The choice of the most appropriate treatment in early-stage glottic cancer with anterior commissure involvement remains controversial. Its therapeutic management is complex because it is a significant prognostic indicator of local control with 37% recurrence, due to the difficulty in establishing tumour extension with understaging of up to 40%, and due to the comparison of results in series on tumours that behave variably as they progress, such as T1a, T1b and T2a with commissure involvement. Furthermore, the complexity of the surgical approach using transoral CO2 laser microsurgery requires surgical skill, appropriate equipment and experience. Aspects to be reviewed in this document are: an updated anatomical definition of the anterior commissure, tumour progression based on histopathological studies, usefulness of videostroboscopy and NBI in diagnostic accuracy, validity of imaging tests, oncological results published in series reviews, systematic reviews and meta-analyses, tumour margin treatment and voice evaluation. Finally, by way of a summary, the document includes a series of recommendations for the treatment of these tumours.
La elección del tratamiento más adecuado en el cáncer glótico en estadio precoz con afectación de comisura anterior sigue siendo controvertida. La complejidad en su manejo terapéutico está justificada por ser un significativo indicador pronóstico de control local con un porcentaje de recidiva del 37%, por la dificultad en establecer la extensión tumoral con un infraestadiaje que llega a alcanzar el 40%, y por la comparación de resultados en series formadas por tumores de diferente comportamiento evolutivo como son T1a, T1b y T2a con afectación comisural. A estos datos se suma la complejidad del abordaje quirúrgico mediante microcirugía transoral con laser CO2 que requiere habilidad quirúrgica, equipamiento adecuado y experiencia.
Los aspectos a revisar en este documento son: definición anatómica actualizada de la comisura anterior, progresión tumoral en función de estudios histopatológicos, utilidad de la videoestroboscopia y la NBI en la precisión diagnóstica, validez de las pruebas de imagen, resultados oncológicos publicados en revisión de series, revisiones sistemáticas y metaanálisis, tratamiento de los márgenes y evaluación de la voz.
Finalmente y a modo de resumen el documento incluye una serie de recomendaciones para el tratamiento de estos tumores.
The choice of the most appropriate treatment in early-stage glottic cancer with anterior commissure involvement remains controversial. Its therapeutic management is complex because it is a significant prognostic indicator of local control, has 37% recurrence, tumour extension is difficult to establish, it is understaged in up to 40%, and due to outcome comparisons in series comprising tumours that behave variably as they progress, such as T1a, T1b and T2a with commissure involvement.1,2 In addition to these data, the complexity of the surgical approach using transoral CO2 laser microsurgery requires surgical skill, adequate equipment and experience.3
The aspects reviewed in this document are: an updated anatomical definition of the anterior commissure, tumour progression based on histopathological studies, the usefulness of videostroboscopy and narrow band imaging (NBI) in diagnostic accuracy, the validity of imaging tests, oncological results published in series reviews, systematic reviews and meta-analyses, treatment of margins and voice assessment.
1.1Anatomy of the Anterior CommissureIn 1975, Olofsson defined the anterior commissure as the ventral region between the two vocal cords, bounded at the top by the anterior angle of the ventricular junction, with a lower and lateral extension of 2−3 mm, including the macula flava or elastic condensation of the vocal ligament.4
Later, in 1981, Andrea and Guerrier published a study using serial sections of human larynges describing "plane 0", which consists of a small area above the vocal cords, without glands or vessels which separates the anterior commissure from the thyroepiglottic ligament. However, at the subglottic level they observed that the glands and vessels were in continuity, making it difficult to identify them independently. Therefore, according to the authors, the anterior part of the larynx was defined by 2 regions, a supraglottic or vestibulo-epiglottic region and a glottic-subglottic region, to which the anterior commissure belonged.5
Prades et al., in 2017, carried out a comparative study of laryngeal specimens from foetuses and adults, expanding on the work by Rucci and Gammarota6 on the embryology of the anterior commissure, which help to understand patterns of tumour spread in this anatomical area.7
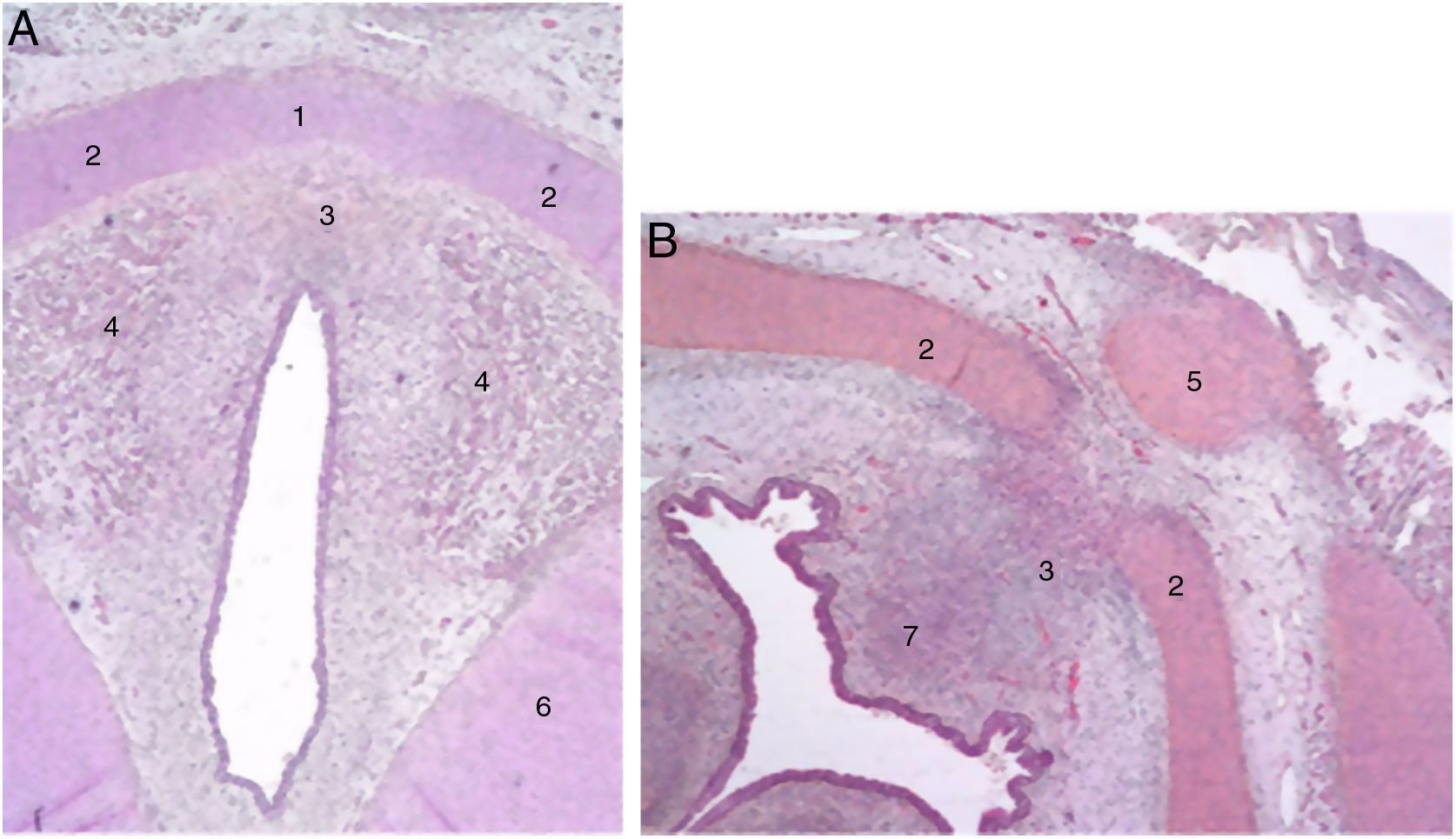
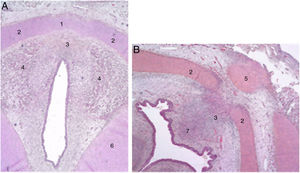
The authors describe a mesenchymal band in human embryos between the two lateral laminae of the thyroid cartilage and an accumulation of connective tissue in the median zone located dorsally (Fig. 1).
Cross sections from an 11-week foetus (haematoxylin-eosin) near the glottic plane (A) and the hyoid bone (B). 1. Intermediate lamina of thyroid cartilage; 2. lateral lamina of the thyroid cartilage; 3. process in middle area; 4. muscle fibres of the glottic plane; 5. hyoid bone; 6. cricoid cartilage; 7. future pre-epiglottic space (images reproduced by permission of the author).
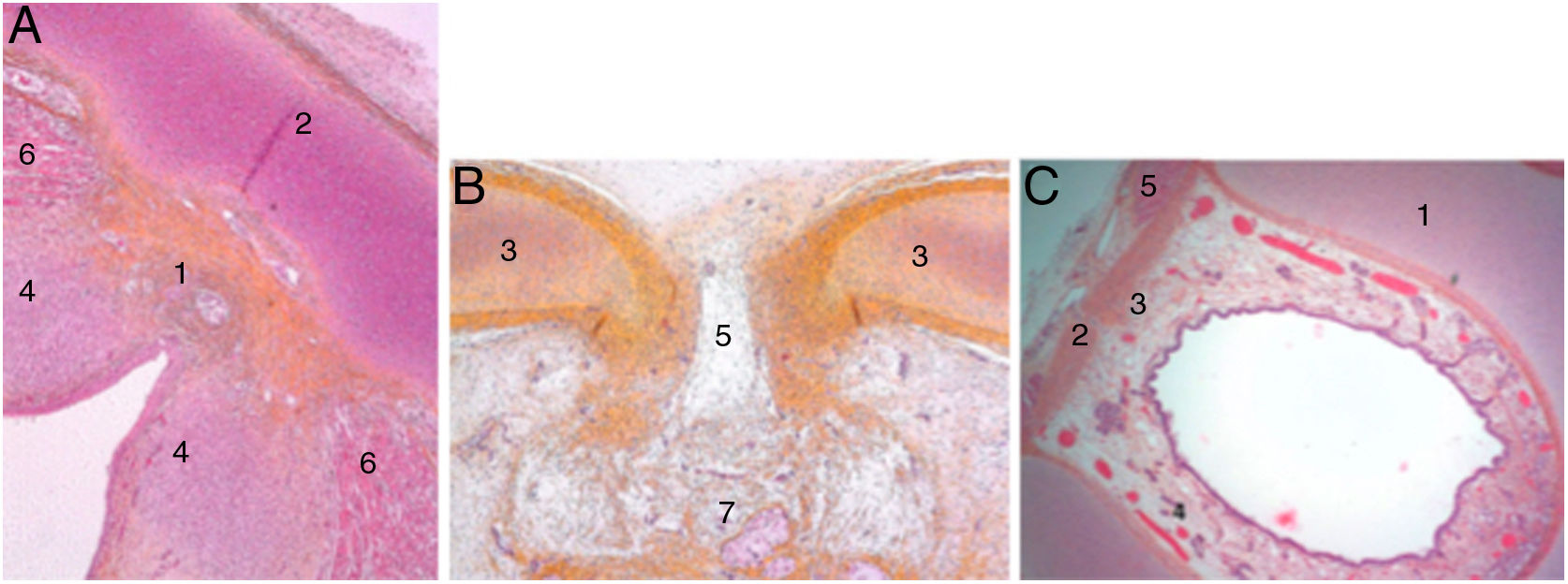
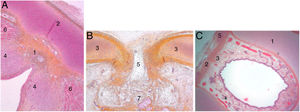
At the end of the embryological period, within this connective tissue, some fibres parallel to the intermediate lamina will interweave and form Broyle’s ligament cranially and the conoid ligament caudally, and others in a perpendicular direction corresponding to the insertion of the vocal folds (Fig. 2). This area of fibres devoid of vessels forms a kind of chiasm called Bagatella and Bignardi's x-space8 or Guerrier’s plane 0.
Cross sections of a 25-week foetus (haematoxylin-eosin) near the glottic plane (A), at the upper edge of the thyroid cartilage (B) and near the upper edge of the cricoid cartilage (C). A and B. 1. Mesh of collagen fibre bundles in the anterior commissure; 2. Intermediate lamina of thyroid cartilage; 3. Lateral lamina of thyroid cartilage; 4. Macula flava; 5. Thyroid cartilage notch; 6. Glottic muscle fibres; 7. Future pre-epiglottic space. C. 1. Cricoid cartilage; 2. Cricothyroid membrane; 3. Mesh of vertical collagen fibres; 4. Circular collagen fibres; 5. Cricothyroid muscle (images reproduced by permission of the author).
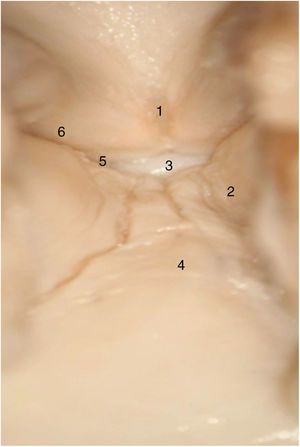
In adult larynges, endoscopically, the anterior commissure apparently resembles a transition area between the supraglottis and the subglottis. In the middle of the commissure, and located ventrally, there is a horizontal space above the glottic plane 3.9 mm wide by 2.3 mm high in continuity with the ventricles and separated from the thyroid cartilage only by very thin fibrous tissue and mucosa. The upper edge of this space is in the middle of the lower Broyle’s tendon insertion, which can impede vision of this area (Fig. 3).
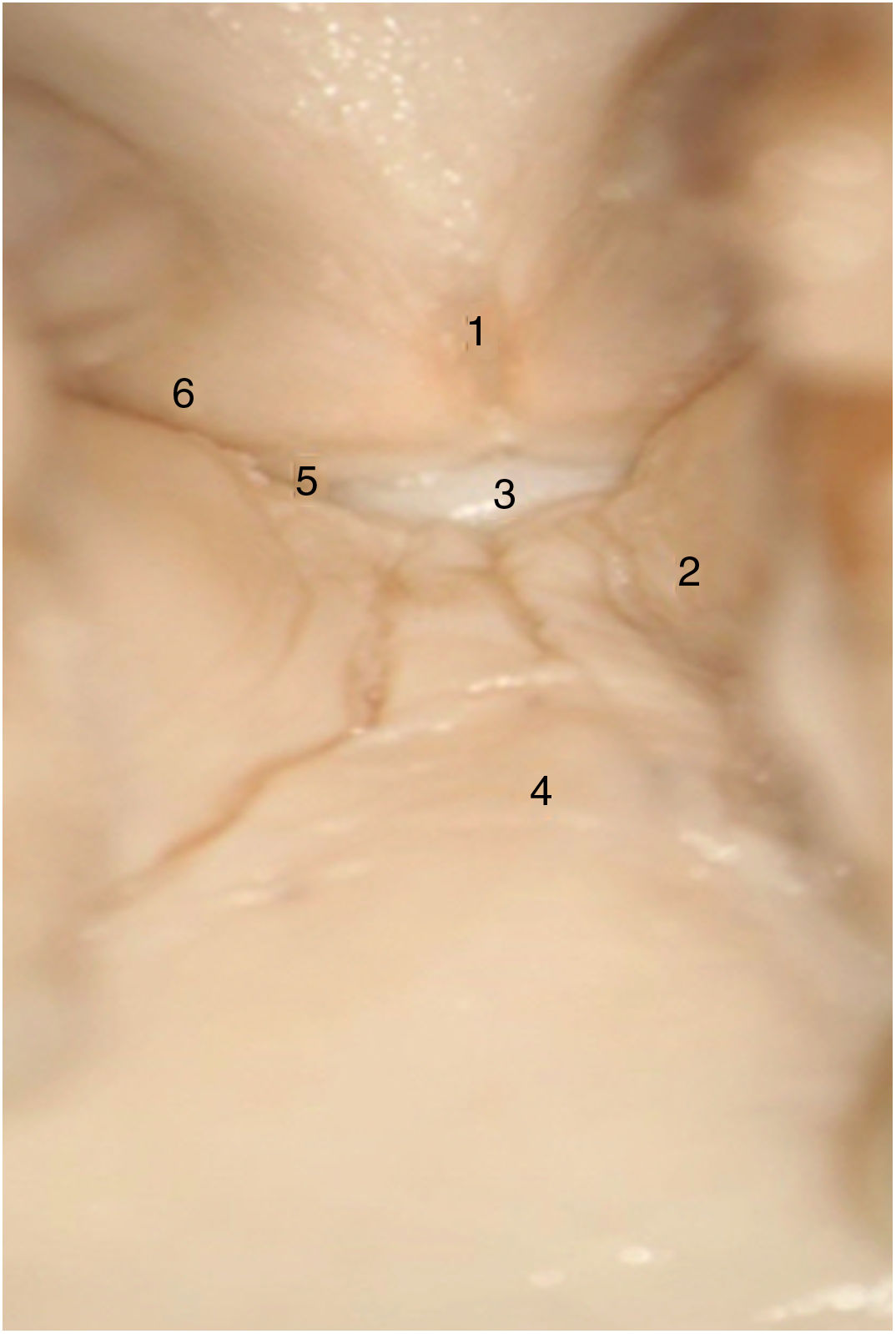
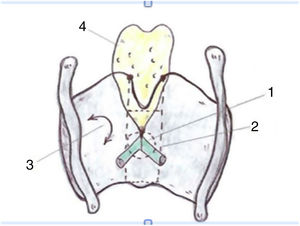
Taking the organogenesis of the larynx into account and the findings in the adult, Prades et al. conclude that the "developed" anterior commissure would be defined ventrally by the intermediate lamina of the thyroid cartilage immediately below the thyroid notch, and dorsally by the insertion of the vocal folds, Broyle’s ligament and the conoid ligament. The vertical limit would be defined by the height of the thyroid cartilage and the width of the notch would define the lateral edge. The posterior limit would be defined in the glottis by a parasagittal plane at the level of the macula flava, in the supraglottis by the lower edge of the epiglottis, and in the subglottis by the lower edge of the thyroid cartilage (Fig. 4).
Proposed definition of the anterior commissure (AC) of the larynx (posterior view). 1. “Classical” AC: insertion of the glottic level excluding thyroid cartilage; 2. “Developmental” AC: 3 levels of insertion (supra, glottic and subglottic) including intermediate thyroid lamina. 3. Lateral thyroid lamina; 4. Epiglottic cartilage (images reproduced by permission of the author).
In the patterns of carcinoma spread involving the anterior commissure the role of the vocal ligament, Broyle’s ligament and the conoid ligament has been widely debated. It could be considered that the poor vascularisation and absence of glandular tissue in this area would favour the tumour remaining confined within a fibrous structure. However, in reality these tumours invade the space surrounding the glottic plane, which is rich in blood vessels and glands, and can extend inferiorly to the conoid ligament and cricothyroid membrane, and superiorly to the laryngeal ventricle and pre-epiglottic space.6
This fact, together with the distance of a few millimetres between the mucosa of the anterior commissure and the thyroid cartilage, makes it possible for small tumours to reach and invade the cartilage, transforming rapidly from T1 to T3-T4. There are a number of histopathological findings that give us a deeper understanding of how this invasion takes place.
Broyle’s ligament acts as an effective barrier to tumour extension, since although the dorsal area of the ligament may be affected in some tumours, no invasion of the intermediate lamina of the thyroid cartilage is observed following a dorsal-ventral direction. Moreover, when anterior involvement of the thyroid cartilage is observed, the tumour surrounds the ring-shaped ligament. In this case spread of the tumour to the subglottic plane is more frequent than to the supraglottic plane.
Tumour spread can also occur from the vocal fold. In the glottis, the location of the collagen fibres that cross the perichondrium at the insertion points of the ligament and vocal muscle give rise to natural disruption of the perichondrium, these areas being especially vulnerable to tumour invasion. As the tumour grows it separates the collagen fibres from the vocal ligament, directly reaching the cartilage. This gives rise to focal ossification when the cartilage comes into contact with neoplastic cells. This ossification, which leads to an increase in vascularisation, favours tumour spread. Infiltration of the vocal muscle, rich in blood vessels and glands, also facilitates the involvement of thyroid cartilage without crossing the ligament. This invasion is further intensified if the involvement of the vocal muscle is bilateral.9
1.2Anatomical Distribution and Morphology of Glottic LesionsSeveral studies have been carried out in an attempt to establish the relationship of a certain factor with the development of glottic tumour in a specific anatomical area. The possibility that in patients who smoke there is a specific site in vocal fold cancers as opposed to non-smokers has been studied by some researchers.
Shoffel et al. establish in their study that the transition area between the medial and superior vocal fold aspects is most frequently affected by dysplasia and carcinoma in situ, with no clear relationship to smoking. The extension of the lesions towards the superior and anterior aspect seems to be more frequent in patients who smoke, although no significant differences could be established.10
With regard to the different morphology taken on by glottic cancers, tumours of the superior aspect are flat, irregular, with higher rates of anterior commissure involvement recurrence. In contrast, tumours of the medial aspect are more voluminous, polypoid in form and with a better defined border between tumour and healthy tissue.11 On the other hand, pushing and infiltrating carcinomas have a differentiated pattern of spread with multiple tumour nests growing surrounded by fibrosis, and tumours of this particular area are often understaged.12
Epidermal growth factor receptor expression and p53 expression in relation to tumours of the anterior and posterior commissure have also been studied. The findings indicated that epidermal growth factor receptor expression was higher in the posterior commissure than in the anterior commissure, a fact that could contribute to the worse results obtained with radiotherapy treatment in this type of tumour. No significant differences in p53 expression were observed.13
1.3Videostroboscopy and Narrow-band ImagingVideostroboscopy is considered a useful tool in the diagnostic evaluation of glottic lesions with involvement of the mucosa. Impaired or absence of mucosal wave is observed in the presence of carcinomas, scars or inflammatory processes
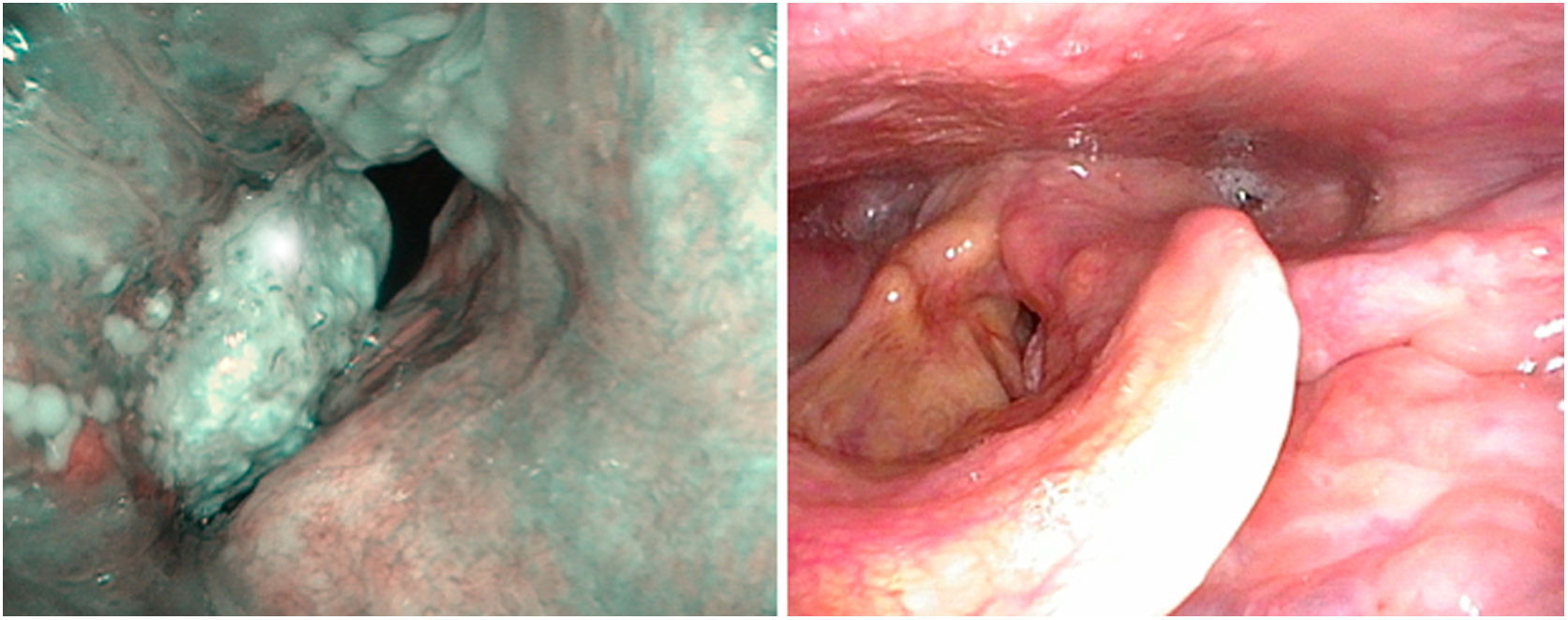
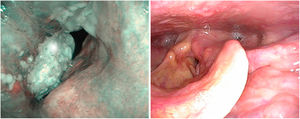
There are no specific papers on the value of videostroscopy in the diagnosis of tumours of the anterior commissure. This is probably because the difficulty in diagnosing these tumours is not so much associated with differentiating the fold’s impaired mobility as with establishing ventral infiltration in a craniocaudal direction. However, it should be noted that in the diagnosis of early glottic cancer videostroboscopy shows high sensitivity but variable and generally low specificity, resulting in an overestimation of non-invasive lesions.14 NBI systems are currently used to improve the diagnostic accuracy of neoplastic and non-neoplastic lesions. To distinguish the nature of findings, the patterns of vascular changes observed in the laryngeal mucosa and their relationship to histopathological changes established by Ni in 201115 are applied. These patterns have been completed with the descriptive guideline on vascular changes in the vocal cord, published by the European Society of Laryngology in 2018, establishing that changes in the perpendicular direction of vascular loops are associated with glottic neoplasia.16 Their usefulness in clinical diagnosis has been highlighted by several authors in retrospective series.17–19 There are no specific studies on tumours involving the anterior commissure, although there is a systematic review and meta-analysis that studies the cut-off value between premalignant and malignant lesions and the iv and v patterns of Ni’s classification. Both patterns predict the presence of neoplasia with differences in specificity, which is higher in pattern v and higher sensitivity in pattern iv. Thus, pattern iv sometimes corresponds to mild or moderate dysplasia20 (Fig. 5).
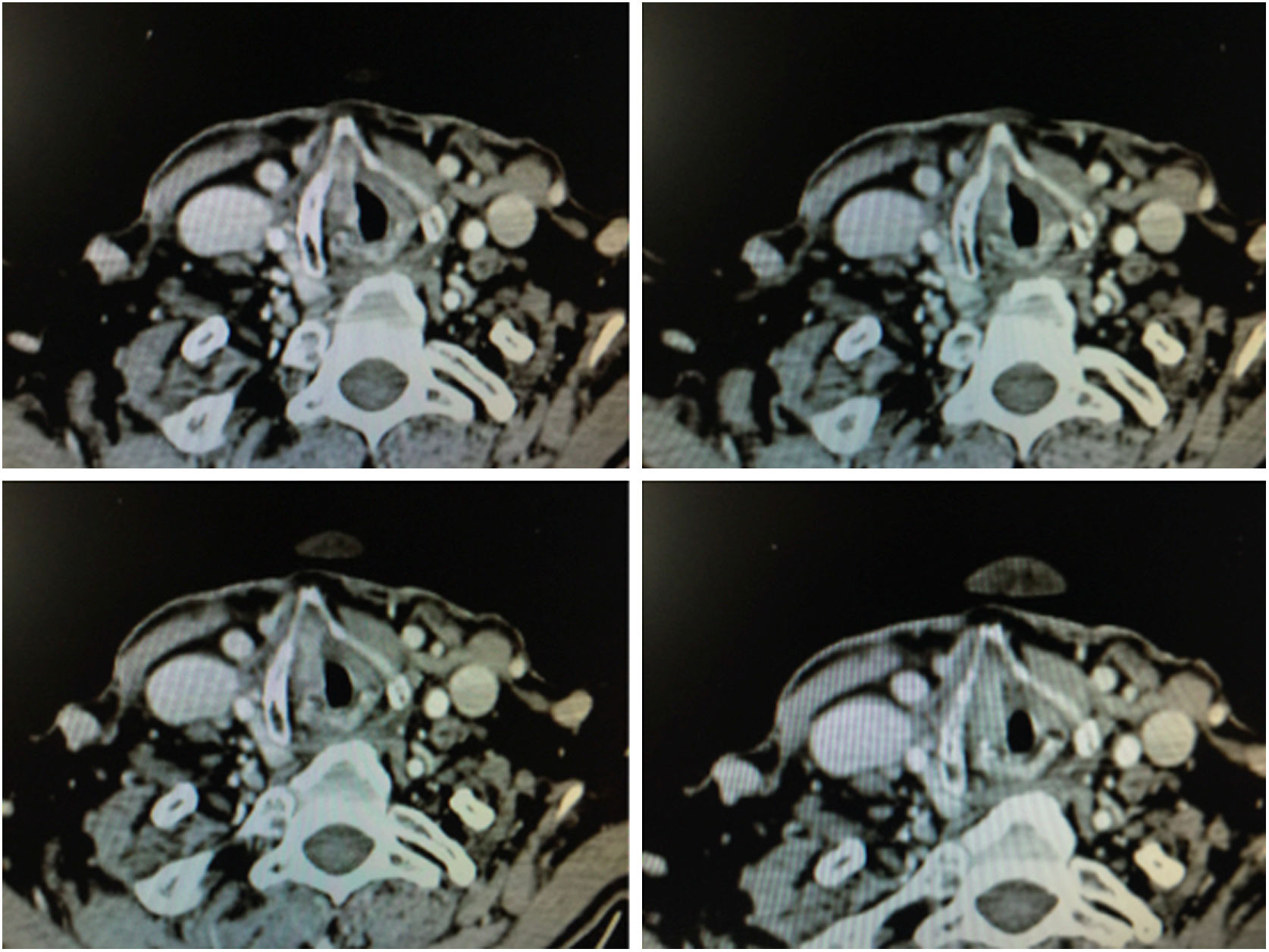
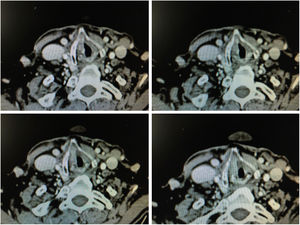
1.4Imaging TestsImages obtained by means of 1 mm thick helicoidal CT sections improve diagnostic accuracy with respect to endoscopic imaging in early stage tumours with involvement of the anterior commissure.21
The radiographic criteria for considering the presence of anterior commissure involvement are: thickening of the anterior commissure greater than 1 mm on at least 2 consecutive CT sections in a horizontal plane, or in a vertical plane (sagittal reconstruction) the presence of commissure thickening advancing towards the pre-epiglottic space superiorly, towards the thyroid cartilage anteriorly or towards the cricoid cartilage inferiorly (Fig. 6).
MRI is more sensitive than CT in studying anterior commissure involvement, but less specific. The presence of peritumoural inflammation can confuse the limits of involved tissue, and as a result the neoplasm can be overstaged.22 On the other hand, although thyroid cartilage involvement in the early stages is less than 9%, cartilage involvement must be discerned within the process of diagnosis.
CT sensitivity is 91% and specificity 68% in the presence of sclerosis, erosion or lysis of thyroid cartilage, all indirect signs of its involvement. If the presence of sclerosis is not considered, specificity is 92%, although sensitivity reduces to 61%. In the case of MRI, sensitivity is 86%–94% and specificity between 74% and 88%.23
Hartl et al. attempt to establish the characteristics of anterior commissure tumours that help predict thyroid cartilage invasion. Among those studied are supraglottic and subglottic extension, bicordal lesions or altered fold mobility. Only in the presence of paresis or paralysis of the vocal fold is an increased risk of cartilage invasion observed.24
1.5Classification of Early Anterior Commissure TumoursThe AJCC classification of malignant tumours of the glottis establishes 3 differentiated anatomical areas: vocal folds, anterior commissure and posterior commissure. Glottic T1 is defined as a cancer limited to the normally mobile vocal fold and can involve the anterior or posterior anterior commissure, T1a for a tumour limited to one vocal fold and T1b when involving both folds. T2 is defined as a tumour involving the glottis and extending to the supraglottis and/or subglottis and/or vocal fold mobility involvement. In the case of impaired mobility it is staged as T2b.25 Therefore, tumours of the anterior commissure do not have a specific classification.
Different articles have been published that established subclassifications with the aim of establishing homogenous groups that allow better prediction of their outcome and response to different treatments. Rucci et al., in 1996, made a proposal for staging based on subsites with better prognostic prediction; tumour confined exclusively to the anterior commissure (AC0), tumour involving one fold and the anterior commissure (AC1), tumour involving the anterior commissure and part of both vocal folds (AC2) and tumour involving most of the vocal fold, the anterior commissure and crossing involving a variable part of the contralateral fold (AC3).26 Peretti et al. also classify T2 glottic tumours into 4 types according to their site and depth. T2 (iii) is defined by supracommissural or subcomissural anterior extension.27 However, these proposals are not widely used.
1.6TreatmentThe treatment options for early stage glottic tumour (T1-T2) with anterior commissure involvement are radiotherapy, transoral CO2 laser microsurgery and partial laryngectomy by external approach.28 Radiotherapy has better functional voice outcomes than transoral CO2 laser microsurgery, although the percentage of patients with final larynx preservation is lower.
Of the surgical options, transoral CO2 laser microsurgery (CO2 TLMS / CO2 TOLMS)29 is not without difficulty in laryngeal exposure and requires experience and skill on the part of the surgeon. Open partial laryngectomy is a therapeutic option with excellent oncological results, although the impact on swallowing and phonation is greater than in CO2 TLMS. Finally, total laryngectomy improves local control but significantly reduces quality of life.
Radiotherapy and Anterior CommissureThe influence of the anterior commissure on oncological outcomes after treatment with radiotherapy remains controversial. Several publications suggest that its involvement is a predictor of lower response compared with glottic tumours without anterior commissure involvement.30–32
Local control at 5 years in patients with early stage tumours extending to the anterior commissure and treated with radiotherapy is between 55% and 80%, lower than that achieved in tumours without anterior commissure involvement, which ranges from 82% to 90%.33 Along the same lines, in the latest published systematic reviews and meta-analyses on risk factors for tumour persistence or recurrence in glottic cancer treated with radiotherapy, anterior commissure involvement is identified as an unfavourable factor (RR: .904).34 However, in other studies the anterior commissure is not identified as a parameter of worse local control.35 Thus, in a multivariate analysis of predictors of long-term cancer outcomes in T1-T2N0 glottic cancers treated with radiotherapy, where the involvement of the anterior commissure, maintenance of smoking during and after treatment and T2 tumours are the only variables in relation to worse local control.36
The causes of the discrepancy between these results do not seem clear. Some authors indicate that doses per session or hyperfractionation could influence local control.37 This has led to proposed doses of 2.25 Gy with hyperfractionation in T1 glottic cancer and radiotherapy and chemotherapy protocols for T2 glottic cancers with anterior commissure involvement to improve local control.33,38 With regard to the latter treatment regimen, there is no clear evidence of improved oncological outcomes with respect to radiotherapy in monotherapy, although this is a current line of research.
Open Surgery and Anterior CommissureAlthough there have been fewer publications in recent years on oncological outcomes of open partial laryngectomy as a therapeutic option in glottic tumours with anterior commissure involvement, horizontal supracricoid partial laryngectomy is still considered an excellent treatment option, especially in cases where there is limited laryngeal exposure and subglottic extension of less than 5 mm. It is not only recommended by different authors in these cases, but also in T2b glottic tumours with extension to the anterior commissure. Arguments are raised that these are tumours with worse local control, worse survival outcomes and a greater number of adverse findings (infiltration of the perichondrium, invasion of cartilage, perineural infiltration and prelaryngeal metastases) in relation to T2a tumours. Their outcomes are comparable to T3 tumours.39 This latter argument is shared by Blanch et al., although they see no contraindication in indicating transoral CO2 laser microsurgery provided it is performed by an expert surgeon.40 Vertical partial laryngectomy has fallen into disuse in favour of horizontal supracricoid laryngectomy mainly due to better oncological control and better functional outcomes.
Published rates of overall survival, disease-specific survival and relapse-free survival at 5 years in T1-T2 tumours with anterior commissure involvement, treated with supracricoid partial laryngectomy with cricohyoid-epiglottopexy are 93.7%, 95.6% and 87.7% respectively.41 The last series published by Pescetto et al., in 2018, that includes T1, T2 and T3 with anterior commissure involvement, achieves overall, cause-specific and recurrence-free survival outcomes of 86%, 95% and 75% respectively at 5 years.42
Bearing in mind that the oncological results obtained are generally excellent, the impact on swallowing and phonation is the main limitation with this therapeutic option. Evaluation of dysphagia using Pearson’s scale and the Dysphagia Outcomes and Severity Scale in the studies by Schindler et al. and Pescetto et al. are similar, with 96% of patients managing oral feeding and 19% with intermittent liquid false passage.42,43 Age over 70 and arytenoid resection are considered risk factors that predict swallowing difficulty.44
Transoral CO2 Laser Microsurgery and Anterior CommissureLaryngeal Exposure. Appropriate exposure of the anterior commissure is decisive in performing adequate resection with free margins if the therapeutic option chosen is transoral CO2 laser microsurgery. The incidence of laryngeal exposure difficulty can range from 1% to 24%.45
The clinical parameters that can predict difficult laryngeal exposure independently, studied in a multivariate analysis by Pinar et al. are a neck circumference greater than 40 cm, a hyoid-mental distance in full extension less than 6.05 cm and sternum-mental distance in full extension less than 13.9 cm. Body mass index was an influential but not independent factor. 45
With the aim of determining difficulties prior to surgery, Piazza et al. defined in 2014 a series of parameters to be assessed on clinical examination of the patient to help predict laryngeal exposure difficulty. These are interincisors gap, thyro-mental distance, trismus, mandibular prognathism, macroglossia or micrognathia, degree of neck flexion-extension, history of previous surgery, the Mallampati modified score and body mass index.46
With regard to the anterior commissure, 4 classes of laryngeal exposure are established in relation to the laryngoscope bore position and external pressure. Class 0 where the anterior commissure is visualised with a large-bore laryngoscope and Boyce-Jackson position with no external pressure, class i the aforementioned requirements but in addition external pressure is required, class ii where the flexion-flexion position (chin-chest) has to be modified, class iii requiring a change to a small-bore laryngoscope, flexion-flexion position and external pressure corresponding to a score of 6 on the clinical assessment scale and class iv, where it is not possible to visualise the commissure and corresponding to a score of 9. The different degrees of difficulty in exposing the anterior commissure correlate with the involvement of margins in the tumour resection.47
Although this is not a generally used evaluation scale, there is consensus that head flexion and external laryngeal pressure are manoeuvres that improve visualisation of the tumour and help to direct the laser beam in a coaxial axis with respect to the anterior commisure.40,48
Learning Curve. Experience in transoral CO2 laser microsurgery is considered an important factor when dealing with this type of tumour. Bernal-Sprekelsen et al. relate the surgeon’s experience levels with the frequency of complications, involved margins, recurrence rate, type of rescue surgery and disease-specific survival, both in early and advanced stage tumours. The authors believe that a type v cordectomy requires an expert surgeon who has performed at least sixty transoral CO2 laser microsurgery procedures.49
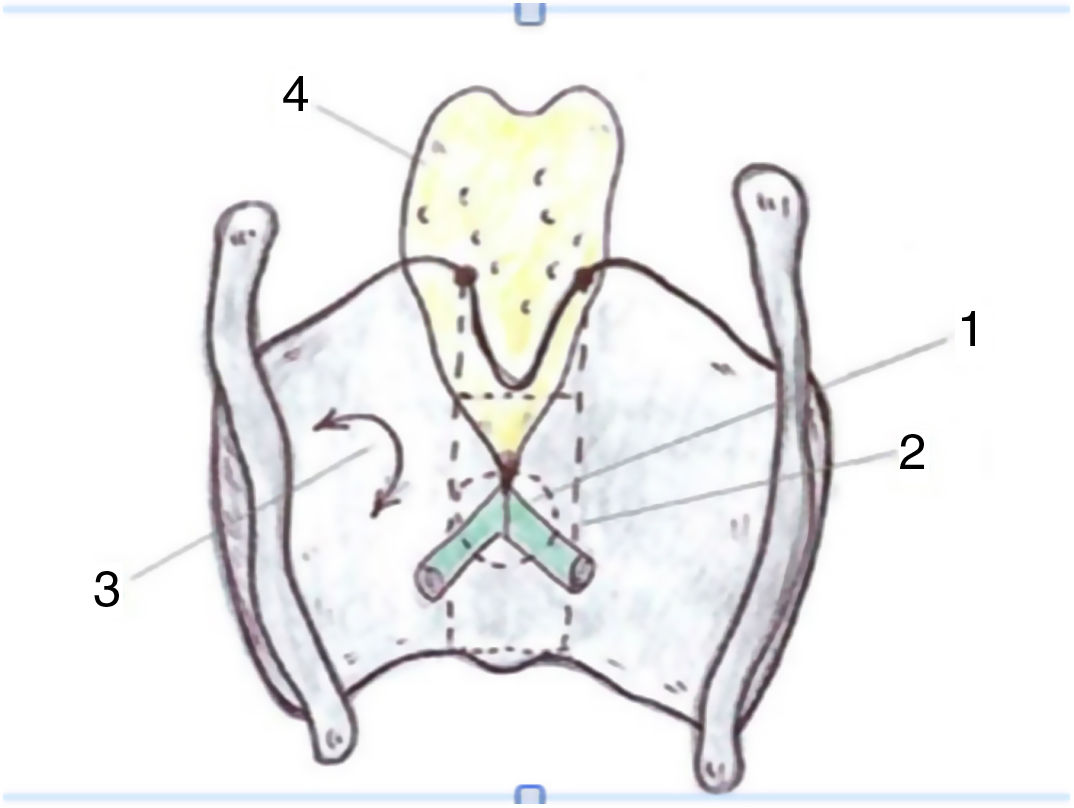
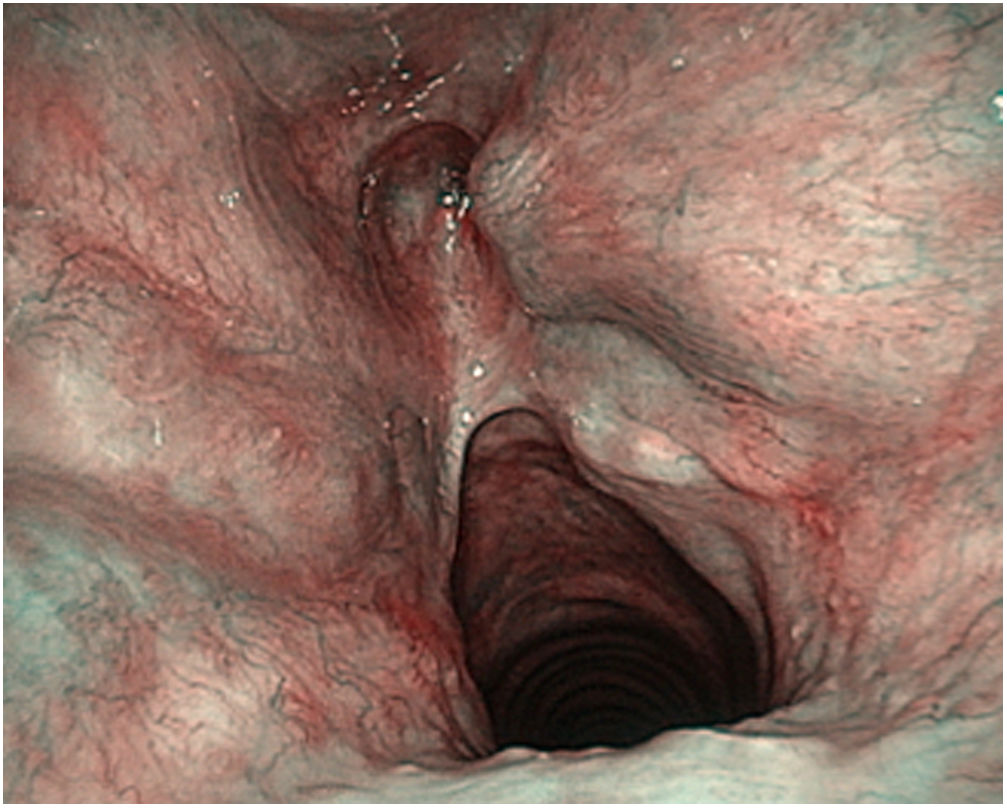
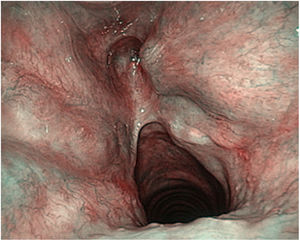
These authors highlight the importance of the learning curve in the treatment of T2 tumours with vertical cranio-caudal expansion. In these circumstances the local recurrence rate decreases the greater the surgeon’s experience, but especially if there is adequate resection of the anterior third of the false vocal cords, the epiglottic petiole and the lower pre-epiglottic fat in the form of a keyhole.40 (Fig. 7)
Classification of Cordectomies. The European Laryngological Society classifies cordectomies into 6 types, dividing type 5 into 4 subtypes. Type Va cordectomy is indicated in vocal fold neoplasia that superficially affect the anterior commissure. Resection encompasses the anterior commissure and the vocal fold partially or completely. Type VI cordectomy is a resection of the anterior commissure and anterior third of both vocal folds. It is accompanied by resection of the epiglottic peciole, the ventricular bands in their anterior third and the subglottic mucosa.50
The difference between type Va and type VI is the origin of the tumour. In type Va the tumour develops in the fold and involves the anterior commissure, following the horizontal plane, whereas in type vi the tumour originates in the anterior commissure and grows following a vertical plane.
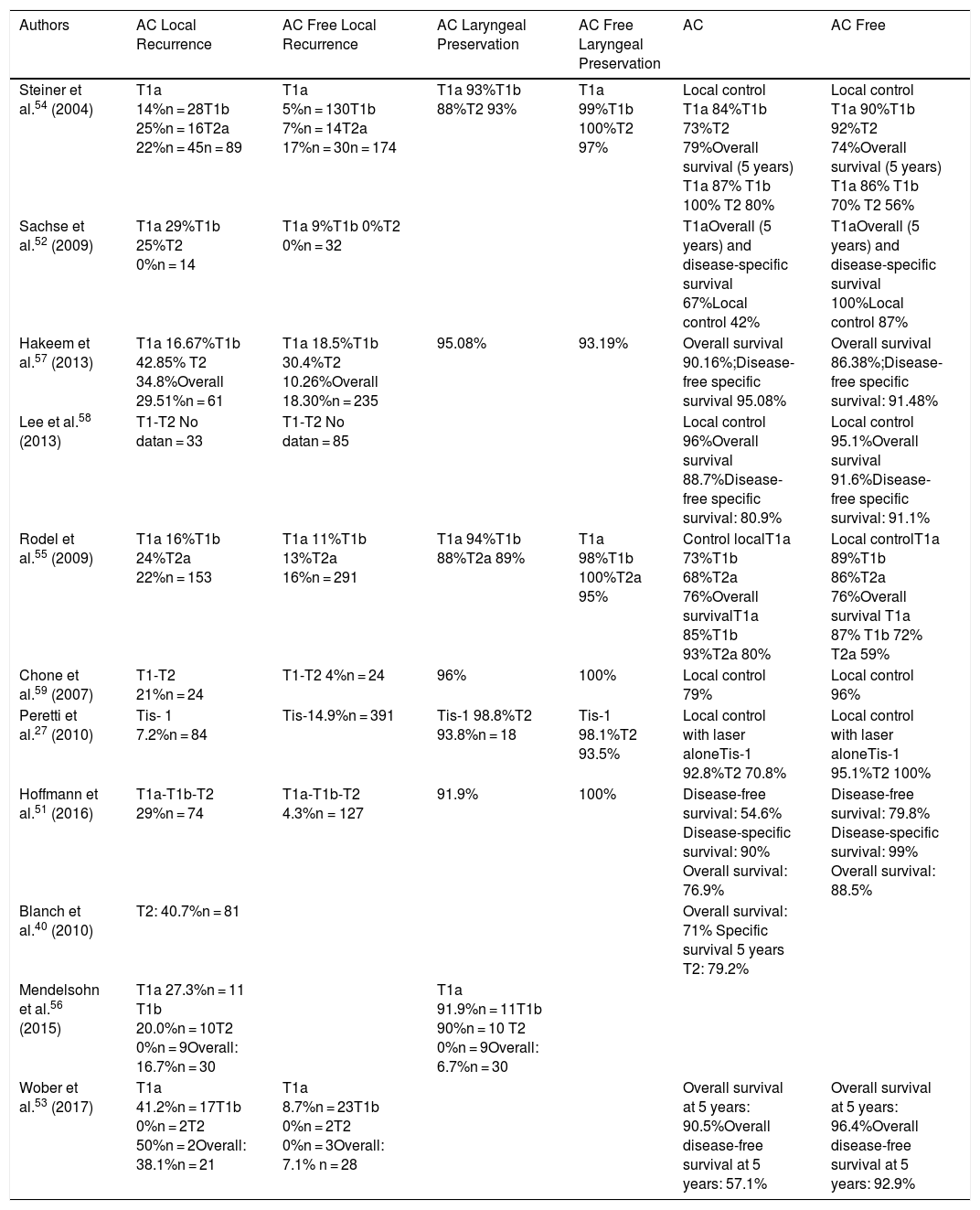
Oncological Results. The difference in the results in local control observed in the publications studied could be a consequence of differing surgeon experience, the non-homogeneous classification of the various subgroups and heterogeneous patient selection (Table 1).
Representative Series of Tumours With Anterior Commissure Involvement Treated With Transoral CO2 Laser Surgery.
| Authors | AC Local Recurrence | AC Free Local Recurrence | AC Laryngeal Preservation | AC Free Laryngeal Preservation | AC | AC Free |
|---|---|---|---|---|---|---|
| Steiner et al.54 (2004) | T1a 14%n = 28T1b 25%n = 16T2a 22%n = 45n = 89 | T1a 5%n = 130T1b 7%n = 14T2a 17%n = 30n = 174 | T1a 93%T1b 88%T2 93% | T1a 99%T1b 100%T2 97% | Local control T1a 84%T1b 73%T2 79%Overall survival (5 years) T1a 87% T1b 100% T2 80% | Local control T1a 90%T1b 92%T2 74%Overall survival (5 years) T1a 86% T1b 70% T2 56% |
| Sachse et al.52 (2009) | T1a 29%T1b 25%T2 0%n = 14 | T1a 9%T1b 0%T2 0%n = 32 | T1aOverall (5 years) and disease-specific survival 67%Local control 42% | T1aOverall (5 years) and disease-specific survival 100%Local control 87% | ||
| Hakeem et al.57 (2013) | T1a 16.67%T1b 42.85% T2 34.8%Overall 29.51%n = 61 | T1a 18.5%T1b 30.4%T2 10.26%Overall 18.30%n = 235 | 95.08% | 93.19% | Overall survival 90.16%;Disease-free specific survival 95.08% | Overall survival 86.38%;Disease-free specific survival: 91.48% |
| Lee et al.58 (2013) | T1-T2 No datan = 33 | T1-T2 No datan = 85 | Local control 96%Overall survival 88.7%Disease-free specific survival: 80.9% | Local control 95.1%Overall survival 91.6%Disease-free specific survival: 91.1% | ||
| Rodel et al.55 (2009) | T1a 16%T1b 24%T2a 22%n = 153 | T1a 11%T1b 13%T2a 16%n = 291 | T1a 94%T1b 88%T2a 89% | T1a 98%T1b 100%T2a 95% | Control localT1a 73%T1b 68%T2a 76%Overall survivalT1a 85%T1b 93%T2a 80% | Local controlT1a 89%T1b 86%T2a 76%Overall survival T1a 87% T1b 72% T2a 59% |
| Chone et al.59 (2007) | T1-T2 21%n = 24 | T1-T2 4%n = 24 | 96% | 100% | Local control 79% | Local control 96% |
| Peretti et al.27 (2010) | Tis- 1 7.2%n = 84 | Tis-14.9%n = 391 | Tis-1 98.8%T2 93.8%n = 18 | Tis-1 98.1%T2 93.5% | Local control with laser aloneTis-1 92.8%T2 70.8% | Local control with laser aloneTis-1 95.1%T2 100% |
| Hoffmann et al.51 (2016) | T1a-T1b-T2 29%n = 74 | T1a-T1b-T2 4.3%n = 127 | 91.9% | 100% | Disease-free survival: 54.6% Disease-specific survival: 90% Overall survival: 76.9% | Disease-free survival: 79.8% Disease-specific survival: 99% Overall survival: 88.5% |
| Blanch et al.40 (2010) | T2: 40.7%n = 81 | Overall survival: 71% Specific survival 5 years T2: 79.2% | ||||
| Mendelsohn et al.56 (2015) | T1a 27.3%n = 11 T1b 20.0%n = 10T2 0%n = 9Overall: 16.7%n = 30 | T1a 91.9%n = 11T1b 90%n = 10 T2 0%n = 9Overall: 6.7%n = 30 | ||||
| Wober et al.53 (2017) | T1a 41.2%n = 17T1b 0%n = 2T2 50%n = 2Overall: 38.1%n = 21 | T1a 8.7%n = 23T1b 0%n = 2T2 0%n = 3Overall: 7.1% n = 28 | Overall survival at 5 years: 90.5%Overall disease-free survival at 5 years: 57.1% | Overall survival at 5 years: 96.4%Overall disease-free survival at 5 years: 92.9% |
AC: anterior commissure.
Hoffman et al. in a study of a large cohort of 201 patients treated with transoral CO2 laser microsurgery found that anterior commissure involvement is the only predictor of unfavourable outcomes in disease-free survival rate and local control.51 These outcomes are consistent with the series published by Sachse et al., where anterior commissure involvement is a factor that reduces local control, and by Wober et al., where anterior commissure involvement increases recurrence rates.52,53
The study by Steiner and Ambrosch in 2004 is one of the most extensive series on glottic tumours with anterior commissure involvement. In the breakdown by subgroup, the greatest difference in local control is shown in T1a according to anterior commissure involvement. Taking stages T1a, T1b and T2 into consideration the authors found no differences in local control with or without anterior commissure involvement, although there are certainly limitations, since the study’s statistical power is less than 80%.54
In the series by Rodel et al., published 4 years later, the difference in local control involves both T1a and T1b.55 Along the same lines, Mendelsohn et al. found that local recurrences mainly involved T1a and T1b.56 However, in the series by Hakeem et al. differences in local control fundamentally involve T2. Taking all the cases into account the authors found no significant differences in overall survival, disease-specific survival and laryngeal preservation between neoplasms with or without anterior commissure involvement.57
The local control results in T1a and T1b urge consideration of the difficulty of surgical treatment, even in small lesions that superficially involve the commissure. However, the results are not similar in all the series. Thus, Lee et al. found no significant effect of tumour spread to the anterior commissure on local control or survival, which they associate with appropriate patient selection, tumour exposure and experience of the surgeon.58
Chone et al. also found no differences in local control between neoplasia with and without anterior commissure involvement, although they observed a trend towards local recurrence in T1 extending to the commissure. The small number of the series is a limitation for establishing significant differences.59
Peretti et al., with the aim of observing the different behaviour of glottic T2 treated with transoral CO2 laser microsurgery, classify them into 4 subcategories: pT2 (iii) includes supracommissural and/or subcommissural extension. Patients in this subgroup have worse local control, which does not affect the laryngeal preservation rate if rescue is by open partial surgery in the presence of early recurrence. 27 Blanch et al. study the results in T2 and T3 with anterior commissure involvement, finding no differences between either category or the likelihood of local recurrence. These results support the aggressiveness of T2 with similar biological behaviour to T3.40 The series by Canis et al. also concludes that glottic pT2b behave in a similar way to pT3.60
There are no published series on treatment for anterior commissure tumours by transoral surgery using CO2 laser fibre.
Surgical Margins. The National Comprehensive Cancer Network Guideline defines as an adequate margin in transoral CO2 laser microsurgery a distance of 1.5−2 mm between the tumour invasive front and the edge of the surgical specimen, considering a distance between 0 and 1 mm to be a close edge.61 Most of the series consider 2 mm an adequate margin.
On occasion, the limit between involved margin and near margin is difficult to establish due to heat damage produced at the edges, small tumour size and its reduction in the process of fixing the surgical specimen.
The positive margins of the different series range from 9% to 25.9%.62–64 However, margins considered histologically positive do not always correspond to tumour persistence. In the review by Jackel et al. only 18% of the patients who underwent a second surgery due to doubtful or positive margins had residual carcinoma.65 Canis et al. also support these data considering false positives of close or positive margins to be around 80%.66
These findings, and also because the papers do not define whether the positive margins correspond to surface or deep margin, or whether positivity is at one or at various points, makes it more difficult to assess impact on local control and disease-free survival rate.
Charbonnier et al. relate the deep positive margin of the vocal muscle in T2b with lower disease-free survival rates. In their series they do not find a high rate of positive margins in tumours with anterior commissure involvement, since in the case of deep involvement they opt for open partial laryngectomy.67
Some surgeons use intraoperative frozen section analysis to limit the risk of positive margins. Remacle et al. consider this proposal useful due to its negative predictive value.68 Fang et al. also find a relationship between positive intraoperative margins in frozen sections, local recurrence and overall survival in the first year of follow-up. However, this data is disconcerting, since this relationship is maintained even when the surgical field has been extended. This is explained by the difficulty of clearly identifying tumour in a submucosal extension or existence of a wider undefined field of cancerisation.69
However, the generalised use of intraoperative samples is not straightforward, since diagnostic precision from frozen intraoperative samples depends on experience and technical support.70
The study by Fiz et al. provides greater clarity on the importance of involved margins. The study stratifies them into negative, positive and close, superficial and deep, and single or multiple point involvement. In their series, close superficial margins have no impact on disease-free survival. By contrast, in all other instances the possibility of recurrence increases.71 Gallet et al. also confirm these results in a multivariate analysis of risk factors related to local control and risk of recurrence. Positive or doubtful margins and commissure involvement are independent risk factors with worse outcomes.72
In the abovementioned paper by Jackel et al., involved margins that can be treated by further resection affect local control, but not overall survival. In the case of involved margins in the second resection, overall survival is compromised.65 Re-excision of doubtful margins achieves good final oncological outcomes similar to those obtained with free margins in the first resection in the papers by Wilkie and Karatzanis.70,73
The studies by Valletti et al. consider that deep positive and multifocal margins are related to a lower laryngeal preservation rate, while involved superficial margin in a cordectomy has impact on local control, but not on survival rates.74 On these same lines, Lucioni et al. associate a higher recurrence rate and lower disease-specific survival in the case of deep margin involvement.75
There remains controversy, therefore, as to the most appropriate attitude if margins are not clearly negative - clinical observation or margin re-excision? We should not forget that a second re-excision results in tissue loss with increased scarring and poor voice quality. In this situation the experience of the surgeon and the intraoperative assessment appear to be relevant aspects that will influence the final decision.76
Narrow-band imaging, intraoperatively and during patient follow-up, improves the diagnostic accuracy of involved margins or early relapse.77,78 The European Laryngological Society and the Korean Society of Thyroid, Head and Neck Surgery recommend its use, although the evidence supporting this suggestion is of low quality.79,80
Vaporisation of the surgical bed by photocoagulation has also been the subject of study and diverse opinions. In the series published by Lucioni et al. the authors conclude that the use of photocoagulation improves local control in close or positive superficial margins, and deep margin involvement appears to have no influence.62
Although there are no large series on the role of radiotherapy in the case of doubtful or positive edges in tumours with anterior commissure involvement, in their paper Stephenson et al. found adjuvant radiotherapy to be useful in these types of lesions, as it is related to good local control and high laryngeal preservation rates on follow-up. The study has limitations in that it does not identify whether the involved margins are superficial or deep.81 Peretti et al. also mention in their paper that in the case of positive deep margins after endoscopic resection, open surgery or radiotherapy is a prudent option to improve laryngeal preservation rates.27
By contrast, in the study published by Ansarin et al., comparing the use of adjuvant radiotherapy on positive margins and clinical follow-up without radiotherapy, no significant differences were obtained in the percentage of patients who relapsed.64
In general, multimodal therapy for early-stage glottic tumours is not recommended, and it seems reasonable that if there is doubt about resection with adequate margins it could be treated with radiotherapy from the outset, avoiding biological costs to the patient.
Comparison of SeriesComparative studies between different therapeutic options for early-stage glottic tumours with anterior commissure involvement are generally based on retrospective series with an absence of comparative prospective randomised studies.
We present the most interesting series and systematic reviews.
Surgery Versus Radiotherapy. In the 1990s some comparative series between open partial laryngectomy and radiotherapy began to be published with variable results.
Thus, in the series by Zohar et al. local control was better in patients initially treated with the surgical option.82 However, in the series by Rucci et al. local control was superior in patients initially treated with radiotherapy in anterior commissure tumours. However, rescue surgery after recurrences in patients initially treated with surgery had better oncological outcomes than rescue surgery after recurrence in patients treated with radiotherapy.83 Later, Bron et al. comparing surgery (endoscopic cordectomy with and without laser, laryngofissure cordectomy and cricohyoidoepiglotopexy) with radiotherapy observed better local control and final laryngeal preservation in patients treated surgically than in those treated with radiotherapy.84
Comparison of T1b treated with radiotherapy and transoral CO2 laser microsurgery in the Canadian multicentre study shows no statistically significant difference in local control or laryngeal preservation rate. The authors attribute these results to the small size of the series, although they note that there are clinical differences with better results in favour of CO2 TLMS in both parameters.33
A recent comparative study by Alkan et al. of T1a and T1b patients treated with radiotherapy or transoral CO2 laser microsurgery found no statistically significant differences in local control or overall survival. However, the recurrence rate was greater and disease-free survival lower in the patients treated with CO2 TLMS. The lack of prior imaging tests and the higher percentage of patients treated with the radiotherapy option could constitute a bias for the study results.85
In relation to systematic reviews, the absence of a clear distinction between T1b and T2 with extension to the anterior commissure, the heterogeneity of cancer outcome rates assessed and the lack of randomised comparative studies are major limitations to a methodological quality analysis of results.
The systematic review on glottic T1a and T1b tumours by O’Hara et al., comparing CO2 TLMS versus radiotherapy, found no demonstrable differences between local control rates in T1a, although it does observe a trend of better local control in T1b in patients treated with radiotherapy. The author himself sees limitations to the review in these tumours due to the small number of cases.86
A meta-analysis of oncological and voice quality outcomes comparing transoral CO2 laser microsurgery and radiotherapy in glottic Tis and T1a patients was published in 2018. The overall survival, disease-specific survival and laryngeal preservation results were better in the patients initially treated by transoral CO2 laser microsurgery.87 These results are consistent with those obtained by Huang et al. in their meta-analysis with respect to laryngeal preservation; however, they contribute nothing for tumours with anterior commissure involvement.88
The latest systematic review published in 2018 on oncological and functional outcomes of T2 glottic tumours treated with surgery versus radiotherapy includes the studies by Hoffmann et al., Peretti et al., Blanch et al., Canis et al. and Rodel et al. on T2 with anterior commissure involvement cited above. In this review it is concluded that laryngeal preservation for T2 tumours is better in patients treated with CO2 TLMS than with radiotherapy. Anterior commissure involvement does not have worse oncological outcomes if adequately staged and treated.89
Open Partial Laryngectomy Versus CO2 TLMS. The comparison of oncological outcomes between open partial surgical techniques and TLM has changed over time. Frontolateral partial laryngectomy and external cordectomy have given way to supracricoid laryngectomy.
Sachse et al. conducted a retrospective study comparing open surgery (external cordectomy or frontolateral partial laryngectomy) and transoral CO2 laser microsurgery in T1a-T2 patients with anterior commissure involvement without finding significant differences in local recurrence rate.52 Mantsopoulos et al. also found no significant difference in local control and disease-specific survival and CO2 TLMS in glottic T2 invading the anterior commissure.90
Wolber et al. conducted a comparative study between the results of CO2 TLMS and open surgery in tumours with anterior commissure involvement. They found significant differences with a greater number of local recurrences in patients treated with CO2 laser with no differences in overall survival or disease-free survival. The limitations of the study include the small size of the series and the variety of open surgery techniques used.53
Open Partial Laryngectomy Versus Radiotherapy Versus CO2 TLMS. There are few studies that compare the three therapeutic modalities in glottic tumours, and even fewer specifically in tumours with anterior commissure involvement.
In 2011 Hartl et al. published a review on the evidence to date for the different treatment options in glottic cancer. Specifically, in the section on glottic tumours involving the anterior commissure they conclude that there is no high quality evidence indicating the superiority of one treatment over another.91
Giocchini et al. published a systematic review on 2360 patients comparing the three treatment options for glottic T1b, although they did not differentiate whether or not there was anterior commissure involvement. The authors conclude that CO2 TLMS has higher recurrence rates compared to radiotherapy and open techniques. However, there is no difference in overall survival rates between the 3 therapeutic modalities.92
Transoral Robotic Surgery. Transoral robotic surgery (TORS) for glottic tumours of the anterior commissure currently offers no clear benefit over transoral CO2 laser microsurgery. The size of the robotic arms that have to work in a reduced space, the difficulty in exposure that requires adapted retractors and the use of monopolar cautery are the greatest handicaps.
TORS enables 3D vision of the surgical field angles with different degrees of rotation of the instrument. The 8 mm and 30° endoscopes allow a wide field of vision. Therefore they could offer improvements over CO2 TLMS, in cases where direct vision is not possible, such as bordering angled spaces “around the corner”.93
In a preliminary study of 6 patients with T1b and T2 glottic cancers with anterior commissure involvement treated using TORS, 2 patients relapsed (33%).94 The future role of TORS in the treatment of glottic tumours with commissure involvement has yet to be defined.95,96
1.7Voice QualityAlthough there are numerous measuring instruments for voice evaluation, a standard method for assessing the impact on the voice of the patient treated with CO2 TLMS has not been established. A multidimensional analysis would include questionnaires that reflect the impact on the patient’s quality of life such as the Voice Handicap Index (VHI), the Voice Related Quality of Life Measure or the Voice Symptom Scale, perceptual assessment of voice quality with the GRBAS scale and acoustic analysis. Both objective and subjective dimensions are recommended by the Committee of Phoniatrics of the European Laryngological Society.97
However, most of the studies do not offer a full assessment of all the dimensions.
Meta-analyses have been published that include voice outcomes in patients treated with radiotherapy versus patients treated with CO2 TLMS in glottic T1a tumours, without specifying possible extension to the anterior commissure. The authors conclude that, although there are no significant differences in VHI, jitter and shimmer between the two therapy options, radiotherapy maintains better voice quality parameters with a longer maximum phonation time and lower fundamental frequency than patients treated with CO2 TLMS.98,99
A systematic review was published in 2012 that aimed to compare functional voice and swallowing outcomes in extensive T1a tumours and limited T2 tumours treated with CO2 TLMS versus radiotherapy. The authors found several limitations in drawing clear conclusions. The comparison between 2 types of treatment is sometimes not homogeneous, comparing resections of different depth and extent, the series are sometimes small in size and heterogeneous measures are used to assess the results.100
In relation to tumours with anterior commissure involvement, Peretti et al. performed an analysis of functional voice outcomes in patients treated with type V cordectomy extended to ventricle and subglottic area. VHI mean score, one year following cordectomy, was 20, which indicated subjective perception of mild dysphonia. In the perceptual GRBAS score it was observed that the majority of patients had mild dysphonia (82%) and less than 20% moderate dysphonia. Mean jitter was 7.87%, shimmer 24.8% and the noise-to-harmonics ratio .37. Age was associated with worse results in the voice quality parameters studied.101
The impact of rehabilitation with speech therapy after treatment, whether surgical or with radiotherapy in tumours involving the anterior commissure, has not been sufficiently studied either. In general, for glottic tumours, the study by Tuomi et al. on voice rehabilitation in patients treated with radiotherapy concludes that an early structured programme can improve vocal function. In their study, the rehabilitation group with an individual structured programme improved maximum phonation time and harmonics-to-noise ratio.102
The presence of anterior laryngeal synechiae is one of the causes of decreased voice quality in patients. Different types of techniques have been used to correct this: laser resections, skin or mucous membrane flaps, silastic or Teflon keels and mitomycin. There are no comprehensive series to establish which is the most effective in functional outcomes.103
1.8Clinical Guidelines and Opinion ArticlesPreferences in treatment options for glottic cancer with anterior commissure involvement depend largely on the development of different techniques in particular countries. Generally speaking, radiotherapy is a predominant choice in Canada, Northern Europe, Australia, South Africa and some centres in the United States, while surgery is preferred in Southern Europe.104
The American College of Radiology considers radiotherapy at a dose of 2.25 Gy the first therapeutic option for a T1b tumour involving the anterior third of both vocal folds and only considers CO2 TLMS an appropriate option if performed by an experienced surgeon and whenever possible changing the initial approach to open surgery if the circumstances of tumour spread require it.105
In the same vein, the guideline for surgical treatment of the Korean Society of Thyroid Head and Neck Surgery recommend transoral CO2 laser microsurgery for T1-T2 glottic cancer with anterior commissure involvement, as long as the resection margins are adequate (weak recommendation and moderate quality evidence.80
The clinical practice guideline on the therapeutic option in T1 glottic tumours of Cancer Care Ontario Canada, concludes that in the case of anterior commissure tumours, voice involvement may be a factor to consider when recommending radiotherapy rather than surgery.106
Similarly, the United Kingdom multidisciplinary clinical guideline considers radiotherapy the most appropriate therapeutic option, rather than transoral CO2 laser surgery for tumours diffusely infiltrating the mucosa of the vocal fold and involving the anterior commissure with a large tumour volume.107
Peretti et al. published an opinion article in 2016 on the reasonable limits of CO2 TLMS in laryngeal cancer. They reflect on the importance of distinguishing between tumours of the folds that involve the commissure in a horizontal plane from those that extend and grow in a cranio-caudal plane, in such a way that the unexpected difficulty in the visualisation of the lesions that affect the anterior commissure can lead to an incomplete transoral resection, being an insufficient prognostic factor of oncological and functional results. Therefore, precise programming of the surgical approach is required for adequate resection margins. The authors believe that the indication is clear for superficial lesions of the larynx, with limited in-depth invasion of the lateral and anterior region, and that it would not be advisable in lesions involving the posterior paraglottic space or tumours infiltrating the laryngeal cartilages.108
2Recommendations of the Group of Experts on the Diagnostic Management of Early Glottic Tumour With Anterior Commissure Involvement and Treatment With Transoral CO2 Laser SurgeryThe recommendations are made based on the literature reviewed and expert opinion
The use of rigid optics at 0°, 30° and 70° can improve anterior commissure tumour involvement accuracy in the case of hypertrophic bands and doubts about extension to the subglottis. However, the current flexible video-endoscopes incorporating camaras with image magnification have reduced their routine use.
NBI examination improves diagnostic accuracy in flat and irregular tumours involving the anterior third of the vocal folds and the anterior commissure, facilitating the location of tumour nests in early stages. The perpendicular vascular changes of the lesion are assessed and in the case of leukoplasia the submucosal microvascular abnormalities of the tissue surrounding it.
Videostroboscopy does not improve diagnostic precision of anterior commissure involvement. It can be useful in assessing the mobility of the fold with commissure involvement to establish the difference between T1a and T2.
A multisection helicoidal CT of 1 mm with 3-plane reconstruction is recommended as the first option in glottic tumours with extension to the anterior commissure.
Complementing the study with MRI is recommended if there are doubtful images of cartilage erosion that could change the therapeutic attitude.
Cervical ultrasound is not an elective imaging test.
PET-CT is not a recommended imaging test for initial assessment.
It is recommended that AJCC glottic T1a, T1b and T2 tumour staging is complemented with the description of anterior commissure involvement according to its extension.
- •
Tumour confined exclusively to the anterior commissure (AC1).
- •
Tumour involving one fold and the anterior commissure (AC2).
- •
Tumour involving the anterior commissure and part of both vocal folds (AC3).
- •
Tumour involving most of one vocal fold and crossing the anterior commissure involving the other vocal fold (AC4).
It is considered of great importance to reflect whether growth is in the horizontal plane or the vertical plane of the glottis.
The decision on the choice of treatment will be made on an individual basis according to the endoscopic and imaging findings that define its extent and depth, the patient’s wishes taking into account personal expectations, age, general and functional condition and the experience of the multidisciplinary team.
The patient will be informed of the different therapeutic options, including sequelae and oncological outcomes. Voice professionals will be informed precisely about foreseeable functional sequelae that will affect the voice in the different possible treatments.
Whenever CO2 TLMS is used for T1-T2 glottic cancer with anterior commissure involvement adequate resection margins must be ensured.
In general, involvement of the posterior paraglottic space and infiltration of laryngeal cartilages is considered a limitation for CO2 TLMS.
It is recommended that the difficulty of exposure prior to surgery be known by evaluating the following parameters: thyroid-mental distance, dental status, presence of trismus, mandibular prognathism, macroglossia, micrognathia, degree of neck flexion-extension, previous surgical treatments, modified Mallampati classification and body mass index.
Laryngeal exposure of the anterior commissure is facilitated with the use of rigid endoscopes of different sizes and shapes (the more difficult the exposure the smaller the size), flexion-flexion manoeuvre and external pressure at the level of the cricothyroid area that improves the coaxial direction of the laser beam and the anterior glottis.
Intraoperative evaluation using 30° and 70° angled optics and light filters to determine surface extension are recommended. If there are reasonable doubts as to the possibility of resecting the tumour with adequate margins, an alternative therapy will be chosen.
The use of light filters (NBI) is recommended to establish superficial resection margins more accurately and minimise the removal of healthy tissue.
In T2 cancers involving the anterior commissure in the vertical plane, tumour extension is usually in a craniocaudal direction, therefore vertical resection is recommended including the infrapetiolar area of the epiglottis, inferior pre-epiglottic fat, anterior third of bands and anterior glottic commissure with extension to the subglottis and towards the cartilage (keyhole image).
The subtype of cordectomy performed, Va or type VI and the details of its enlargement must be recorded.
Vaporisation of the surgical bed will take place at the surgeon’s discretion, bearing in mind that it does not improve oncological outcomes if the deep margins of the surgical specimen are involved.
In T1b tumours 2-stage resection is not advised in order to reduce the possibility of anterior synechiae.
Orientation of the surgical specimen or specimens is necessary, clearly establishing the superficial and deep margin. Intraoperative analysis of margins is recommended whenever possible.
When histopathological results indicate close margins, the experience of the surgeon and intraoperative evaluation will be considered in order to recommend clinical observation or margin re-excision.
When the histopathological results indicate a positive margin, enlargement in a second surgery stage is recommended. If the deep margin is positive and enlarging the margin is not possible, it is recommended that other therapeutic options are evaluated such as supracricoid partial laryngectomy or total laryngectomy if extension to the subglottis contraindicates partial techniques or radiotherapy.
Patient follow-up will consider whether the margins obtained were close and whether or not a second re-excision was performed.
For appropriate margins in T1a-T1b clinical control is recommended every 3 months for the first year.
In close surgical margins where clinical observation has been decided, closer follow-up is recommended for the first year.
Control cervical CT or MRI (depending on the initial diagnostic study) is recommended every 6 months for T2 and for doubtful surgical margins regardless of the T. If the images are not conclusive, PET-CT can be considered.
Surgical treatment of anterior synechiae will be based on the results of voice evaluation, patient preferences and real expectations for improvement, taking thickness and length into account.
In general, surgical treatment of anterior synechiae is not recommended earlier than 6 months following oncological treatment.
There is no standard surgical treatment to improve functional voice outcomes in anterior synechiae.
Use of mitomycin C has not shown better results in the prevention of anterior synechiae or efficacy in isolation to prevent recurrence.
Speech therapy rehabilitation treatment is recommended for all patients treated with an individual tailored programme.
Although specific questionnaires for voice assessment in patients treated with CO2 TLMS have not been defined, it is recommended to evaluate the impact on the voice before and after surgical treatment according to the ELS recommendations.
The VHI questionnaire, perceptual evaluation of voice quality with the GRBAS scale (especially R evaluation in anterior synechiae), aerodynamic measures that include maximum phonation time and phonation quotient and acoustic parameters including fundamental frequency and frequency range are recommended.
The Spanish Society of Otorhinolaryngology and Head and Neck Surgery (SEORL-CCC) funded the translation and publication of this supplement.
Conflict of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Porras Alonso E, Vilaseca González I, García Teno M, Barberá Durbán R, Viscasillas Pallàs G, Sancho Mestre M, et al. Tumores glóticos precoces con afectación de la comisura anterior. Revisión bibliográfica y documento de consenso. Comisión de cabeza y cuello y base de cráneo. SEORL-CCC. Acta Otorrinolaringol Esp. 2020;71:1–20.