Transplantation is the treatment option that offers improved survival and quality of life as compared to organ failure. Psychiatric and psychological aspects of transplant candidates are important, especially in the pre-assessment stage, as the influence of these factors can hinder post-surgical outcome in both the implanted organ survival and the quality of life of the transplanted person. Of particular importance, the factors related to pathology are due to substance use, psychopathology, and psychosocial support. There are currently few guidelines on the correct evaluation of patients eligible for these complex procedures.
MethodNineteen psychiatrists and clinical psychologists from six public hospitals in Catalonia conducted a systematic consensus to determine the design of a Unified Protocol psychological and psychiatric evaluation. An annual work plan was implemented, during which; the objectives were defined, a literature review was conducted, the inclusion and exclusion criteria were discussed, questionnaires were selected, and a structured interview was developed.
ResultsWith the implementation of the work plan, the Assessment Protocol presented in this article was designed.
Conclusionssystematic work and improving cooperation between psychiatrists and clinical psychologists, has led to homogeneity and consensus on a unified evaluation protocol.
El trasplante es la opción terapéutica que ofrece mayor supervivencia y calidad de vida frente al fallo de un órgano. Los aspectos psiquiátricos y psicológicos de los candidatos a trasplante son relevantes, especialmente en la etapa de evaluación previa, pues la influencia de estos factores puede dificultar la evolución posquirúrgica, tanto en la supervivencia del órgano implantado como en la calidad de vida de la persona trasplantada. Son de especial importancia los factores vinculados a la patología por uso de sustancias, los trastornos psicopatológicos y el soporte psicosocial. Actualmente, existen pocas Guías que orienten sobre la correcta evaluación de los pacientes candidatos a estos procedimientos complejos.
MétodoDiecinueve psiquiatras y psicólogos clínicos de 6 hospitales públicos de Cataluña realizaron un trabajo sistemático de consenso para llegar al diseño de un protocolo unificado de evaluación psicológica y psiquiátrica. Se implementó un plan de trabajo anual, se definieron objetivos y se realizó una revisión bibliográfica, se discutieron los criterios de inclusión y exclusión, se seleccionaron los cuestionarios y se elaboró la entrevista estructurada.
ResultadosCon el cumplimiento del plan de trabajo, se diseñó el Protocolo de Evaluación que se presenta en este artículo.
ConclusionesEl trabajo sistematizado y la colaboración intercentros de psiquiatras y psicólogos clínicos ha facilitado homogeneizar y consensuar un protocolo unificado de evaluación.
Upon failure of a vital organ, transplantation is the therapeutic option that offers greater survival and quality of life, compared to other kinds of substitute treatments.1 The organ transplantation programme in Catalonia dates back almost 50 years. In 1965, the first kidney transplantation was performed; in 1984, the transplantation programme was created in the Health Department, and in 1985 the Catalan Transplant Organisation (Organización Catalana de Transplantes, OCATT) was established. The experience acquired over the years makes it clear that organ transplantation requires a multidisciplinary team able to offer coverage in all stages of the process.
The shortage of organs available for donation has led to a selection of candidates that allows for better post-transplantation results.2 In this sense, several studies outline the influence of psychiatric and psychological factors in post-surgical progression for both the survival of the implanted organ and the quality of life of the subject.3 Factors related to the pathology due to substance use, psychopathological disorders and psychosocial support are particularly important.4,5 These mental characteristics of candidates began to gain importance in the 80s, but it was not until approximately 15 years ago when different protocols were established. Although they are similar as regards intention and implementation, it is advisable to reach an agreement and to unify professional criteria.
In Spain, there are few guidelines governing these psychological and psychiatric assessments6,7 and, though these have been performed for many years, there is no agreed operation protocol to date. In this context, and given the heterogeneity of existing models, it was decided to form a working group with professionals who currently perform this task in hospitals of Catalonia. The main objective was to draft a Unified Protocol for Pre-transplantation Psychiatric and Psychological Assessment. This was intended to help the transplantation team perform interventions that promote change and adherence to medical standards and, in this way, improve health care quality and procedure efficiency within the health care system.
MethodThe working group was formed in December 2011 and was conformed by 19 professionals, clinical psychiatrists and psychologists, from 6 public hospitals in Catalonia. The tasks performed to reach the objective included sharing institutional experiences, establishing common criteria and selecting the instruments that, together with the support from publications made by other specialists, set the foundations to transfer all this knowledge to the protocol design.
Over a term of 10 months of work, the protocol for the assessment of candidate patients was developed. For its completion, the following partial objectives were initially established to guide the work plan from a chronological point of view.
- 1.
To agree on basic and common psychiatric and psychological criteria for the inclusion of candidates for transplantation.
- 2.
To design a Unified Protocol for the psychiatric and psychological assessment of recipient candidates of different organs and tissues.
- 3.
To determine which psychological assessment instruments would form part of their basic set for assessment.
- 4.
To promote the use of a computerised assessment and its inclusion in the patients’ electronic clinical history, so that it could be easily accessed by other professionals involved and, thus, avoid the repetition of health care and/or administrative acts.
- 5.
To inform about the use of the Assessment Protocol and its results to the centres associated with the OCATT and to other related organisms.
The protocol design was performed in 3 consecutive stages that lasted approximately between 3 and 4 months each. During the first stage, the objectives of the working group were defined, the existing bibliography on the matter was reviewed and working models for each health care centre were submitted. During the second stage, common and minimum contents to be included in the Protocol were agreed upon and the accompanying questionnaires were selected. During the last stage, the Protocol was drafted and the initial substantiating applications began to be implemented.
ResultsDescription of the Unified Protocol for psychiatric and psychological assessment of candidates for transplantation of organs and/or tissuesDuring the first stage of design, the objectives for the psychiatric and psychological assessment of candidates for transplantation were established (Table 1).
Objectives of the psychiatric and psychological assessment of candidates for transplantation.
| 1. To determine the candidates’ capacity to understand the process and to give their informed consent |
| 2. To assess the treatment adherence capacity and collaboration with the transplantation team |
| 3. To diagnose comorbid psychopathological aspects that may influence the pre and post-transplantation process, thus favouring follow-up |
| 4. To detect substance use and its consequences. To assess the capacity for long-term abstinence maintenance |
| 5. To assess their transplantation process coping skills and intervene when patients present limitations |
| 6. To identify health behaviours, particularly unhealthy life habits that may influence post-transplantation morbidity and mortality. |
| 7. To explore the candidates’ level of social support and socio-economic conditions |
| 8. To establish objective measurements for the baseline functioning level, so as to obtain objective data for their standardisation and for future investigations |
It was decided that, to access the psychiatric-psychological assessment, the patient must have been previously informed by the medical team about the characteristics of the treatment and its risks. The assimilation and understanding of this information shall be assessed in the interviews. Another requirement is the performance of a toxicological screening, preferably prior to the first psychiatric visit.
The Unified Assessment Protocol for recipients (see Appendix as additional online material) is composed of 2 separate and complementary sections: a semi-structured interview and a set of questionnaires. The time required for the assessment process is estimated to be between 2 to 3 visits, so as to collect all the information possible and to facilitate the return of results and an intervention plan.
In the semi-structured interview, the following data are collected: socio-demographic data of the candidate, personal and family medical and psychopathological history, past and present substance use, lifestyle description and self-motivation for change. As for the medical problem, it is important to gather what the patient knows about the history of the disease that has led to transplantation, the patient's knowledge and understanding of the medical–surgical procedures involved, as well as the treatment result expectations. Furthermore, due to demographic changes in last two decades, there are also specific sections that should be assessed if the patient has an ethnic origin different from that of the local population (see additional online material).
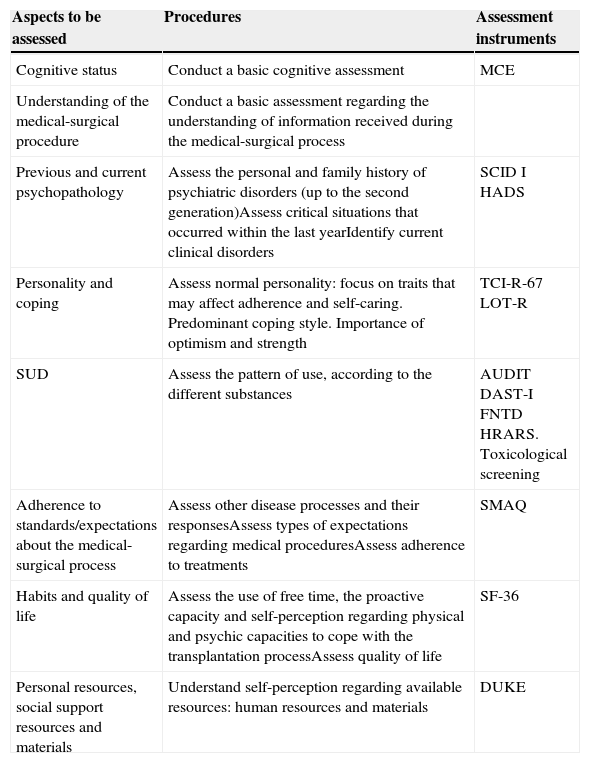
Regarding criteria for the selection of psychometric instruments, the working group decided that questionnaires are to be validated for the Spanish population, and they need to be adequately reliable, easily comprehensible and brief, so that they can be corrected in a simple and direct manner. The experience of experts regarding the use of these instruments was also essential for their selection (Table 2).
Psychometric procedures and instruments for the psychiatric and psychological assessment prior to organ transplantation.
| Aspects to be assessed | Procedures | Assessment instruments |
|---|---|---|
| Cognitive status | Conduct a basic cognitive assessment | MCE |
| Understanding of the medical-surgical procedure | Conduct a basic assessment regarding the understanding of information received during the medical-surgical process | |
| Previous and current psychopathology | Assess the personal and family history of psychiatric disorders (up to the second generation)Assess critical situations that occurred within the last yearIdentify current clinical disorders | SCID I HADS |
| Personality and coping | Assess normal personality: focus on traits that may affect adherence and self-caring. Predominant coping style. Importance of optimism and strength | TCI-R-67 LOT-R |
| SUD | Assess the pattern of use, according to the different substances | AUDIT DAST-I FNTD HRARS. Toxicological screening |
| Adherence to standards/expectations about the medical-surgical process | Assess other disease processes and their responsesAssess types of expectations regarding medical proceduresAssess adherence to treatments | SMAQ |
| Habits and quality of life | Assess the use of free time, the proactive capacity and self-perception regarding physical and psychic capacities to cope with the transplantation processAssess quality of life | SF-36 |
| Personal resources, social support resources and materials | Understand self-perception regarding available resources: human resources and materials | DUKE |
AUDIT: Alcohol Use Disorders Identification Test; DATS-I: Drugs abuse Test Screening; DUKE: Functional Social Support Questionnaire; FNTD: Fageström Test for Nicotine Dependence; HADS: Hospital Anxiety and Depression Scale; HRARS: High Risk Alcoholism Relapse Scale; LOT-R: Life Orientation Test-revised; MCE: Mini-Cognitive Examination; SCID: Structured Clinical Interview for DSM-IV Disorders; SF-36: Health Survey Short Form 36; SMAQ: Simplified Medication Adherence Questionnaire; TCI-R-67: Temperament and Character Inventory-Revised-67; SUD: substance use disorders.
A relevant aspect of the assessment is the detection of possible substance use patterns. The performance of a baseline screening together with the use of specific scales would represent the baseline values. To predict the progression or capacity for medium or long-term abstinence maintenance, the measurement used is the number of previous months of abstinence. A minimum of 6 months is required for alcohol and a minimum of one year for regular use of other drugs. The clinical interview intends to measure the patient's awareness on toxicity and dependence, to determine if the patient is aware of the negative influence that the use of toxic substances poses on organic disorders, as well as to establish the patient's incapacity for substance use control and its negative consequences in the patient's life.
Description of psychometric assessment instrumentsMini-Cognitive ExaminationThe Mini-Cognitive Examination (MCE) is an hetero-applied instrument designed to detect cognitive impairment cases. It is a widely used scale in hospitals for various medical diseases. It explores the areas of temporal and spatial orientation, immediate memory, concentration and calculation, deferred memory, and language and praxis. The MCE is brief and easy to implement. In this protocol, we used the version adapted and validated by Lobo et al.8
Structured Clinical Interview for DSM-IVThe Structured Clinical Interview for DSM-IV Disorders (SCID) is a semi-structured interview which consists of 2 sections and is used for clinical diagnoses (i axis) and personality disorder diagnoses (ii axis) set forth in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). The studies conducted with this instrument have a high reliability and validity. The extensive nature of diagnostic criteria of the DSM manual promoted the creation of different versions. The last version was published in 1992, and it was translated and adapted in 1999 by the authors themselves.9
Items are scored upon progression and identification of affirmative responses from the patient under assessment. There are 3 possible options: positive, negative or question, if it was not possible to verify the situation. The SCID-I is administered in the Protocol.
Hospital Anxiety and Depression ScaleThe Hospital Anxiety and Depression Scale (HADS) is a self-applied scale, designed to assess anxiety and depression symptoms in patients with medical diseases and it is widely used in hospitals. The scale consists of 14 items (7 measure depression symptoms and 7 measure anxiety symptoms) and, unlike other questionnaires designed to measure psychopathology, HADS does not assess symptoms that could be due to physical causes, so it is particularly adequate for people with somatic morbidity.
In the protocol, we included the Spanish version, which presents acceptable psychometric properties (87% specificity and 72% sensitivity for the depression subscale, and 85% specificity and 80% sensitivity for the anxiety subscale).10
Upon comparing the above-mentioned questionnaire with other questionnaires, we decided to include this one because it was also validated in a population with medical pathology and because it combines anxiety and depression items in a summarised and comprehensible manner.
Temperament and Character Inventory-Revised-67The Temperament and Character Inventory-Revised-67 (TCI-R-67) is a summarised version of Cloninger's Temperament and Character Inventory-Revised (TCI-R). The TCI-R-67 is a self-applied instrument and is used to estimate the temperament and character traits proposed by Cloninger's model. It presents good psychometric qualities compared to the entire test and it is validated in the Spanish population.11
The TCI-R-67 measures the 4 temperament dimensions of the complete instrument (novelty seeking, harm avoidance, reward dependence and persistence) plus exploratory excitability, which is included as an independent scale, and 3 character dimensions (self-directedness, cooperativeness and self-transcendence).
It consists of 62 items plus 5 validity items, and it is responded on a 5-point Likert scale. Each scale keeps 8 items from the original scale, except for exploratory excitability that keeps 6.
Upon comparing the above-mentioned questionnaire with other personality questionnaires, we chose Cloninger's model over others because it represents one of the most complete models on normal personality and because it is brief and easy to understand for the type of patients under assessment.
Optimism Questionnaire: Life Orientation Test-RevisedThe Optimism Questionnaire: Life Orientation Test-Revised (LOT-R) measures generalised predisposition towards positive result expectations and, according to the studies, it seems to be directly related to the different types of coping with stressful situations. It is a self-applied questionnaire and it consists of 6 items (plus 4 supplementary items) that provide a dispositional optimism score. In this protocol, we included the version validated in Spain.12
Alcohol Use Disorders Identification TestThe Alcohol Use Disorders Identification Test (AUDIT) is a screening instrument developed by the World Health Organisation. It is self-applied and it allows for the identification of alcohol dependence. It may be administered in a very short period of time and it is easy to score. It consists of 10 questions with a response scale ranging from 0 to 4, thus obtaining a total score from 0 to 40. Scores ranging from 8 to 15 indicate a medium level of alcohol problems, while scores equal to or above 16 represent a high level of problems, and a score above 20 indicates probable alcohol dependence. It has been validated in the Spanish population13. With a cut-off point of 7, sensitivity to detect hazardous use amounted to 91.7% and specificity amounted to 91.9%. According to the DSM-IV criteria, sensitivity was 88.3% and specificity was 88.3%.
Drug Abuse Screening TestThe Drug Abuse Screening Test (DAST) is a self-administered adaptation of the Michigan Alcoholism Screening Test to identify the use of substances other than alcohol and tobacco. The application time ranges from 2 to 5min. Both the DAST-20 version and the DAST-10 version have a high internal consistency (Cronbach's alpha of 0.93 and 0.89, respectively). The cut-off points>5/6 (DAST-20) and ≥3 (DAST-10) showed a high level of concordance with DSM-IV-TR criteria, correctly classifying 98.07% and 95.36% of subjects, respectively. These are valid and reliable instruments for the detection of drug abuse among adults.14
Fageström Test for Nicotine DependenceThe Fageström Test for Nicotine Dependence is a self-administered questionnaire consisting of 6 items. It is a summarised version of the original Fagerström Tolerance Questionnaire and it measures nicotine dependence. Items have 2 different types of responses: 4 of them have a dichotomous response (yes or no) and the other 2 items are responded on a 4-point Likert scale (0 to 3 points). The total score ranges from 0 to 10 points. Becona and Vázquez have validated this instrument15 and have demonstrated that it is a valid and reliable instrument to detect nicotine dependence. The internal consistency coefficient (Cronbach's alpha) obtained in 3 clinical samples ranged from 0.56 to 0.64.
High Risk Alcoholism Relapse ScaleThe High Risk Alcoholism Relapse Scale was designed by the group of Yates et al.16 and applied in a group of veteran patients to assess the risk of relapse after alcohol rehabilitation treatment. It has been used to compare profiles of patients with end-stage hepatic disease under a transplantation process with profiles of the veteran population (not necessarily with alcoholic hepatic disease). Based on the results obtained, information on the duration of alcohol use (a measure of chronicity), daily quantity of alcohol use, and previous alcohol treatments, this scale was incorporated as a method of selection of candidates for transplantation in several hepatic transplantation programmes, such as those from the University of Iowa, in the United States, and Hospital Clínic, in Barcelona.
It consists of 3 items: daily alcohol use in standard drinking units, years of hazardous use and number of previous alcohol treatments.
It has a predictive validity for early relapse within the first 6 months after treatment, with 69% sensitivity and 65% specificity.
Simplified Medication Adherence QuestionnaireThe Simplified Medication Adherence Questionnaire (SMAQ) is a short and hetero-applied instrument related to patients’ medication-taking habits. It was developed as a modification of the Morisky-Green questionnaire to measure adherence to antiretroviral treatments in patients with acquired immunodeficiency syndrome (AIDS) and, later on, its Spanish version was validated in renal transplantation patients.17 It consists of 6 questions that assess different aspects of compliance with treatment: forgetfulness, routine, adverse effects, and a quantification of omissions. Patients are classified as non-compliant if they answer any of the questions with a non-adherence response, and if patients have lost more than 2 doses during the last week or have not taken medication during more than 2 complete days during the last 3 months.
Health Survey SF-36The Health Survey SF-36 is one of the most widely used generic self-applied scales for the assessment of clinical results and quality of life. This questionnaire was developed for the analysis of results in medical interventions and may be used for both the general population and patients. It provides a health status profile using 36 items with a different score that assess physical, social and emotional functions, as well as role limitations due to health problems.
In the protocol, we included the validated Spanish version with the reference values of the general population.18 This choice was based on the fact that this is one of the scales that include mental health items in self-perceived quality of life.
Functional Social Support Questionnaire-11The Functional Social Support Questionnaire is a self-applied instrument that consists of 11 items on a Likert-type scale (from 1 to 5 points), which allows for the assessment of social support perceived regarding 2 subscales: confidential support and emotional support. The higher the score, the higher the social support perceived. The Spanish adaptation has good reliability and validity indexes.19
DiscussionAlthough there are many other factors involved in transplantation success (for example, even with a perfect adherence, organ rejection may occur), many studies4 indicate that psychiatric and psychological variables have an impact on the patient's prognosis.
According to Maldonado et al., if we can predict the transplantation result with better psychosocial criteria and, therefore, intervene prematurely, we will be able to achieve lower post-transplantation morbidity, reduced organ rejection, improved transplantation survival, better quality of life for patients and, overall, a global reduction of costs.2
The pre-transplantation psychological and psychiatric assessment must be used to identify the psychosocial adjustment of patients, their cognitive functioning and emotional status, as well as to implement a treatment plan to address current mental health problems.
This protocol summarises the experience of teams from different hospitals in Catalonia, with considerable experience in the assessment of candidates for transplantation. This has made it possible to reach an agreement upon unifying assessment variables and trying to promote a comprehensive view of patients by means of an interdisciplinary collaboration among centres.
An exploratory implementation of the protocol and its questionnaires is currently being conducted. The authors consider that the results from the continuous and systematic implementation of the protocol will enable the creation of assessment standards. Further investigations are required to broaden the evidence on the importance of psychological and psychiatric factors in the transplantation process prognosis and their use as inclusion and exclusion criteria.
Ethical responsibilitiesProtection of persons and animalsThe authors state that no experiments were performed on human beings or animals as part of this investigation.
Data confidentialityThe authors state that this article does not contain patient data.
Right to privacy and informed consentThe authors state that this article does not contain patient data.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Fidel Kinori SG, Alcántara Tadeo A, Castan Campanera E, Costa Requena G, Diez Quevedo C, Lligoña Garreta A, et al. Protocolo unificado para la evaluación psiquiátrica y psicológica de candidatos a trasplante de órganos y tejidos, PSI-CAT. Rev Psiquiatr Salud Ment (Barc.). 2015;8:130–136.
All authors are members of the Psychiatry and Psychological Assessment Working Group of the Catalan Transplant Organisation (OCATT). The Working Group is made up of 19 clinical psychiatrists and psychologists, and 10 of them have participated in the preparation of this article. The first author is the coordinator of the Working Group and responsible for preparing the article. She is the person who designed it. The other 9 authors contributed equally in the writing of the article and its critical review.