The introduction of the first antidepressants in the 50s of the 20th century radically changed the treatment of depression, while providing information on pathophysiological aspects of this disease. New antidepressants drugs (agomelatine, tianeptine, vortioxetine) are providing data that give rise to pathophysiological hypotheses of depression that differ from the classic monoaminergic theory. In this sense, tianeptina, an atypical drug by its mechanism of differential action, contributes to clarify that in depression there is more than monoamines. Thus, tianeptine does not modify the rate of extracellular serotonin, so it does not increase or decrease the reuptake of serotonin. Chronic administration of tianeptine does not alter the density or affinity of more than a hundred classical receptors related to depression. Recently, a weak action of tianeptine on Mu opioid receptors has been described that could explain the release of dopamine in the limbic system and its participation in the modulation of glutamatergic mechanisms. These mechanisms support the hypothesis of the possible mechanism of action of this antidepressant.
Tianeptine is an antidepressant, with anxiolytic properties, that can improve somatic symptoms. Tianeptine as a glutamatergic modulator, among other mechanisms, allows us to approach depression from a different point of view than other antidepressants.
La introducción de los primeros antidepresivos en la década de los cincuenta del sigloxx modificó de forma radical el tratamiento de la depresión, a la vez que aportó información sobre aspectos fisiopatológicos de esta enfermedad. Los nuevos fármacos antidepresivos (agomelatina, tianeptina, vortioxetina) están aportando datos que dan lugar a hipótesis fisiopatológicas de la depresión que difieren de la clásica teoría monoaminérgica. En este sentido, la tianeptina, un fármaco atípico por su mecanismo de acción diferencial, contribuye a clarificar que en la fisiopatología de la depresión hay algo más que monoaminas. Así, la tianeptina no modifica la tasa de serotonina extracelular, por lo que no aumenta ni disminuye la recaptación de serotonina. La administración crónica de tianeptina no altera la densidad ni la afinidad de más de un centenar de receptores clásicos relacionados con la depresión. Recientemente se ha descrito una acción débil de la tianeptina sobre receptores opioidesMu que podría explicar la liberación de dopamina en el sistema límbico y su participación en la modulación de mecanismos glutamatérgicos. Estos mecanismos sustentan la hipótesis del posible mecanismo de acción de este antidepresivo.
La tianeptina es un antidepresivo con propiedades ansiolíticas que puede mejorar síntomas somáticos. La tianeptina como modulador glutamatérgico, entre otros mecanismos, permite abordar la depresión desde un punto de vista diferente al del resto de antidepresivos.
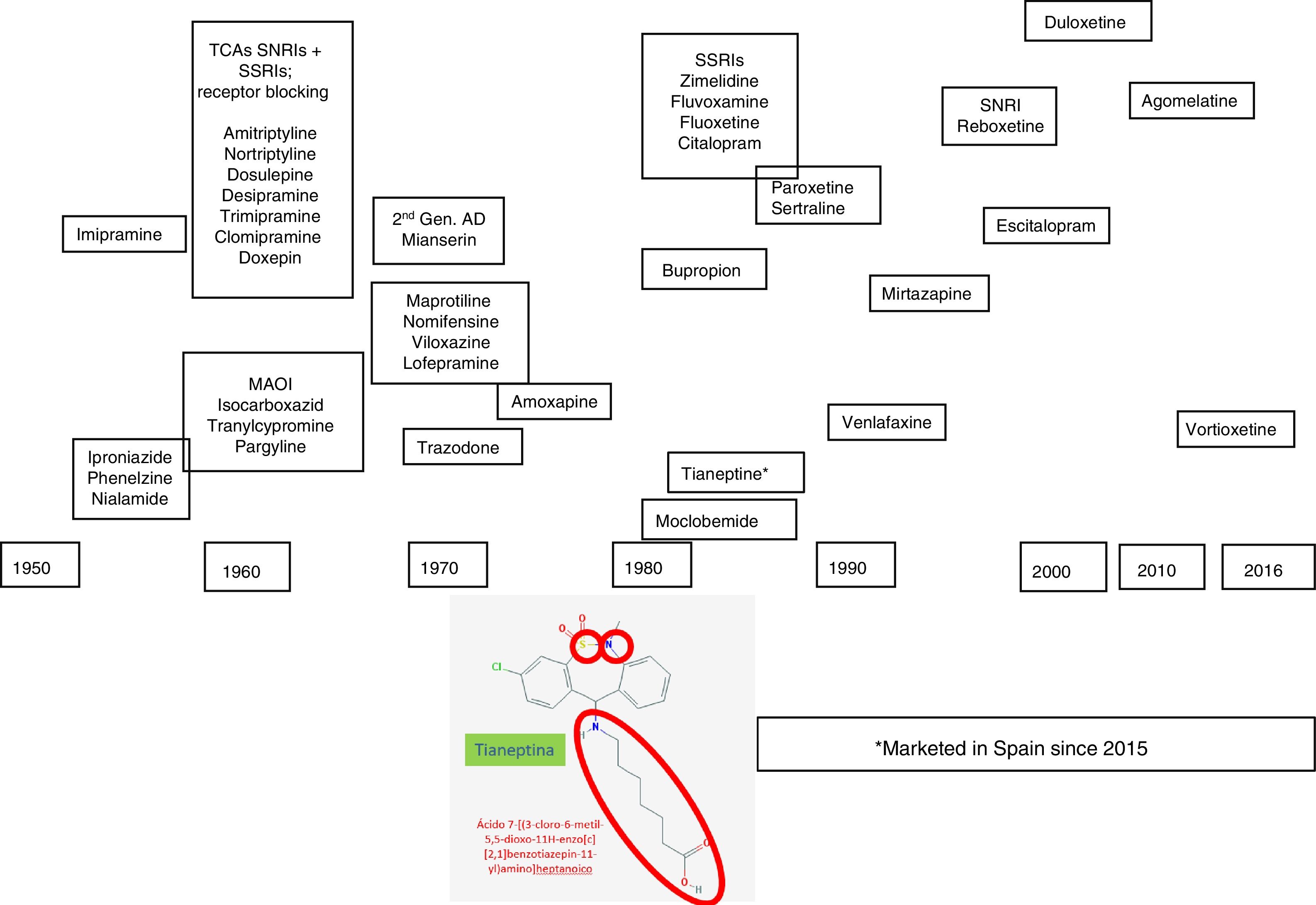
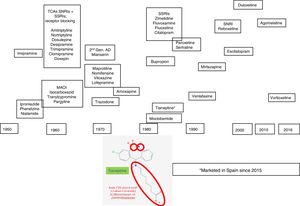
Depression is a complex and heterogeneous disorder, and its pathophysiology is difficult to uncover. We have to resort to research studies based on animal models, genetic and pharmacological techniques to explain the mechanisms underlying its pathogenesis,1 which are not always easy to carry over to and interpret in clinical practice. However the pharmacological response to agents with similar mechanisms of action, monoaminergic drugs essentially, has been helpful in clarifying some aspects of the pathophysiology of depression. The clinical introduction of antidepressant drugs to the therapeutic arsenal started during the nineteen fifties, which was when the so-called “psychopharmacological revolution” took place. It was during this decade that the antidepressant properties of iproniazid and imipramine were discovered, the first exponents of the monoamine oxidase inhibitor family (MAOIs) and tricyclic antidepressants (TCAs), respectively. New heterocyclic antidepressants started to appear in the seventies, called “atypical” or “second generation” antidepressants (maprotiline, mianserin, trazodone, viloxazine, and nomifensine), and this constituted the transition to the introduction, at the end of the eighties, of the noradrenaline and serotonin reuptake inhibitors (NSRI), such as venlafaxine, and more recently duloxetine and desvenlafaxine, the presynaptic aminergic receptor inhibitors, the prototype of which is mirtazapine, and the selective noradrenaline reuptake inhibitors (SNRIs), with reboxetine as the sole representative2,3 (Fig. 1).
From a pharmacological perspective, all these antidepressants have explored monoaminergic mechanisms. In short, initially they increase monoamines in the synaptic cleft, and this gave rise to the monoaminergic hypothesis of depression.4 However, there can be a total lack of response or partial response with persistent residual symptoms, with the agents that act according to this classical theory of depression, and they can be poorly tolerated in relation to serotonergic functionality, with adverse cardiovascular effects or an increased suicide risk.5
In this regard, we can point out that the real progress made by the new antidepressant agents is based more on a differential profile of adverse effects than on greater therapeutic efficacy – some do not even surpass the classical agents6 – and there are still problems to resolve. We now need new antidepressants to tackle the problem of depression with a mechanism of action other than that of the “monoaminergic” antidepressants.6
We now know that the abovementioned monoaminergic increase in the synaptic cleft will act as an “interrupter” that will trigger a series of delayed presynaptic and postsynaptic events, transduction mechanisms, that resemble the hard disc of a computer, and that will ultimately be responsible for the antidepressant effect.6,7
Agomelatine was marketed in the first decade of the 21st century, an antidepressant whose onset of effect is not exclusively monoaminergic. The antidepressant arsenal has recently been increased with vortioxetine, which has a multimodal mechanism as it affects the functionality of several monamines (serotonin, noradrenaline, histamine, etc.), and finally, tianeptine, an antidepressant with a differential action mechanism, has been marketed in Spain, which is the reason for this paper (Fig. 1). In this review we will describe how tianeptine does not use monoaminergic interrupters, and therefore triggers differential transduction mechanisms that could ultimately be responsible for the antidepressant effect.6–8
Intense efforts have been made to find other antidepressants that initiate their effects through mechanisms independent of monoaminergics, however few satisfactory results have been achieved so far. Indeed, studies of neurotrophin analogues, CRH antagonists, NMDA receptor modulators, substance P antagonists, all on an experimental and scientific, essentially preclinical basis, which supported a potential antidepressant effect have not yielded the expected results from a clinical perspective, and therefore have not been able to be marketed.9,10
Tianeptine is an atypical antidepressant that constitutes an exception to the monoaminergic approach to depression,11 the experimental pharmacological features5 of which we shall develop in this review.
Tianeptine in the antidepressant arsenalTianeptine was synthesised by Deslandes and Spedding and marketed in Europe in the nineteen eighties,12 although it was only introduced in Spain very recently. Tianeptine has a heterocyclic structure which means that it is often classified as a tricyclic antidepressant.13,14 However, tianeptine is characterised by incorporating 2 heteroatoms: sulphur (S) hence its name “tia”=“sulphur”) and nitrogen (N) instead of carbon (C) in the central ring, and it carries an aminoheptanoic side chain. The tricyclic nucleus has an electron donor heteroatom in position 5, and an electron–acceptor atom in position 3 in the aromatic ring. The side chain has an optimal length of six links, essential for its efficacy (Fig. 1). It should be noted that these chemical characteristics differentiate it from other antidepressants with a tricyclic nucleus, from a structural point of view as well as in its pharmacodynamic and clinical features and therefore, tianeptine is not a tricyclic antidepressant. This different structure makes tianeptine an antidepressant with an experimental and clinical pharmacological profile that differs from that of other antidepressants.
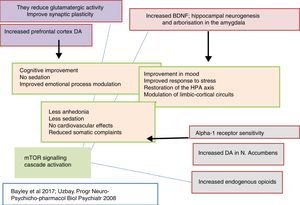
Thus, clinically, tianeptine shows an additional anxiolytic effect as well as an antidepressant effect in common with other antidepressants, but with the difference that does not involve sedation. Furthermore, tianeptine improves the cognitive component and acts on the somatic symptoms of depression, digestive symptoms in particular. Since the comorbidity of depression, anxiety and somatisation is extremely frequent, the use of tianeptine results in a broader spectrum of clinical efficacy.5,15
Pharmacokinetic aspects of tianeptineTianeptine is well absorbed from the gastrointestinal tract and, since it avoids first hepatic step metabolism, has high bioavailability.16 Food has a modest effect, with a half-hour increase in Tmax and a 25% decrease in Cmax. The magnitude of these effects seems to be of little clinical significance.17 Plasma protein binding” is around 95%, especially to albumin.18
Tianeptine is rapidly eliminated, mainly by the kidney, and has a short half-life (T1/2) of about 2.5h. Its main metabolites are tianeptine analogues with a C5 and C3 side chain and an N-methylated-derivative.16 A prolongation by 1h of the elimination half-life was observed in kidney failure and elderly patients.16,18 The half-life of the C5 metabolite almost tripled in patients with chronic renal failure. Since this C5 metabolite is pharmacologically active, the dose of tianeptine should be reduced in patients with kidney failure. Tianeptine and its metabolite C5 are not very dialysable, and therefore haemodialysis is an ineffective way of accelerating their elimination in the event of overdose.18
Acute administration of alcohol reduced the absorption rate of tianeptine and its plasma levels by approximately 30%, but did not affect the pharmacokinetics of the C5 metabolite. The pharmacokinetic parameters of tianeptine in individuals with alcoholic hepatitis did not significantly differ from those of healthy controls.16,19
In the elderly (72–81 years) the pharmacokinetic profile of tianeptine was similar to that of young volunteers, but levels of C5 metabolite were higher, suggesting the need for a reduction in dose.20 In this regard, the dosage guidelines for those aged over 70 years is two doses per day (25mg), instead of the three doses (37.5mg) recommended for adults.21–23
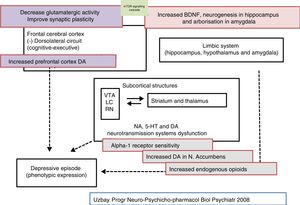
Pharmacodynamic aspects of tianeptineAccording to the Anatomical Therapeutic Chemical classification system, tianeptine belongs to the N06AX group (Psychoanaleptics [N06]; Antidepressants [N062]; Other antidepressants [N06AX]), and has differential pharmacodynamic features that determine a totally different profile to that of other antidepressants, manifested from its receptor ratio,24,25 to its clinical features, through markedly different aspects on mechanisms of transduction, neuroprotection, etc.1,2 Tianeptine is an atypical, non-monoaminergic antidepressant, since, unlike most of the antidepressants marketed, it does not inhibit the reuptake of monoamines (serotonin, noradrenaline and dopamine) in the central nervous system.24,27
Chronic administration of tianeptine does not alter the concentration or affinity of more than one hundred receptors studied: noradrenergic receptors α1A, α1B, α2A, α2B, α2C, β1, β2; dopaminergic receptors D1, D2, D3, D4, D5; serotoninergic receptors 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5A, 5-HT6, 5-HT7; glutamatergic receptors NMDA, AMPA, kainate; benzodiazepine receptors; GABAB receptors.5,24,27 The only effect observed with tianeptine on these receptors is increased sensitivity of the α1 adrenergic receptors, which only manifests in chronic treatment.28
In addition, tianeptinde does not inhibit the activity of the monoamine oxidase enzyme systems MAO-A and MAO-B, as has been demonstrated in the hypothalamus, the hippocampus and cerebral cortex.29 This receptor and enzyme cleansing of tianeptine, observed at an experimental level, might transfer to the clinical situation, since it is widely acknowledged that many of the adverse effects of the conventional antidepressants are due to their affinity for different types of receptors or the inhibitory capacity of MAO.
In relation to serotonin, the SSRIs, dual antidepressants such as venlafaxine, duloxetine, and devenlafaxine, as well as many of the ADTs, inhibit serotonin reuptake, thereby increasing its rate in the synaptic cleft. This was the basis for the serotoninergic hypothesis of depression.2,6 Based on initial experiments carried out with tianeptine, it was postulated that this antidepressant, unlike the SSRIs, reduced the rate of extracellular serotonin, by increasing serotonin reuptake,30,31 which had some resonance because it called into question the serotonergic hypothesis of depression. However we now know that tianeptine does not increase the number of serotonin transporters or its levels of mRNA in the dorsal raphe nucleus. Furthermore, tianeptine has low affinity for these serotonin transporters, therefore it seems unlikely that it can increase reuptake.32 These contradictory data were due to the technical limitations of the time, and have now been overcome.5,33 In fact, subsequent research studies have demonstrated that tianeptine, following both acute and prolonged administration, neither significantly increases nor reduces extracellular levels of 5-HT in the cortico-limbic structures of conscious rats. Moreover, electrophysiological studies show that sustained administration of tianeptine does not modify the spontaneous firing rate of serotonergic neurones in the dorsal raphe,34 or modify the activity of the post-synaptic 5-HT1A receptors, or the efficacy of the pre-synaptic autoreceptor antagonists to increase the function of the synaptic terminal. In addition, the administration of tianeptine, both acute and chronic, did not modify extracellular serotonin levels, whereas under identical conditions they increased after administration of paroxetine.34
In light of the above, it seems that tianeptine does not modify extracellular serotonin levels, and therefore neither increases nor reduces serotonin re-uptake. Therefore serotonergic mechanisms do not seem to be involved, or at best, are insufficient to explain the antidepressant efficacy of tianeptine.26
Given that tianeptine acts on mechanisms that are well differentiated from those of the SSRIs, its efficacy in patients who are resistant to these antidepressants could be explained, as shown in clinical studies.35 Likewise, this differential approach to depression could explain its efficacy in Parkinson's patients,36 whose motor symptoms can be worsened by the SSRIs, in patients with post-traumatic stress disorder,37,38 and in the elderly.39
In addition, patients treated with SSRIs suffering sexual dysfunction as an adverse effect have been observed to improve, maintaining the antidepressant effect.40
Furthermore, the only effect detected with tianeptine, after systemic administration, on monoamine levels is a moderate increase in dopamine release in the nucleus accumbens and, at higher doses, in the prefrontal cortex.29 However, it is not clear how tianeptine reinforces dopamine transmission at this level, since it lacks affinity for the dopamine transporter, and therefore does not inhibit dopamine uptake, which completely differentiates it from amineptine.41 In fact, tianeptine, following acute or chronic administration, does not modify reuptake of dopamine or noradrenaline by rat cortical synaptosomes or hippocampus. Presumably, independent actions of the dopamine neurons could be involved, such as their tonic inhibition by GABAergic and glycinergic terminals, in the moderate dopaminergic release by tianeptine.13,42
Tianeptine: opioidergic mechanisms and antidepressant effectThe opioid receptors have been implicated to a varying extent in depression and its treatment. However some experimental data that implicate the Delta receptors in anxiety and depression43 have not been accompanied by efficacy in human clinical trials.44 Similarly, some studies investigating the role of Kappa receptor modulators in depression – as these receptors are the target of the dynorphins released by stress45 – have not yielded clinically relevant results.46
Tianeptine, and its active MC5-metabolite, behave as Mu, and to a lesser extent, Delta opioid receptors, without affecting the Kappa receptor.47,48 A noteworthy fact is that tianeptine, despite its affinity for these receptors, did not cause tolerance, as it does not lose its efficacy after continued treatment, or physical dependence, as after suppression or administration of naloxone no withdrawal syndrome was observed. These two characteristics clearly differentiate tianeptine from the other opioids, such as morphine. In all likelihood, tianeptine, despite acting on the Mu receptor, will trigger mechanisms of neuronal transduction that are different to those induced by morphine and other opioids, since these cause tolerance and withdrawal syndrome after they are discontinued. This differential fact has raised the possibility that antidepressants could be developed that act on the transduction mechanisms modified by tianeptine.5,48
On the other hand, we should point out that the potency of tianeptine on Mu receptors is 6 times less than that of morphine, and that addiction to the antidepressant has been limited to a few isolated cases,48 essentially polydrug-dependent individuals. In fact, tianeptine does not cause tolerance or withdrawal syndrome, two inescapable characteristics of opioids that cause dependence. Furthermore, along the same lines, it has been demonstrated that supratherapeutic doses of tianeptine showed low potential for abuse.49 Thus, tianeptine significantly decreased morphine tolerance and suppressed the withdrawal syndrome caused by the administration of naloxone in the mouse, and therefore it can be claimed that tianeptine, despite having affinity for the opioid receptors, behaves as an inhibitor of morphine tolerance and dependence. In fact, these authors point out that the administration of tianeptine could benefit patients who require prolonged administration of morphine.50 These pharmacological properties of tianeptine have aroused great interest, not only in the field of depression, but also for the potential development of Mu receptor agonists that modify transduction mechanisms in a similar way to tianeptine, in order to obtain analgesics that cause less dependence than the classic opioids.48
Another fact to indicate that tianeptine has differentiating characteristics with opioids is that, without modifying the analgesic activity of morphine, it is capable of antagonising the experimental respiratory depression induced by opioids. Because tianeptine does not behave as a Mu receptor antagonist, respiratory depression antagonism is thought to be indirect and secondary to positive AMPA receptor modulation, as we will discuss later.51 As per Bailey et al.,5 the antidepressant actions of tianeptine in mice rely on the Mu receptor, which could in turn activate mTOR signalling to enhance associated AMPA signalling.
Furthermore, Kalkman and Feuerbach52 described how some antidepressants inhibit inflammation and microglial M1 polarisation. In experimental depression/stress models they showed that chronic stress caused M1 polarisation of microphages and microglia. In this sense, in the M1-polarised form, the microglia and macrophages generate reactive oxygen species and nitrogen radicals to eradicate microbial pathogens, but they can also irreversibly oxidise tetrahydrobiopterin (BH4), which generates neopterin, a recognised biomarker of depression. Moreover, this BH4 is a critical cofactor for the synthesis of dopamine, noradrenalin and serotonin, and its loss could explain some depressive symptoms.
In addition, it is known that the Mu receptor agonists have anti-inflammatory properties and that these receptors are expressed in human monocytes, macrophages and microglia,53,54 increasing their expression with M2 polarisation of the cytokine, IL4.55 Morphine, as an Mu receptor agonist, inhibits the release of TNFα by macrophages induced by bacterial membrane lipopolysaccharides,56 while it reduces phagocytic activity and the production of reactive oxygen species and prostaglandins.53 These data enable us to postulate that tianeptine, by stimulating the Mu opioid receptors, might limit M1 polarisation of macrophages and microglia, diminishing the process.
In conclusion, we can point out that tianeptine's relationship with the Mu opioid receptors is characterised by triggering transduction mechanisms that are probably different to those of the conventional opioids, and this might be indirectly involved in the mechanism of its antidepressant action. The special action of tianeptine on opioid receptors might explain the release of dopamine in the limbic system and also participate in the modulation of glutamatergic mechanisms. In addition, tianeptine, by stimulating Mu opioid receptors, could limit M1 polarisation of macrophages and microglial cells.52 It is interesting to note that the properties of tianeptine as a very unique opioid might contribute to its antidepressant properties. In fact, Nobile et al.57 highlight that the opioid agonists might potentially reduce the risk of worsening suicidal ideation at the start of antidepressant treatment. In this regard, tianeptine was associated with a lower risk of suicidal ideation compared to noradrenalin and serotonin reuptake inhibitors or TCA, in the first six weeks of treatment.57
Tianeptine: glutamatergic mechanisms and antidepressant effectA set of experimental data has been accumulated in recent years that support the role of glutamatergic functionality, the brain's main excitatory neurotransmitter, and its multiple and varied ionotropic and metabotropic receptors, in the pathophysiology of depression. Likewise, evidence has accumulated to indicate that some points of the functional glutamatergic system might be the target of the therapeutic effect of some antidepressants.14,26,58
Evidence of high levels of glutamate in depression and the antidepressant efficacy of antiglutamatergic agents suggests that the condition might be associated with glutamatergic hyperfunction. Glutamate is the brain's main excitatory neurotransmitter, where it is widely and ubiquitously distributed. In the human brain, the glutamatergic neurones project their axons from the cortex to subcortical regions, such as the locus coeruleus, raphe nuclei, and the substance nigra, where they modulate monoaminergic pathways, which allows the glutamatergic system to participate in a wide range of physiological functions, including memory and cognition. In addition, glutamate participates in physiological functions relating to neurotrophicity and neuronal plasticity. However, excess glutamate, as produced in conditions of continuous stress, can become a neurotoxic substance. On the other hand, the presence of glutamate in a number of brain structures relating to the pathophysiology and symptoms of depression supports its role in these symptoms. In fact, there are many data that indicate that glutamatergic hyperfunction could be at the root of depression.6,26
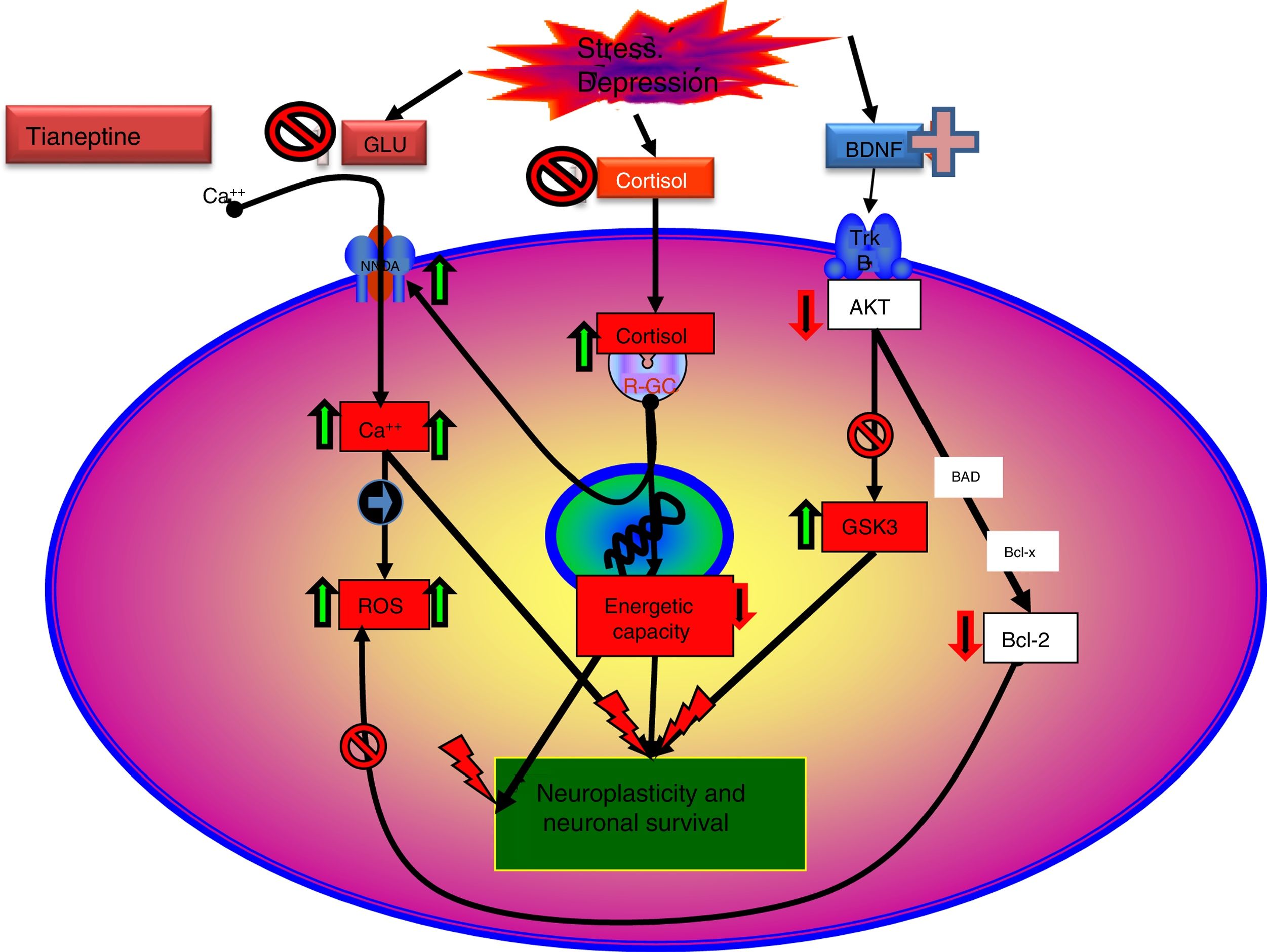
Indeed there is evidence to indicate that glutamatergic functionality might be associated with depression, and its modulation with the antidepressant effect. Therefore, chronic stress and depressive states are usually associated with NMDA receptor hyperfunction59 and the antagonists of these receptors tend to behave as antidepressants. In this sense, there is evidence that normalising and stabilising glutamatergic neurotransmission are the objectives of potentially effective drugs for the treatment of depressive disorders. Some antidepressants attenuate the release of glutamate in the corticolimbic structures,59,60 and the long-term administration of different groups of antidepressants such as the TCAs SSRIs or MAOIs, modifies the density or sensitivity of the NMDA receptors61 downwards by desensitising the glycine site.
On an experimental level, a set of NMDA receptor antagonists have shown antidepressant effects.62 The administration of ketamine, an anaesthetic that noncompetitively antagonises NMDA receptors has shown anxiolytic and antidepressant effects in animal models of anxiety and depression. There is current evidence to show that ketamine behaves clinically as a fast-acting antidepressant, while reducing autolytic ideas. Ketamine's rapid antidepressant effect substantially differentiates it from the monoaminergic antidepressants, which require weeks before their antidepressant effect starts.10
Tianeptine seems to modulate some aspects of glutamatergic functionality. Therefore, acute stress increases the levels of extracellular glutamate in the basolateral nucleus of the amygdala, this increase is normalised by the administration of tianeptine. In addition, tianeptine modulates the changes in the expression of glial glutamate transporters induced by stress. It is important to point out that the glial transporter is the most important mechanism for completion of glutamate activity in the excitatory synapses; therefore normalisation of the glutamate transporter by tianeptine would reduce the “toxic” levels of extracellular glutamate.63
The NMDA receptor is extremely complex in its functioning since, in addition to glutamate, it is essential that glycine is present for its channel to open. In this regard, it has been proven that a glycine-B site antagonist (L-701.324) enhances the experimental antidepressant effect of tianeptine whereas, by contrast, d-serine, a glycine-B site agonist antagonises the antidepressant effect of tianeptine. These results suggest an important participation of the glycine-B site of the NMDA receptor as a target of the experimental antidepressant effect of tianeptine.64 It should be noted that several antidepressants such as imipramine, fluoxetine and reboxetine, also desensitise the glycine-B site65; this speaks in favour of this site as a target for antidepressant drugs.
Electrophysiological studies in the rat have shown that repeated stress enhances excitatory post-synaptic currents (EPSC), dependent on the NMDA receptor, in the synapses of association with CA3 pyramidal neurons. When rats were treated with tianeptine, the ratio of the currents mediated by the NMDA receptor to those mediated by AMPA/kainite normalised. It is considered that this normalisation of the functional ratio between both receptors might contribute to the neuroprotective properties of tianeptine against stress.66
Without discounting the role of NMDA receptors, there is growing evidence that AMPA receptors play an important role in the pathophysiology and treatment of depression.60,67 On the one hand, the existence of a high density of AMPA receptors in structures responsible for mood regulation, such as the prefrontal cortex and the hippocampus, point to their importance in the pathophysiology of affective disorders. And on the other hand, positive AMPA receptor modulators have been shown to behave as antidepressants, in animal depression models, with efficacy comparable to that of theTCAs or SSRIs.60,68 Furthermore, the AMPA receptor is involved in most rapid transmission processes and seems to be a central mechanism in synaptic plasticity, a phenomenon that is becoming increasingly important in the pathophysiology of depression.68
Likewise, the phosphorylation of AMPA receptors, which, as we shall mention, involves their activation and sensitisation, appears to play an important physiological role. In the hippocampus, some physiological states, such as sleep69 or learning,70 could alter the phosphorylation of the GluA1 subunit of these receptors, thereby increasing their activity and expression, which appears to indicate an important role in the coding and processing of memory.71,72
It is known that tianeptine enhances the function of the AMPA receptor through two interrelated mechanisms. On the one hand, enabling the phosphorylation of the GluA1 subunit, and on the other, increasing the synthesis and traffic of the AMPA receptors towards the membrane.73,74 Both mechanisms increase the sensitivity of the AMPA receptors.72 Phosphorylation of the GLuA1 subunit in the PKA site (serine 845) increases the efficiency of the channel controlled by the AMPA receptor by increasing peak response open probability,75 whereas phosphorylation of the CaMKII/PKC site (serine 831) increases AMPA channel conductance.76 In addition, phosphorylation of the GluA1 subunit of the AMPA receptor increases the concentration of receptors on the surface.77,78 In short, by facilitating phosphorylation of the GluA1 subunit, tianeptine increases the efficacy of the AMPA receptor ensuring that there are more channels on the surface of the membrane and that they are more effective.73 In addition, it has been possible to demonstrate that induced phosphorylation on the GluA1 subunit of the AMPA receptor by tianeptine does not alter the recovery of the receptor, therefore, with sustained stimulus it does not desensitise and maintains its response.75
It has been demonstrated that, in accordance with the electrophysiological effects of tianeptine, this antidepressant, facilitating the phosphorylation of the GluA1 subunits of the AMPA receptor, facilitates the neuroprotective function attributed to this receptor. It should be noted that treatment with other antidepressants, such as fluoxetine or imipramine, also increases the phosphorylation of the AMPA receptor,27 and that this molecular process is closely related to synaptic plasticity. These data suggest that AMPA receptors are linked to the therapeutic and neuroprotective effects of some antidepressants, especially tianeptine.26,69
It is noteworthy that the experimental antidepressant activity of tianeptine, in the forced swimming depression model in mice, depends on the presence of phosphorylated serine residue in the GluA1 subunit of the AMPA receptor, and therefore the GluA1 subunit of the AMPA receptor could be the initial target responsible for the antidepressant and neuroprotective effect of tianeptine.26,27 The importance of the AMPA receptor in the antidepressant effects of tianeptine seems evident, since blocking of these receptors by NBQX inhibits their antidepressant activity.64
Acute stress increases the extracellular levels of glutamate in the basolateral nucleus of the amygdala, an effect that is inhibited by tianeptine, which supports the hypothesis that tianeptine's mechanism of antidepressant action involves the normalisation of glutamatergic tone in the amygdala and in the hippocampus. By contrast, fluoxetine increases the levels of glutamate in this nucleus both in the presence and in the absence of stress. This fact highlights another difference in the mechanism of action of tianeptine compared to that of the SSRIs.79
Glutamatergic functionality is currently a target of maximum interest in research and the future development of new antidepressant drugs.80 Tianeptine can be considered the present, since its ability to block the glycine-B site in the NMDA receptor participates in its antidepressant activity, and potentiating through phosphorylation of the GluA1 subunit of the AMPA receptor. The interaction of tianeptine with these two glutamatergic receptors appears to play a key role in its antidepressant action.64
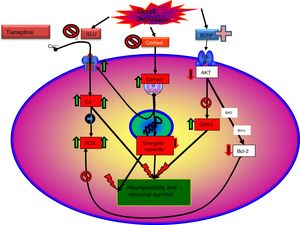
Tianeptine: neurotrophins and antidepressant effectIt is known that stress decreases neurotrophins of brain-derived neurotrophic factor (BDNF), an important mediator of neuronal plasticity, mainly in the hippocampus, which might contribute to the atrophy and neuronal loss observed in key areas of the brain of some patients with depression. By contrast, antidepressants increase the phosphorylation of cAMP response element binding protein (CREB), thereby increasing the expression of neurotrophic factures such as BDNF.81
In this regard, there are experimental data that indicate that tianeptine might counteract the neurodegenerative effects of stress by increasing the genetic expression of various neurotrophic factors. Indeed, tianeptine increases BDNF levels and nerve growth factor (NGF) levels in both the hippocampus82 and the amygdala,83 which manifests morphologically, electrophysiologically and behaviourally, improving amygdalar plasticity.83 In addition, experimentally, chronic treatment with tianeptine demonstrated its efficacy and in parallel increased BDNF levels in the prefrontal cortex and hippocampus.
On the other hand, in rats subjected to continuous stress, the administration of neuroinflammatory agents (lipopolysaccharides) caused a decrease in BDNF levels in the amygdala and hippocampus, and an increase in adrenal corticosterone accompanied by depressive symptoms. In this model, administration of the antidepressants desipramine and fluoxetine, as controls, reversed the changes caused by lipopolysaccharide. Tianeptine administration exhibited a powerful neuroprotective effect, which was accompanied by an increase in BDNF and a decrease in corticosterone, superior to that shown by desipramine (noradrenaline reuptake inhibitor) and fluoxetine (SSRI).85
Intermittent cold stress in rats is an experimental model of fibromyalgia accompanied by decreased levels of BDNF and phosphorylated CREB in the hippocampus and the prefrontal cortex, along with a significant increase in corticosterone levels. In this experimental model, tianeptine administration antagonised hyperalgesia while normalising BDNF, phosphorylated CREB, and corticosterone levels. The authors point out that tianeptine might have efficacy in depression that goes with pain, and if it behaves the same way in clinical practice it could be a pharmacological alternative to the difficult treatment of fibromyalgia.86
In light of the above, the antidepressant effect of tianeptine has been associated with an increase in BDNF levels in areas of the CNS associated with depression, therefore it is postulated that this neurotrophin could be involved in the antidepressant effect of tianeptine.
Tianeptine: inflammation and antidepressant effectIn the complex pathophysiology of depression there is increasing evidence of its association with inflammatory processes involving various immunomediaries. In the model of depression caused by prenatal stress in rats, alterations in chemokines (CXCL12 and CX3CL1) are produced, as well as in their receptors (CX3CR), which are the main regulators of inflammatory processes in the brain. Alterations in the chemokine axis tend to be normalised by the chronic administration of certain antidepressants (venlafaxine, fluoxetine, and tianeptine) in the hippocampus, while tianeptine and venlafaxine also normalised the level of CXCL12 in the frontal cortex. Furthermore, tianeptine normalised levels of CX3CL1 and its CX3CR1 receptor, both in the hippocampus and in the frontal cortex of the brain. On the other hand, both fluoxetine and tianeptine reduced the level of the chemokine receptor CXCR7 in the frontal cortex. In addition, tianeptine modulated brain levels of TGF-beta in the animal model of depression induced by prenatal stress. In this model tianeptine has shown antidepressant and anxiolytic efficacy.87
Furthermore, inflammation and oxidative stress are intimately related to each other and can therefore participate in the pathophysiology of depression. In the experimental model of depression in rats, subjected to chronic unpredictable stressful stimuli for 40 days, alterations occurred in the production of reactive oxygen species (ROS), and nitrogen species (RNS) which, under normal conditions, are controlled by antioxidant elements such as catalase and superoxide dismutase (SOD). Stress increased levels of malondialdehyde, an oxidative marker, which was normalised by the administration of tianeptine. In addition, this antidepressant reversed the decrease in activity of SOD in the hippocampus, prefrontal cortex, amygdala, and nucleus accumbens of rats subjected to stress. Likewise, it was observed that the antioxidant activity of catalase decreased in the prefrontal cortex hippocampus, prefrontal cortex, and nucleus accumbens of stressed rats, this effect was reversed by tianeptine. These data suggest that tianeptine has an antioxidant action by increasing the activity of antioxidant agents, such as catalase and superoxide dismutase. Moreover, these findings imply new targets for the development of new antidepressant agents, while helping to understand the pharmacological activity of tianeptine, particularly with regard to its ability to attenuate oxidative stress.88
Tianeptine: function of the hypophyseal-adrenal hypothalamusBoth major depression and continuous stress are associated with hyperactivity of the hypothalamic–pituitary–adrenocortical (HPA) axis. In clinical practice, depressed patients have elevated plasma and CSF cortisol levels that indicate hyperfunction of this axis secondary to desensitisation of central corticosteroid receptors.89 This hyperfunctioning of the HPA axis is characterised by hypersecretion of corticosteroids, both at an experimental and a clinical level.90 Indeed, various types of experimental stress constitute models of depression characterised by neuroendocrine disorders, essentially disordered control of the HPA axis, and behavioural disorders in the experimental animal. In addition, prenatal administration of corticosteroids in rodents causes neuroendocrine and behavioural symptoms similar to those observed in patients with depression.91 Therefore, it is postulated that poor adaptation of the HPA to stress is involved in triggering the onset of subsequent depression.92,93
Many antidepressants have been shown to be effective in experimental models of stress due to depression. Tianeptine is able to antagonise behavioural impairments that cause stress in experimental animals, while it reduces the hyper-response of the HPA axis.94,95 Furthermore, some morphological neuronal changes, such as dendritic atrophy in the rat hippocampus, secondary to elevated corticosterone due to stress, can be prevented by the administration of tianeptine.96
HPA dysfunction has recently been associated with abnormal activity of the prefrontal cortex in depressed patients with functional impairments of the hippocampus. Tianeptine attenuated HPA axis hyperactivity, and secondary neuronal impairments observed in the hippocampus and the prefrontal cortex.97 In addition, it has been demonstrated that the continuous use of tianeptine tends to “normalise” the HPA system in situations of stress, which facilitates better performance in stressful environments. Treatment with tianeptine inhibits modifications in corticosterone-induced gene transcription and reduces stress-induced plasma levels of ACTH and corticosterone. Tianeptine also reduces the baseline activity of the neurones that produce corticotropin-releasing factor (CRF) and their sensitivity to stress.98 All these findings indicate a bidirectional relationship between stress and depression through HPA axis hyperfunction. Tianeptine, like some other antidepressants, normalising the functionality of the HPA axis can counteract this negative interaction.
Tianeptine: mitochondrial dysfunction hypothesis in depressionIn addition to the classic monoaminergic hypothesis of depression,4 there are sufficient arguments to consider that, at least in some types of depression, HPA axis hyperfunction, immune system dysfunction or a neurotrophic factor deficit3 may be involved.
Recent data enabled a new theory to be elaborated: “mitochondrial dysfunction hypothesis in depression”,99,100 which does not exclude, but rather complements, the neuroprotection hypothesis. According to this hypothesis, anomalous function of the mitochondria would result in a decrease in the production of ATP, with impairment of calcium homeostasis, together with an increase in the production of free radicals and oxidative stress, which would facilitate the initiation of the apoptosis process.101 Moreover, mitochondria can control neuroplasticity in the CNS, including neural differentiation and growth, dendritic remodelling and neurotransmitter release.102 On the other hand, there is a relationship between mitochondrial functions and certain epigenetic processes of significance in the pathogenesis of some mental disorders, including depression. Therefore, there are arguments for considering that poor mitochondrial function is crucial in the metabolic disorders that can lead to depression.99
According to this hypothesis, changes in morphology and in the mitochondrial respiratory chain have been observed in depression, and an increase in polymorphisms and mitochondrial DNA mutations,103 together with hyporegulation of nuclear mRNA and mitochondrial proteins. In addition, changes in the number of mitochondria and their distribution in areas of the brain relating to the pathogenesis of depression have been observed by electron microscopy.104 It is noteworthy that in the brains of depressive patients high energy phosphates and pH are decreased.105
The influence of antidepressants on mitochondrial function could support this theory, particularly tianeptine, which seems to participate in modifying some of the mechanisms involved in the mitochondrial dysfunction hypothesis in depression. In this regard, it has been possible to prove in vitro that tianeptine inhibits the activity of mitochondrial complex I,101 while, in chronic administration to adult rats subjected to maternal deprivation, it modulates levels of mitochondrial complex I and complexes II and III of the respiratory chain, while reducing creatinine concentration in the amygdala and hippocampus.84
Stress in pregnant rats causes impairments to cerebral mitochondrial biogenesis and the mitoproteoma of descendents when they become adults.100 As we mentioned earlier, this experimental model of depression is very well characterised and is accompanied by behavioural impairments and neuroendocrine and immune abnormalities characteristic of depressive states.106 Chronic administration of tianeptine to adult rats, descendents of pregnant rats subjected to stress, is accompanied by an experimental antidepressant and anxiolytic effect. These behavioural effects of tianeptine were accompanied by an increased expression of isocitrate dehydrogenase (IDH), a limiting step in the Krebs cycle, together with an increase in 2-oxoglutarate dehydrogenase complex (OGDHC)), essential for the generation of energy in the form of NADH and succinyl CoA,107 whose deficit has been related to the pathogenesis of depression.108 Furthermore, tianeptine in the hippocampus enhances the expression of succinate dehydrogenase (SDH), which is the most important marker of mitochondrial efficiency, producing ATP in the Krebs cycle and respiratory chain. These actions of tianeptine, at the level of the hippocampus and prefrontal cortex, are associated with an antidepressant and anxiolytic effect in these experimental animals.107
In conclusion, we can state that tianeptine causes changes in the microproteome that could be implicated in its antidepressant effect, since they are the opposite to those produced by stress in models of depression or anxiety.100
Tianeptine: neuroplasticity, cytoprotective and antidepressant effectSo far we have mentioned a series of aspects related to the pathophysiology of depression and the role of tianeptine that enable us to relate the effects of the antidepressant with mechanisms of neuroprotection and neuroplasticity. This relationship is not surprising, given the evidence associating depression with loss of volume of the hippocampus. In fact, depressive disorder could be a manifestation of the degeneration of this area. The relationship between stress, depression and neuronal degeneration, especially in the CA3 pyramidal neurons of the hippocampus have been extensively described and reviewed. Neurobiological tests indicate that affective disorders, such as major depression, are characterised by dendritic contraction in the neurons, loss of glial cells and diminished neuronal neuroplasticity.26
Therefore, it is not surprising that one of the most meticulously studied hypotheses on the antidepressant efficacy of tianeptine are the effects it exhibits on the neuroplasticity of certain areas of the brain related to depression. In fact, according to the literature, tianeptine appears to be the most extensively researched antidepressant in relation to its neuroprotective effect against stress-induced neuronal deterioration. Some authors consider tianeptine the only antidepressant capable of inducing neurogenesis,109 although there are other agents that also show this capacity, to varying degrees.110
The more marked effects of tianeptine on neurogenesis appear to be due to the increased phosphorylation of glutamate receptors, specifically the GluA1 subunit of the AMPA receptor, and intracellular kinases dependent on them. In fact, phosphorylation of intracellular kinase is the main signal for the growth and structural stability of the dendrites. The calcium/calmodulin dependent protein kinase II (CaMK II) thus participates in the stabilisation of these structural changes.111
Tianeptine appears to have intense effects on neuroplasticity in the granular layer of the dentate gyrus and in the adjacent subgranular area of the hippocampus. These areas are of great significance in experimental models of depression.112 Treatment with tianeptine positively affects cytogenesis and cell apoptosis, and consequently the entire regeneration process of the adult dentate gyrus. In addition, tianeptine, in accordance with its antiapoptotic effect, prevented the reduction of stress-induced brain levels of N-acetylaspartate, producing a recovery in the overall work of the neuronal networks of the hippocampus by improving their dendritic and axonal terminals and glial function. This effect indicates that tianeptine reverses the reduction in neuroaxonal density and function caused by stress.113
As we have discussed, tianeptine has the ability to “normalise” stress-altered glutamatergic neurotransmission by antagonising the function of the NMDA receptor and enhancing the activity of the AMPA receptor. These effects have been linked to the ability of the antidepressant to prevent or reverse structural and cellular changes in the brain caused by stress. In the hippocampus and amygdala, tianeptine prevents stress-induced dendritic atrophy, promotes neurogenesis, abolishes apoptosis, and normalises metabolite concentrations and the volume of the hippocampus.61
Moreover, depressive disease is associated with changes in the volume of the amygdala and its ability to connect with the hippocampus. Chronic stress has been shown to cause a decrease and atrophy of hippocampal dendritic arborisation. However, an increase in dendritic arborisation of pyramidal and stellate neurons occurs at the level of the basolateral nucleus of the amygdala, which are presumably excitatory projection neurons.114 It is interesting to note that tianeptine prevents excessive dendritic arborisation of stress-induced basolateral excitatory neurons,1 an effect that has been associated with a preventive effect of experimental anxiety in male rats. Recently, tianeptine has shown anxiolytic properties in different experimental models involving stress, fear or aggressiveness. All of these experiments indicate that the actions of tianeptine on stress-induced behavioural changes may be due to the morphofunctional changes it induces in the amygdala.26 In addition, it has been shown that tianeptine can reverse the adverse effects of stress on hippocampal processing without adversely affecting the synaptic function of the amygdala in animal models of stress.115
Tianeptine, in addition to normalising the rate of neuronal regeneration in the brain, might have cytoprotective effects in animals subjected to chronic stress. In this regard, it has been shown that tianeptine reduces stress-induced apoptosis in the granular layer and the subgranular area of the dentate gyrus, probably in non-neuronal cells. These effects affect both the hippocampus and the temporal cortex. In both areas tianeptine had an antiapoptotic effect in stressed animals as well as in those not subjected to stress.116 In addition, chronic administration of tianeptine has a cytoprotective effect at cortical level against proinflammatory cytokines, and inhibits the negative influence of cytokines on mood.117
As discussed, the interaction of tianeptine with the NMDA receptor glycine modulatory site could modify target of rapamycin (mTOR) signalling and affect the distal mediators such as BDNF and the AMPA receptor.5
Therefore, tianeptine has the capacity to control, in a general way, the negative effects of chronic stress, and also participates in cytoprotection by inhibiting the harmful actions of cytokines. These mechanisms are potentially responsible for its antidepressant activity.
Tianeptine and procognitive effectCognitive deficits, such as decreased attention, memory and problem-solving ability, are common in patients with depressive disorders. The mechanisms by which this cognitive deficit can occur in depression are diverse.118 Disruption of the HPA axis, mediated by loss of hippocampal volume, together with changes in the amygdala could underlie some of the cognitive deficits that accompany major depression. By contrast, prevention or restoration of morphological and functional changes in the hippocampus would have a pro-cognitive and memory-enhancing effect.
Intense acute stress in rats is known to produce stimulation which is directed from the hippocampus towards the frontal cortex that causes cognitive impairment, characteristic of situations of prolonged stress. Tianeptine, by acting on the intrinsic circuits of the hippocampus, rapidly reverses the inhibitory effects of stress at prefrontal level, which could influence its procognitive effects. These data suggest that impairment of prefrontal cortex-related memory, observed in individuals under stress, could be improved by treatment with tianeptine.119,120
Several experimental studies show the procognitive effect of tianeptine. In various models of depression, the stress caused by predators results in impaired memory, which is accompanied by changes in synaptic plasticity at hippocampal level. In this experimental model tianeptine improves spatial memory, enhances attention focussed on the cat in response to a significant stimulus, and produces positive effects on learning, working memory and reference memory in rodents, while increasing vigilance in rats and monkeys.26
Overall, it can be interpreted that the procognitive effect and the improvement in memory brought about by tianeptine would be due to the partial restoration of the functional plasticity of the hippocampal networks, which would secondarily result in better functioning of the HPA axis. This restoration of the hippocampal networks, which are deficient in depression, can be explained by the acceleration of neuradaptive mechanisms produced by tianeptine.5,26
In light of the available experimental data, tianeptine has particularly favourable effects on cognitive functions, which may be mediated through its upregulation of neurogenesis, of restoration of the functional hippocampal networks, which in turn would result in better functioning of the HPA axis.
Tianeptine as an experimental anxiolytic agentDepressive disorders exhibit high rates of comorbidity with anxiety; therefore the co-existence of an antidepressant with anxiolytic properties is extremely interesting. In this regard, there is experimental and clinical evidence of a reduction in anxiety by tianeptine. In acute treatment, tianeptine counteracts the anxiogenic effect of benzodiazepine and alcohol withdrawal, and is effective in experimental models of social interaction anxiety.119,120 As mentioned, the action of tianeptine on the amygdala would support its anxiolytic efficacy.
Furthermore, chronic tianeptine has been shown to prevent stress-induced aggression.121 In addition, Burghardt et al.122 demonstrated that 3-week treatment with tianeptine was superior to the SSRIs in reducing conditioned fear. In addition, after acute administration, tianeptine, unlike the SSRIs, was not anxiogenic.115
Gassaway et al.47 recently postulated that the antidepressant and anxiolytic actions of tianeptine could participate in its weak agonist effect on Mu and Delta opioid receptors, which could be the initial interrupter of the abovementioned glutamatergic system modulation.
ConclusionsThe literature review sought to gather published information on the mechanism of action of tianeptine, declaring that the information contained therein has been reflected in an honest and transparent manner, as suggested by Catalá et al.123,124 and Dal-Ré125 for the publication of scientific articles.
Tianeptine is an antidepressant with an atypical and differential pharmacological profile that has challenged the monoaminergic hypothesis of depression. It also questions whether all antidepressants must implement monoaminergic mechanisms as a basic and common element of their therapeutic effect (Figs. 2–4).126,127
The generally accepted biological basis of depression, a monoaminergic deficit, primarily serotonergic and/or noradrenergic, cannot explain the antidepressant activity of tianeptine. It is now known that this antidepressant does not exert its effect by modifying monoamine levels. Therefore, there must be mechanisms beyond monoaminergic regulation to explain its clinical properties.
The neurobiological characteristics of tianeptine seem to involve a dynamic interaction between the numerous neurotransmission systems and their ability to restore normal neuroplasticity in limbic regions, while reversing many of the deleterious central effects caused by stressful situations. In this regard, modulation by tianeptine of glutamatergic synaptic transmission, that plays crucial roles in practically every function altered by stress, appears to be the current hypothesis to explain its therapeutic efficacy.
The effects of tianeptine on the glutamatergic system may represent the most proximal target of the cascade of biochemical events that underlie its antidepressant efficacy. Thus, there appears to be strong evidence that tianeptine enhances the function of the AMPA receptor, affecting the intraneuronal transduction cascade. Because tianeptine has rapid onset of antidepressant action (7–14 days), it would be interesting to examine the mechanisms underlying this effect. It is known that tianeptine enhances the function of AMPA receptors relative to that of the NMDA receptors in neuronal circuits related to the pathophysiology of depression.
Irrespective of the subtleties of tianeptine's mechanism of action, there is no doubt that it is an antidepressant that acts differently to the other antidepressants in our current pharmacological arsenal, which, bridging the translational gap between basic and clinical research, can have a translation from a therapeutic perspective.
In this sense, from a clinical perspective, the main characteristic of tianeptine is its antidepressant effect, which is accompanied by an additional anxiolytic effect without causing sedation. Because the comorbidity of depression with anxiety is extremely common, the use of tianeptine in these patients results in clinical efficacy of a broader spectrum. In addition, a set of preclinical data indicate that it would be interesting to study, from a clinical perspective, the effects of tianeptine on depression caused by situations of social stress, in which the SSRIs have little efficacy.
On the other hand, during recent decades the monoamine reuptake inhibitors have been the mechanism most used for treating depression. It is possible that this repetition in these mechanisms is the reason why a significant proportion of patients do not respond to these agents. Tianeptine is a new pharmacodynamic strategy that avoids direct action on the synaptic aminergic terminal to overcome this universal mechanism of the current antidepressants.
Tianeptine as a modulator of glutamatergic mechanisms shows great promise for the treatment of depression. There is growing evidence that many patients with depression will benefit from antidepressants that explore, among others, glutamatergic mechanisms.26,47
Conflict of interestsC. Alamo has collaborated in studies on training and information through presentations at scientific events for healthcare workers sponsored by Adamed, Exeltis, Janssen, Lundbeck, Normon, Otsuka, Pfizer, Sevier, Zambon.
P. García García works in the Medical Department of Exeltis Healthcare S.L.
The other authors have no conflict of interests to declare.
Please cite this article as: Alamo C, García-Garcia P, Lopez-Muñoz F, Zaragozá C. Tianeptina, un abordaje farmacológico atípico de la depresión. Rev Psiquiatr Salud Ment (Barc). 2019. https://doi.org/10.1016/j.rpsm.2018.09.002