory symptoms, children of atopic parents and those with personal atopy have impaired lung function in early life.29 Lowe et al. showed that in young children a combination of sensitization and exposure to a sensitizing allergen resulted in significant deficits in lung function measured as specific airway resistance in plethysmography.31 It has been demonstrated that early allergic inflammation may lead to remodeling which then impairs airway growth with long-term increase in airway resistance.32 In this regard, atopic children have been shown to have significantly higher sRaw levels measured in plethysmography than those who were not atopic.29 Song et al. revealed that more significant findings of baseline and bronchodilator response of IOS were present in atopic subjects aged three to six years than in non-atopic ones. They reported that IOS was a useful diagnostic tool in early detection of asthma development.33 In the present study, R20 predicted levels which reflect the proximal airway resistance and resonant frequency (Fres) values reflecting the inertial properties of airway and capacitance of lung periphery were significantly higher in children with inhalant allergen sensitization than those without. These results suggested that atopic sensitization and parental asthma may affect the lung tissue and therefore the lung function mechanics in different ways in API positive children.
In the present study, R5-R20% and total IgE values were found to be significantly related to the risk of positive mAPI. In addition, the levels of R5-R20% pred>14.4% were found to have a sensitivity of 75% and specificity of 53% for detecting mAPI positive children.
The limitation of our study was its small sample size. The strength of this study was the exclusion of infants with low weight for gestational age and born prematurely because both of these factors impact the lung development and lung mechanics in a different way. To the best of our knowledge, no study has reported the association between API characteristics and R5-R20 in IOS in recurrent wheezers and also suggested the contribution of small airway dysfunction in children with high risk for asthma.
In conclusion, our study provides additional insights about the utility of IOS in recurrent wheezers in relation to mAPI characteristics. We suggest that IOS, especially R5-R20 values, can be used to identify those pre-school children with a high risk of asthma and those whose allergen sensitivities cannot be determined.
Conflicts of interestAll authors declare that there are no conflicts of interest that may be inherent in this submission.
We gratefully acknowledge Mustafa Göçeri for his help with the lung function tests.