Phosphodiesterase-5 inhibitors (PDE5i) are the first choice for treating erectile dysfunction (ED) but are not always effective. The aim of this study was to present our experience in treating patients with ED, refractory to treatment with PDE5i, using intraurethral alprostadil (MUSE).
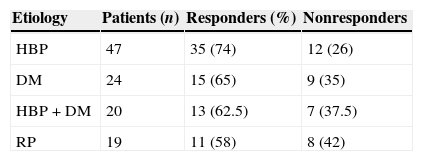
Material and methodsWe conducted a review of 82 patients with ED and no response to PDE5i, from March 2013 to October 2014. Forty-seven patients (57%) had hypertension (AHT), 24 (29%) had diabetes (DM) and 20 (24%) had AHT and DM. Additionally, 19 (23%) had undergone radical prostatic (RP) surgery. The patients were evaluated after the treatment was applied and at 4 weeks using the following validated questionnaires: International Index of Erectile Function (IIEF-5/SHIM), Global Assessment Questionnaire (GAQ), Sexual Encounter Profile (SEP) and Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS).
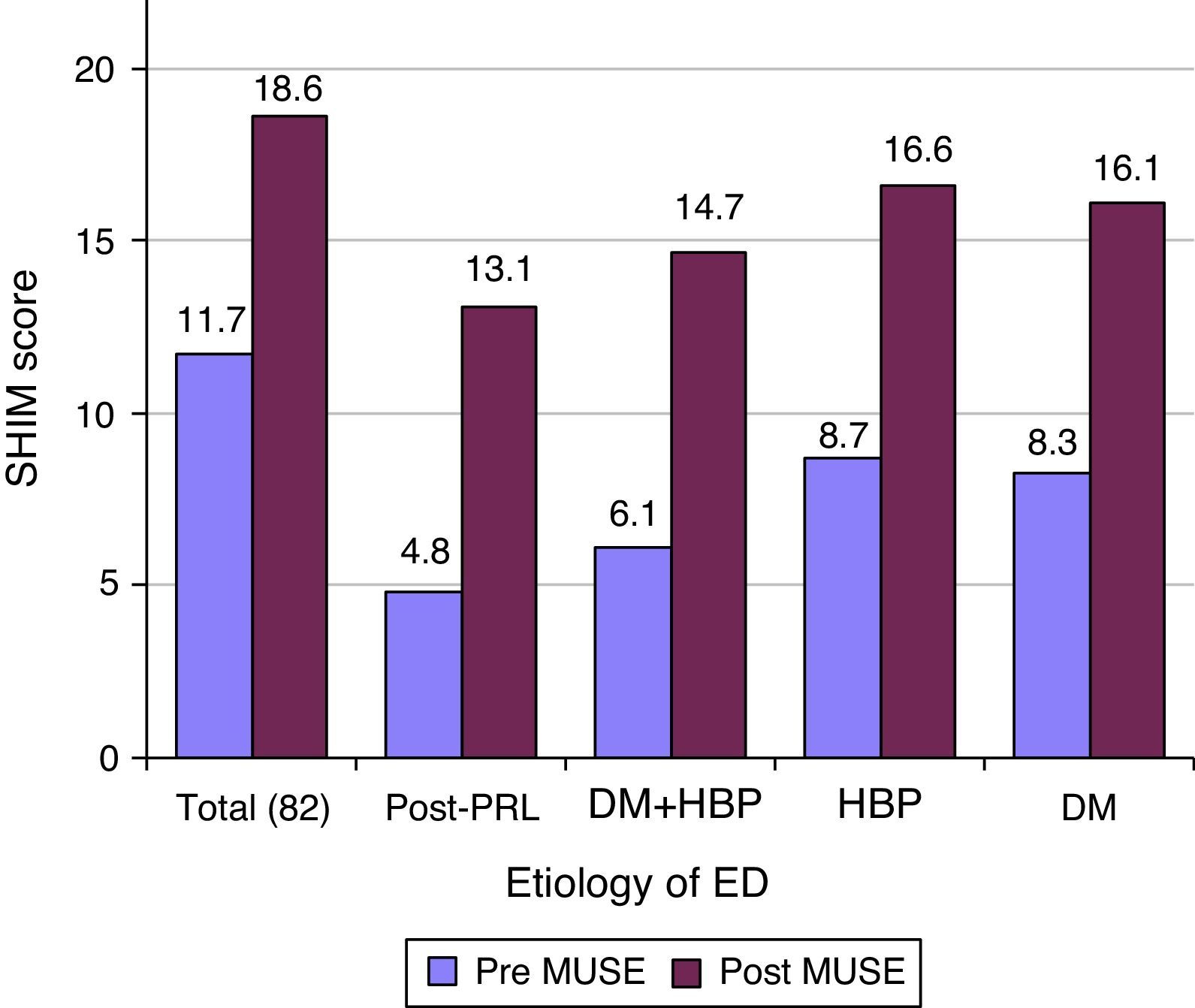
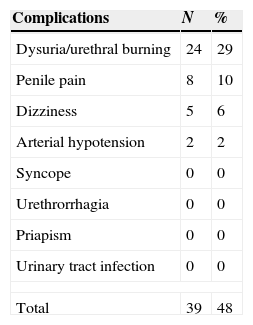
ResultsThe mean patient age was 60.5 years (40–80), and the mean follow-up was 11.3 months (1–20). Sixty-eight percent of the treated patients responded to MUSE® (74% in the AHT group, 65% in the AHT+DM group, 62.5% in the DM group and 58% in the RP group). The mean IIEF-5 score was 11.7±4.7, which increased to 18.6±4.9 after MUSE was administered (P=.027). The mean EDITS score at 4 weeks was 61.6 (6–81.9). The most common adverse effect was urethral burning, which occurred in 24 patients (29%). There were no cases of urinary tract infection, syncope or priapism.
ConclusionsIntraurethral alprostadil is an effective treatment and has a broad safety profile for treating patients with erectile dysfunction refractory to oral treatment with PDE5i.
Los inhibidores de la 5-fosfodiesterasa (IPDE5) son de primera elección para el tratamiento de la disfunción eréctil (DE), pero no siempre son efectivos. El objetivo es presentar nuestra experiencia en el tratamiento de pacientes con DE, refractaria al tratamiento con IPDE5, mediante alprostadil intrauretral.
Material y métodosRevisión de 82 pacientes con DE, sin respuesta a IPDE5, desde marzo de 2013 hasta octubre de 2014. De ellos, 47 (57%) presentaban hipertensión (HTA), 24 (29%) diabetes (DM), y 20 (24%) HTA y DM. Además, 19 (23%) habían sido tratados mediante cirugía radical prostática (PR). Fueron evaluados en la consulta tras la aplicación del tratamiento y a las 4 semanas mediante los cuestionarios validados: International Index of Erectile Function (IIEF-5/SHIM), Global Assessment Questionnaire (GAQ), Sexual Encounter Profile (SEP) y Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS).
ResultadosLa edad media fue de 60,5 años (40-80), con seguimiento medio de 11,3 meses (1-20). El 68% de los pacientes tratados respondieron a MUSE® (74% en el grupo de HTA, 65% en el de HTA+DM, 62,5% en el de DM y 58% en el de PR). La media del IIEF-5 era de 11,7±4,7, y ascendió hasta 18,6±4,9 tras MUSE® (p=0,027). La media de la puntuación del EDITS a las 4 semanas fue de 61,6 (6-81,9). El efecto adverso más frecuente fue el escozor uretral, que ocurrió en 24 pacientes (29%). No se observó ningún caso de infección del tracto urinario, síncope ni priapismo.
ConclusionesEl alprostadil intrauretral es un tratamiento efectivo y con un amplio perfil de seguridad para tratar a aquellos pacientes con disfunción eréctil refractaria al tratamiento oral con IPDE5.