Iatrogenic hyperprolactinaemia (IHPRL) has been more frequently related to some antipsychotic drugs that provoke an intense blockade of dopamine D2 receptors. There is a wide variation in clinical practice, and perhaps some more awareness between clinicians is needed. Due to the high frequency of chronic treatment in severe mental patients, careful attention is recommended on the physical risk. IHPRL symptoms could be underestimated without routine examination.
MethodologyAn intense scientific literature search was performed in order to draw up a multidisciplinary consensus, including different specialists of psychiatry, endocrinology, oncology and internal medicine, and looking for a consensus about clinical risk and detection of IHPRL following evidence-based medicine criteria levels (EBM I–IV).
ResultsShort-term symptoms include amenorrhea, galactorrhoea, and sexual dysfunction with decrease of libido and erectile difficulties related to hypogonadism. Medium and long-term symptoms related to oestrogens are observed, including a decrease bone mass density, hypogonadism, early menopause, some types of cancer risk increase (breast and endometrial), cardiovascular risk increase, immune system disorders, lipids, and cognitive dysfunction. Prolactin level, gonadal hormones and vitamin D should be checked in all patients receiving antipsychotics at baseline although early symptoms (amenorrhea–galactorrhoea) may not be observed due to the risk of underestimating other delayed symptoms that may appear in the medium term. Routine examination of sexual dysfunction is recommended due to possible poor patient tolerance and low compliance. Special care is required in children and adolescents, as well as patients with PRL levels >50ng/ml (moderate hyperprolactinaemia). A possible prolactinoma should be investigated in patients with PRL levels >150ng/ml, with special attention to patients with breast/endometrial cancer history. Densitometry should be prescribed for males >50 years old, amenorrhea >6 months, or early menopause to avoid fracture risk.
La hiperprolactinemia iatrogénica (HPRLi) se ha descrito con más frecuencia con algunos antipsicóticos, dependiendo de su capacidad de bloqueo de los receptores de dopamina D2. Existe gran heterogeneidad de la práctica clínica y posiblemente falta de concienciación sobre este problema entre los médicos. Dada la elevada frecuencia con la que los pacientes con enfermedad mental grave reciben antipsicóticos de forma prolongada, se precisa vigilar posibles riesgos en su salud física. La HPRLi y sus síntomas pueden pasar desapercibidos si no se investigan rutinariamente.
MetodologíaSe realiza una revisión profunda de la literatura para elaborar un consenso multidisciplinario con psiquiatras junto a otros especialistas (de Endocrinología, Medicina Interna y Oncología) con el fin de consensuar los riesgos clínicos y los métodos de detección más adecuados de la HPRLi de acuerdo con los distintos niveles de evidencia científica (I-IV).
ResultadosLos síntomas a corto plazo incluyen amenorrea, galactorrea y disfunción sexual (descenso del deseo y disfunción eréctil por hipogonadismo secundario). A medio-largo plazo y relacionado con la disminución de estrógenos, se pueden inducir baja masa ósea (osteopenia y osteoporosis), hipogonadismo, menopausia precoz, incremento del riesgo de algunos tipos de cáncer (mama y endometrio), aumento del riesgo cardiovascular, alteraciones en la inmunidad, dislipidemia y disfunción cognitiva, entre otros. La petición de niveles de PRL debería realizarse al inicio del tratamiento en todos los pacientes que reciben antipsicóticos, aunque no se observen síntomas precoces (amenorrea, galactorrea) por el riesgo de subestimar otros síntomas que pueden aparecen a medio plazo. Se aconseja determinar también niveles de FSL, LH, testosterona y vitamina D. Se recomienda explorar rutinariamente la función sexual, ya que puede ser un síntoma mal tolerado que podría conducir al abandono del tratamiento. Se propone un especial cuidado en niños y adolescentes, así como en pacientes con PRL>50ng/ml (intensidad moderada), revisando periódicamente si existe hipogonadismo o disfunción sexual. En los pacientes con PRL>150ng/ml debe descartarse siempre un prolactinoma radiológicamente y se debe prestar especial atención a posibles antecedentes de cáncer de mama o endometrio. Se aconseja realizar densitometrías en varones > 50 años y en mujeres con amenorrea > 6 meses o menopausia precoz para detectar osteoporosis y evitar riesgo de fracturas por fragilidad.
There is growing interest in the clinical community in the importance of patients with severe mental illness; this is especially true with respect to metabolic syndrome and the general increase in mortality of these patients.1,2 This undoubtedly includes a real problem often undervalued by clinicians, hyperprolactinaemia (HPRL) provoked by some antipsychotics (APs), and its better and better recognised consequences on the overall health and quality of life of the patient.3–5
Other metabolic and endocrine effects of AP treatment have dominated the literature over the last few years.6 However, HPRL is one of the most common adverse effects associated with APs, especially with most of the typical APs and some of the second-generation ones such as risperidone, paliperidone and amisulpride.7,8 Such effects are detected in 30–70% of the patients that take them continuously7,9,10 and in up to 80% of those that take them on a short-term basis, especially in young populations.11
The increase in prolactin (PRL) serum levels can cause patient distress (sexual dysfunction), be stigmatising (gynaecomastia in males), put overall health at risk and affect functionality and treatment satisfaction. HPRL is associated with short-, medium- and long-term consequences on the organism, and there are many questions as to its effects.12,13
Adherence to treatment can be compromised, as recent studies have revealed; 1 of the main reasons for abandonment in patients treated with APs was poor tolerance, more often than the lack of efficacy, and accompanied by other factors such as the lack of awareness of disease.14 Specifically, the side effects related to HPRL were linked to a drop in adherence levels (OR: 0.69; P=.034) in a survey given to 876 patients with schizophrenia.15 Risk of abandoning treatment due to sexual dysfunction has been described (36% for men and 20% for women) using a specific questionnaire validated for patients with schizophrenia, such as the Psychotropic Related Sexual Dysfunction Questionnaire (PRsexDQ), which measures sexual dysfunction and patient predisposition to abandon the drug because of this adverse effect.16,17
Medium- and long-term silent effects (often not evaluated by doctors if they are not brought to mind) include osteoporosis, low oestrogen levels, greater cardiovascular risk and possible increased risk of breast cancer and endometrial cancer, among others. These effects appear following many years of chronic treatment and there is more and more evidence of them, which we revise in this consensus.
It becomes of great interest, following the latest studies published, to ascertain and adequately assess the true scope of the consequences from chronic iatrogenic HPRL from APs in the severely mentally ill, as well as the possible clinical and therapeutic approaches to such cases.
To do so, we carried out this consensus, which reports the conclusion of internally prestigious Spanish experts in Psychiatry, Endocrinology, Internal Medicine, Oncology and Rheumatology, gathered to study the true scope of the updated scientific evidence available at the moment and to establish recommendations as to the detection and clinical management of Istrogenic HPRL caused by short-, medium- and long-term treatment with APs.
MethodThe current consensus was carried out in 3 phases: (1) review of the scientific literature, (2) a later in-person round table to attempt to achieve consensus among the experts, and (3) a review by all the authors of the final conclusions until complete agreement was reached. The final result was agreed to by all the experts: consensus was reached on the 4th draft of the document. The entire process took place between March 2014 and March 2015.
Review of the scientific literatureThe scientific literature was subjected to an in-depth review to ascertain the published evidence available on AP-associated HPRL, its causes, prevalence and clinical consequences, as well as on questions related to its detection and therapeutic strategies.
The literature search was performed through PubMed and the Cochrane databases, using the terms “prolactin” and “antipsychotic” or “hyperprolactinaemia” included in the title and abstract, in English and non-limited year of publication. This search returned 3341 articles that, after filtering based on content relevance, were reduced to 267. This initial part, focused on clinical risks and HPRL detection, consisted of 179 articles.
Consensus round tableThere was an initial meeting of a group of 18 experts in Psychiatry (15), Endocrinology (1), Internal Medicine (1) and Oncology (1) chosen by the consensus coordinator and coming from different areas of Spain, all having scientific impact and being from the clinical, research and academic environment. This face-to-face meeting was held in Madrid in 2 morning and afternoon sessions. The consensus was sponsored by the Spanish Association of Sexuality and Mental Health ([Spanish acronym] AESexSAME) and included scientific sponsorship by the Spanish Society of Biological Psychiatry ([Spanish acronym] SEPB). The logistics sponsor did not intervene in any of the scientific parts of the consensus, or in the preparation of this manuscript.
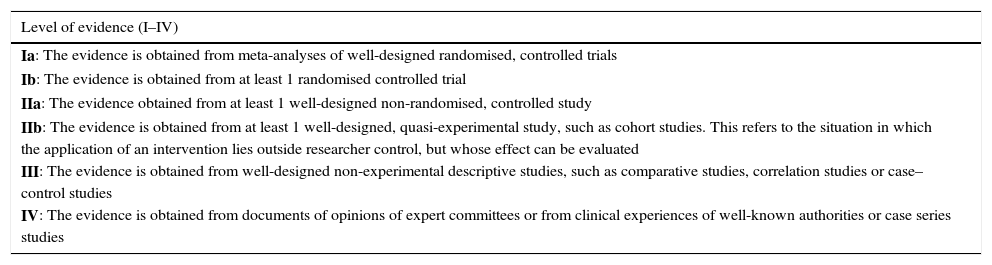
Each of the experts took responsibility to review beforehand the selected literature related to his or her area of clinical experience and investigation, and to prepare a report on the most relevant conclusions. These reports were presented successively in the in-person round table debate at the consensus conference held in Madrid in March 2014. After the in-person debate session, e-mails were exchanged until the review and refinement of the conclusions concluded and they were approved unanimously. To present the results, the levels of evidence and the US Agency for Health Research and Quality recommendation (Table 1) served as reference. After that, a discussion took place on the evidence presented by each speaker to reach a consensus on it and on the final content that should be included in this article. The coordinator prepared an initial overall manuscript, which was revised by all the consensus members, who then e-mailed their comments to the coordinator. Four consecutive manuscript revisions were needed to reach an overall consensus during the 2014–2015 year. All the article authors agreed to the conclusions.
Levels of evidence used and grades of recommendation (according to the US Agency for Health Research and Quality).
| Level of evidence (I–IV) |
|---|
| Ia: The evidence is obtained from meta-analyses of well-designed randomised, controlled trials |
| Ib: The evidence is obtained from at least 1 randomised controlled trial |
| IIa: The evidence obtained from at least 1 well-designed non-randomised, controlled study |
| IIb: The evidence is obtained from at least 1 well-designed, quasi-experimental study, such as cohort studies. This refers to the situation in which the application of an intervention lies outside researcher control, but whose effect can be evaluated |
| III: The evidence is obtained from well-designed non-experimental descriptive studies, such as comparative studies, correlation studies or case–control studies |
| IV: The evidence is obtained from documents of opinions of expert committees or from clinical experiences of well-known authorities or case series studies |
| Grades of recommendation (A–D) |
|---|
| A: Based on a category of evidence I. Extremely recommendable |
| B: Based on a category of evidence II. Favourable recommendation |
| C: Based on a category of evidence III. Favourable but inconclusive recommendation |
| D: Based on a category of evidence IV. Consensus of experts, lacking adequate research evidence |
Prolactin (PRL) is a polypeptide hormone secreted by the lactotroph cells of the anterior pituitary in a pulsing manner, following a circadian rhythm.18,19 Maximum PRL level is reached 4hafter sleep onset, while the minimum level occurs 6hafter waking up.20 PRL receptor expression has been demonstrated not only in the breast, but also in different cells and tissues (that certainly play a role in the iatrogenia of chronic HPRL) such as brain, endometrium, ovary, testicle, prostate, pancreas, liver, kidney, intestine, skin, lung, miocardium, lymphoid cells, adipocytes and endothelial cells.21,22 Likewise, paracrine-type local PRL secretion (not dopamine dependent) is known to exist in some of these tissues.
Circulating PRL levels are the result of a complex balance of stimulating and inhibiting factors, mediated by various neurotransmissors and hormones, of hypothalamus, pituitary and peripheral origin, which act directly or indirectly on the lactotroph cells.2,23 Their main function is initiating secretion of breast milk, and the most important physiological stimuli for PRL secretion are nipple suction, the rise in oestrogen levels, sleep, sexual intercourse and stress. Thyroxine-liberating hormone, histamine and serotonin, among others, also stimulate PRL secretion.25–27 The secretion of PRL is inhibited by the dopamine secreted by the tuberoinfundibular neurons, upon linking to the D2 receptors of the lactotroph cell membranes.19,28 Antipsychotics that strongly block D2 impede the natural brake on PRL and it rises, depending on the intensity of the D2 blockage. Other factors such as GABA, somatostatine, acetylcholine and norepinephrine also have an inhibiting effect, although it is much weaker than that of dopamine.27
Stress increases PRL secretion through a mechanism depending on dopamine secretion. The physiological importance of this fact is unknown, but there are some hypotheses that it would have an immunomodulating-type protective effect.19,29 PRL would have a proliferative and antiapoptotic effect on the lymphocytes by increasing the production of cytokines, immunoglobulins, granulocytes and macrophages and would interact with glucocorticoids in specific tissues; in turn, this would reduce their immunosuppressor effect, normalising the immune response and optimising the adaptive response to stress.30,31
Normal prolactin levelsNormal PRR levels are considered to be lower than 530miU/l (equivalent to 25ng/ml in Spanish laboratory figures) for females and 424miU/l (20ng/ml) for males; 1ng/ml is equivalent to 21.2mIU/l. The clinical repercussion of HPRL is of great relevance, as it can be underestimated if its different levels of severity are ignored.
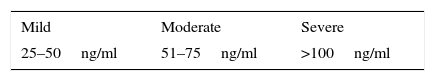
As for these levels of severity, mild HPRL is considered to be below 1000miU/l (50ng/ml), moderate HPRL is between 1000 and 1600miU/l (51–75ng/ml) and severe HPRL, above 2120miU/l (>100ng/ml) (Table 2).32 Taken overall, HPRL is more frequent in females than in males.33
Levels of severity of hyperprolactinaemia.
| Mild | Moderate | Severe |
|---|---|---|
| 25–50ng/ml | 51–75ng/ml | >100ng/ml |
The “severity” of PRL levels refers to the fact that, in general, the degree of hypogonadism is proportional to the degree of PRL increase. Although the levels can hint at the aetiology of the HPRL, there is significant overlap in the figures among different aetiologies; this is especially true among those that are normally seen in microprolactinomas and in those secondary to drugs.
The levels of PRL can be increased pathologically through various causes (Table 3). However, the most frequent non-physiological HPRL cause is drug exposure and, among all of them, the APs are the main culprits by far with respect to all the others.34
Hyperprolactinaemia aetiology.
| Drug related |
| Neuroleptics/antipsychotics (+++) (strongest and most frequent cause) |
| Tricyclic antidepressants, IMAO, ISRS, ISRSN |
| Benzodiazepines |
| Anticonvulsants |
| Anaesthetics |
| Opioids |
| Estrogens: oral anticontraceptives |
| Antyhipertensives: verapamil, methyldopa |
| H2 antihistamines |
| Prokinetics/antiemetics |
| Cholinergic agonists |
| Hypothalamus-pituitary processes |
| Pituitary disease: prolactinomas, acromegaly, plurihormonal adenomas, surgery, radiotherapy, trauma, hypophysitis |
| Hypothalamic disease or compression of the pituitary stalk: tumours (craniopharyngioma, germanium, meningioma, metastasis, Rathke cyst), granulomas, infiltrative diseases, radiotherapy, trauma with stalk section |
| Other processes |
| Primary hypothyroidism (via thyroxine stimulation) |
| Chronic renal failure |
| Hepatic cirrhosis |
| Polycystic ovary syndrome |
| Neurogenic: thoracic trauma, herpes zoster |
| Idiopathic hyperprolactinaemia |
Antipsychotic-related HPRL is certainly the most frequent. It is produced by D2 dopamine receptor blockage, which produces loss of the dopaminergic prolactin inhibitory factor (PIF) in the lactotroph cells in the anterior pituitary. This explains why the APs with a greater D2 occupation index produce higher and more frequent PRL elevations. This is the case of risperidone and its 9-hydroxymetabolite paliperidone,35–37 considered the second-generation APs that cause HPRL most often, with levels even higher than those of haloperidol.34 Another action mechanism involved is their ability to cross the blood–brain barrier: risperidone and paliperidone remain the longest outside of the barrier due to their low liposolubility; consequently, they act for a longer period in the tuberoinfundibular pathway, provoking HPRL.38 The relationship between the concentration of APs in brain and plasma (B/P ratio) using PET is a recent biomarcador for the risk of HPRL. The ratios are lower for risperidone and sulpiride than for olanzapine and haloperidol.39
In contrast to other atypical APs, HPRL secondary to risperidone appears dose-dependent and the increase is produced rapidly, remaining stable during the time the treatment lasts.3,13
In a literature review, frequencies of 69% are shown with risperidone, reaching up to 100% for women.7 Amisulpride is also associated with high HPRL indexes, of up to 100% in some observational studies40 or review studies.41 This effect seems to be independent of the dosage and occurs at dosages as low as 50mg/day.42 Multiple data, although of diverse methodological designs and with varying levels of evidence, indicate HPRL associated with first generation APs43: haloperidol44 and flufenazina provoke the most HPRL45 in observational and prospective studies.
The stable link between HPRL and the use of risperidone, paliperidone, amisulpride and the majority of the first-generation APs has led to this group being called “prolactin-raising” or hyperprolactinemic APs in the literature. A study on 158 treatment-resistant patients that compared the PRL levels of various APs estimated that 60–100% of the women and 40–80% of the men treated with a hyperprolactinemic AP presented HPRL.8
In contrast, other atypical APs, called “prolactin-sparing” APs in the literature, such as aripiprazole, asenapine, clozapine, quetiapine and ziprasidone present a better profile with respect to limited PRL increase5,46–49 (Level of Evidence [LE]: Ib).
Olanzapine (OLZ) produced HPRL less often than risperidone (90% in men and 87% in women with risperidone) in a long-term study in first episodes and remained elevated with risperidone (70%) 1 year after beginning treatment.13 In a controlled clinical trial with placebo vs haloperidol, OLZ provoked HPRL in a dose-dependent manner: 38% (15mg/day), 24% (10mg/day) and 13% (5mg/day). The frequency of HPRL was 72% with haloperidol and 8% with placebo. There was a normalisation at 6 weeks. The frequency of HPRL with OLZ was comparable to placebo50 (LE: Ib). With respect to the most recently approved APs such as iloperidone, they would have a profile similar to that of clozapine, while lurasidone would be closer to that of olanzapine and ziprasidone.51
In short, in a recent meta-analysis on efficacy and tolerability of 15 APs, paliperidone and risperidone have been shown to be the APs most related to HPRL. Aripiprazole and quetiapine have the best HPRL profile (LE: Ia).52
In “drug-naive” patients with various, predominantly observational, designs, it has been observed that up to 20% of the individuals with risk of psychosis and up to 70% present PRL increases when the first episode is diagnosed,13,53–56 that it is more frequent in women57 and that it is not associated (in theory) with age or with the severity of the clinical symptoms. However, these initial increases are much milder than those provoked by APs. This slight initial PRL increase in psychotic patients can be explained by factors other than treatment, such as stress linked to the experience of the disease, the stimulation of the PRL-liberating peptide, anomalous inflammatory-type processes in the serotoninergic pathway or even the discomfort caused by the injection for extracting blood.58 There is strong evidence that the cause of persistent moderate–severe HPRL is the AP treatment itself19,27,28 (LE: Ib). In an open randomised comparative study between risperidone, haloperidol and olanzapine,13 mild HPRL was found in 58.5% of the cases before commencing AP treatment. After beginning the treatment, the rate of moderate–severe HPRL was 60% at 3 months and 36% at 1 year; the rate was greater in the risperidone group (71%) compared with haloperidol (20%) and olanzapine (16%) (LE: Ib).
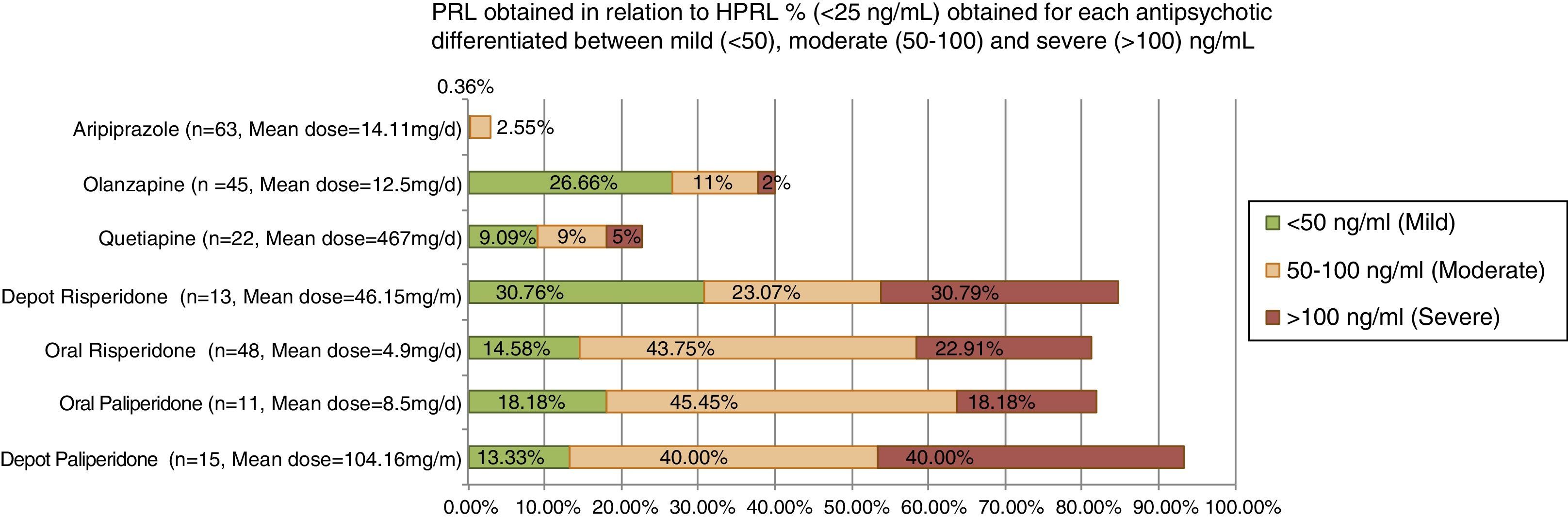
In a recent observational and transversal study, AP-induced HPRL was divided into mild/moderate/severe based on the criteria of Serri, 2003 (Fig. 1). Severe HPRL (>100ng/ml) was found in 40% of patients with paliperidone; 30.8% with depot risperidone; 23% with risperidone and 18% with depot paliperidone at standard clinical dosage.59
In the light of the available evidence, there are solid tests of the direct relationship between HPRL and some AP treatments (risperidone, paliperidone, amisulpride and haloperidol, principally) and that these APs dominate as form as moderates/severe forms over the mild; consequently, the clinical impact on each patient and the medium- and long-term consequences must be known (LE: I and II).
Clinical consequences of antipsychotic-induced hyperprolactinaemiaThe clinical consequences of the effects of HPRL associated with AP treatment in patients with severe mental illness is the same as that indicated for healthy individuals with HPRL. The clinical manifestations clinics of HPRL are not always visible in the short term: it is often a case of adverse effects rarely reported spontaneously by the patients (sexual dysfunction) or that can become evident some time after beginning treatment5,60 (LE: IIb).
The clinical consequences of pathological increases of PRL levels can reveal themselves in the short, medium and long term. The most immediate effects occur on sexual and gonadal function and on the breast, in both females and males. The long-term consequences are to a great extent conditioned by the permanence of these effects over time. However, they are also likely to be a consequence of other direct pleiotropic effects of PRL on different organs and tissues. It is important for clinicians to remember that, even if it is asymptomatic in the short term, sustained HPRL in patients treated with APs is usually clinically relevant because of the possible long-term complications.41 The silent effect having the greatest evidence is the risk of hip fracture associated with osteoporosis due to HPRL (LE: II).61
In the next section, the various clinical manifestations of chronic HPRL are analysed in greater detail.
HypogonadismHPRL interferes with the pulsatile secretion of the gonadotrophin-releasing factor, inhibiting pituitary secretion of LH and FSH and causing hypogonadism, that is, a decrease in oestrogen in women and a decrease in testosterone in men.27,62–64 Clinical repercussion is conditioned by sex, age, the intensity of the HPRL and its duration.32,63,65 The clinical manifestations are more evident and happen earlier in females than in males, but they represent potential health risks for both sexes.
In children and adolescents, HPRL-induced hypogonadism causes a delay in puberty. The main symptoms are amenorrhea in females and eunuchoid habitus, with small, bland testes, in males.66,67 That is why it is important to assess the morbidity from HPRL of the APs prescribed for children and adolescents.
In both adult females and males, the most frequent consequence of HPRL-induced hypogonadism is sexual dysfunction (SD), reviewed in the specific section on SD further on and which includes low sex drive and problems as in excitation and orgasm.
In adult premenopausal women, HPRL causes menstrual disorders that, depending on the intensity of the hormonal increase and individual sensitivity, will feature anovulation, shortening of the luteal phase and oligomenorrhea or amenorrhea or infertility.27,32,68 Chronic HPRL can also appear with symptoms of hyperandrogenism such as acne, hirsutism and weight increase; these are mediated by a secondary increase in dehydroepiandrosterone secretion from the adrenal glands and by a decrease in the protein that carries sexual hormones, with the consequent increases in the levels of free testosterone.69,70
Infertility can be one of the greatest frustrations for some young patients who plan to become pregnant and for their partners. The desire for maternity is seldom explored by physicians before initiating APs with HPRL and, consequently, the infertility associated can interfere with the life plans of the patients.17
In the male, HPRL-induced hypogonadism causes decreased sperm production and can even reach infertility.64 It also causes the appearance of general symptoms secondary to the decreased testosterone levels such as astenia, fatigability and decreased muscle mass and body hair.27,68,71
Sexual dysfunctionA significant percentage of patients in treatment with APs present SD (38–68% depending on the measurement systems)17,72,73 In addition, unfortunately, less than 38% report this spontaneously, so it tends to remain unknown.17 The frequency has been shown to be higher when questionnaires validated for SD detection in these patients, such as the PRsexDQ-SalSEX4 scale are used (LE: IIb).
There are many studies with varying LE that show the association between APs hyperprolactinemic APs (mainly risperidone) and the appearance of SD. This adverse effect is less frequent with other APs such as aripiprazole, quetiapine, ziprasidone and even olanzapine.4,17,74–77 These data have been analysed in a recent metaanalysis78 and in a review article specifically on this subject.79
The most frequent manifestations of HPRL-induced SD are lowered libido17,80,81 and dysfunction in excitation/orgasm17,82 (LE: IIb). The combination of lowered libido with erectile dysfunction is the most frequent pattern in males with HPRL, which sexually active patients generally tolerate poorly. Delay in or lack of orgasm can be associated with this pattern or, more rarely, present alone. In females, there can be dyspareunia secondary to vaginal dryness.
SD causes a deterioration in the quality of life of the patients, especially in the males,83–85 and substantially affects adherence to treatment measured with specific questionnaires validated for sexual dysfunction in schizophrenia (PRSexDQ-SALSEX)4 or by using surveys.86 According to data obtained from patients with schizophrenia selected with inclusion and exclusion criteria, up to 36% of the male patients indicate that they quit their treatment with APs or were thinking about doing so because of SD, a figure that falls to 19% in the females.17 That is why it is important to assess SD presence and intensity systematically in patients treated with APs (LE: IIb).
SD also tends to deteriorate patients’ interpersonal relationships and cause strong difficulties in achieving and maintaining emotional ties.17,87,88 This undoubtedly undermines the clinical state and adds to the isolation and worsening of the negative symptoms. Males in particular suffer from this problem and are at double the risk of non-adherence than women are.17,86
Turning to the physiopathogenic mechanism of AP-induced SD, in spite of the fact that there is a certain controversy about whether PRL is the only factor involved, there are solid data on its role in this adverse effect.89–91 The SD would be greatly conditioned by the situation of hypogonadism secondary to HPRL, although there are also data in favour of a direct HPRL effect on the libido and on erectile dysfunction80,92 (LE: IIb), probably mediated by the dopaminergic system.93 At local level, the erectile dysfunction would be related to endothelial dysfunction secondary to decreased nitric oxide (NO) production from inhibition of endothelial NO synthetase94,95 and vasoconstriction from beta 2-adrenergic effect.96 Nevertheless, in a study specific to SD in patients with HPRL secondary to the use APs, no direct relationship was found with PRL serum levels.97 Consequently, PRL increase is unlikely to be the only factor responsible for SD in patients treated with APs, although surely one of the most involved.
Effects of hyperprolactinaemia on the breastGalactorrhea is one of the possible manifestations of HPRL on the breast, with an estimated prevalence of between 10% and 50% in patients with schizophrenia treated with antipsychotics.98,99 It is more common in premenopausal women than in males and postmenopausal women, because oestrogen and progesterona need to prime the mammary tissue adequately.100,101
Another of the effects of HPRL on the breast in males is uni- or bilateral gynecomastia, present in up to 20% of the patients according to the series102 (LE: IV). Gynecomastia has been found in males treated with risperidone and normal PRL values, which indicates hypersensitivity in the PRL receptor.103
Galactorrhea and gynecomastia are a consequence of the proliferative effect of PRL on the epithelial cells, and of the stimulation of the synthesis of DNA, casein, lactalbumin, lactose and free fatty acids.24
The possible proliferative tumour effect of PRL on the breast is discussed further on, in the section on HPRL and risk of cancer; HPRL would stimulate tumour proliferation, as well as migration and survival of these cells in the tumours with strong expression of the membrane receptor for PRL.104
Effects of hyperprolactinaemia on boneIn patients with psychosis, bone mass is lowered in relation to their age and sex,105,106 especially in the younger population. In addition, the prevalence of osteoporosis is high107 with an increased risk of fracture61,108 (LE: IIa). There are various risk factors involved, such as a sedentary life style and a greater degree of smoking and alcohol consumption109,110 or low levels of vitamin D.105,111 However, the most relevant factor and the one most studied in the last few years is long-term treatment with APs and its relationship with increased PRL levels, even in young people112 (LE: IIb).
There are solid data on the association of HPRL in patients with psychosis, decrease in sexual hormone and bone mass levels and increased risk of fracture (LE: IIa). In a recent longitudinal study, the evolution of bone mineral density (BMD) measured by dual energy X-ray absorptiometry (DXA) densitometry was analysed in patients treated with APs for a mean 26.7±13.8 years (standard deviation), and a tendency to remain more elevated was observed in the group of the non-hyperprolactinaemic APs.113 In another study of cohorts in twins, there was a greater reduction of the BMD in females with schizophrenia treated with APs compared with their twins, which was more notable in the group of PRL-raising APs114 (LE: IIb). In an extensive case–control study, the relationship between treatment with hyperprolactinaemic APs and hip fracture was also seen, both in males (OR 2.6; IC 95%: 2.43–2.78) and in females (OR 1.93; IC 95%: 1.78–2.10)61 (LE: IIb). A transversal study in postmenopausal females with schizophrenia reported that the females that took PRL-raising APs had lower levels of sexual hormones and BMD compared with the olanzapine group.112 In another transversal study with 402 patients diagnosed with schizophrenia and AP treatment longer than 3 months, it was found that 25% of the females and 33% of the males had low bone mass, associated with high PRL levels in the males.115 An increase in bone remodelling markers related to treatment length was observed and, in males, to a drop in testosterone. A decrease in radius BMD was also reported in another transversal study with 74 males with schizophrenia treated with APs, of which 87% presented HPRL correlated with low levels of FSH, LH and estradiol.116 Likewise, there is a negative correlation between treatment length and bone mass.
It is universally admitted that a deficit in sexual steroids constitutes the basic physiopathological mechanism by which HPRL conditions the decreased in BMD. However, some experimental studies point to a possible direct adverse effect of PRL on osteoblastic function.117 This would explain the finding in some clinical studies of a greater BMD deterioration in amenorrheic females with HPRL compared to normoprolactinemic females with similar estradiol levels and duration of amenorrhea,71 as well as the greater risk of vertebral fractures in males with HPRL, regardless of testosterone values118 (LE: III).
Effect of hyperprolactinaemia and risk of cancerIt seems that certain basically hormone-dependent tumours, such as those of the breast, may be related to the molecular signalling pathway of the PRL receptors. Nevertheless, here are important epidemiological limitations when it comes to establishing the relationship between HPRL associated with chronic AP administration and cellular malignization.119 This is the source of the contradictory conclusions of the more than 20 case–control studies published on this matter, many of them with limited samples and hormone determinations after tumour diagnosis, which do not allow confirmation of whether there was prior exposure to chronic HPRL, or its degree.7 In spite of this, there is growing evidence of a possible relationship between chronic long-term HPRL from AP use and some types of cancer, such as breast and endometrial cancer, among others.
Breast cancerDespite the limitations and the impossibility of having type I levels of evidence or clinical trials on the matter, there are an important number of studies, both experimental and epidemiological, that indicate that the increased PRL plasma levels seem to be related to increased risk of cancer, fundamentally breast cancer, especially in postmenopausal women (LE: III).120–125 In an extensive case–control study published recently, a significant increase in the risk of breast cancer was observed in the group of postmenopausal women with higher PRL levels: the most notable relationship was in the case of lobular carcinoma vs ductal type, and independently of the oestrogen receptor (LE: III).126 In another prospective series of previously nested case–control studies, there was also an increase in the relative risk of breast cancer of 1.7 in the group with the highest PRL levels, increased up to 2.1 times in the case of oestrogen-receptor-positive tumours; however, in these samples, the relationship was independent of the menopausal state, type of tumour and its degree of invasiveness (LE: III).127 A very recent observational study with more than 30,000 women in follow-up for 20 years concluded that the high PRL levels measured in the 10 years closest to the tumour diagnosis were strongly associated with the risk of breast cancer in postmenopausal patients, especially for oestrogen-receptor-positive tumours and metastastic disease (LE: IIb).128
There are even survival studies that indicate that the cases of breast cancer with HPRL prior to beginning antineoplastic treatment are associated with higher rates of therapeutic failure, early recurrence and worse overall survival.129
As for the physiopathogenic bases that would explain this greater risk of breast cancer, the PRL itself would stimulate tumour proliferation, as well as migration and survival of these cells, in tumours with strong expression of the membrane receptor for PRL, which include 95% of mammary tumours.104
Other types of cancer: ovarian and endometrialWith respect to cancer of the ovary and endometrium, different studies describe an increase in circulating PRL levels in the patients that present them130–132 (LE: III). In addition, there is an overexpression of the receptors for this hormone in both types of tumour tissue, which might indicate that PRL has some role in the development of the initial neoplastic transformation, possibly through a potential activation of the Ras oncogene.132
Turning to prostate cancer, contradictory data have been published. However, the most solid LE available seems to indicate that HPRL is related to an equal or even lower risk of developing prostate cancer (LE: IIb–III).133,134
With respect to other types of cancer, in a recent cohort study a positive association was observed between high levels of PRL and mortality from cancer in males in general, although not in females3 (LE: IIb). In the Berinder cohort study,134 a generalised increase in the risk of cancer associated to HPRL was found, fundamentally at the expense of upper gastrointestinal tract tumours in both sexes and of hematopoetica origin in females (LE: IIb). However, the specific risk of breast cancer was not significantly increased in this series.
Hyperprolactinaemia and cardiovascular effectsHPRL has been associated with long-term cardiovascular effects, mainly mediated by sexual steroid deficit and by direct PRL action at cardiovascular level. Recently, in a cohort study that included 3929 males and females followed for 10 years, a positive correlation between PRL levels and cardiovascular mortality was found in both sexes3 (LE: IIb). In women with early menopause, a correlation between PRL levels, blood pressure and artery wall rigidity has been shown; this could indicate an acceleration of the ateroscleretic process with increased calculated risk of cardiovascular mortality at 10 years135 (LE: III).
In vitro studies have shown that PRL per se is capable of modulating inflammatory response136–138 (LE: III) and producing endothelial dysfunction by reducing NO production94–96,139 (LE: IIa). PRL could also stimulate angiogenesis indirectly, as it encourages the synthesis of substances such as endothelial growth factor and fibroblast growth factor,140,141 stimulating the proliferation of smooth muscle cells of the vascular wall and the adhesion of mononuclear cells to vascular endothelium. In addition, PRL has been related to an increase in platelet aggregation142 (LE: III), as well as to increased intima thickness at carotid level, compatible with preclinical aterosclerosis143 (LE: III). Lastly, PRL receptor expression has been demonstrated in macrophages within atheroma plaque.144,145
Hyperprolactinaemia and metabolic effectsThere is also limited evidence that HPRL directly influences lipid and hydrocarbohydrate metabolism.146 The association between HPRL and dyslipidemia has been described137 (LE: III); the PRL receptors are expressed in human adipose tissue,147 where PRL reduces lipoprotein lipase activity and inhibits adiponectin secretion, leading in turn to insulin resistence.148,149In vitro studies have also demonstrated the potential influence of this hormone in the development of the β-pancreatic cells and in the secretion of insulin.150,151 Some clinical trials139,152 carried out with a limited number of patients indicate the existence of insulin resistance in patients with HPRL that improves following treatment with bromocriptine (LE: IIa). Other authors find no correlation between PRL levels and different metabolic syndrome parameters153 or obtain insufficient evidence that the levels of PRL play a causal role as a factor of risk for the development of metabolic syndrome or type 2 diabetes154 (LE: IIb).
HPRL has also been associated with ponderal increase, not always reversible, when PRL levels are normalised through drug treatment155–157 (LE: IV). Factors such as reduced dopaminergic tone, leptin resistance or reduced adiponectine levels have been suggested for its pathogenisis.158
Effects on the immune systemSeveral authors have described an association between HPRL and different autoimmune diseases, such as diabetes mellitus type I, rheumatoid arthritis and systemic erythematous lupus, among others30,159–162 (LE: IV). The physiopathological mechanism could be mediated by the antiapoptotic effect of PRL in the B lymphocytes163,164 and the stimulation of interferon-γ and interleucin-2 production by the T lymphocytes.30
Effects on the central nervous system: cognitive and emotional functionA recent study in a non-psychiatric female population indicates that HPRL could have direct negative effects on cognitive function165 (LE: III), which could originate in low levels of gonad steroids (LE: Ib–IIa).166–169 It has also been observed that in males low levels of testosterone are related with deterioration of the memory and of the visual-spatial abilities170–172 and with a greater risk of demencia173 (LE: IIb). Nevertheless, serian necesarios specific studies using populations with HPRL secondary to APs would be needed to assess this possible cognitive effect cognitivo in relation to this adverse effect.
HPRL has also been associated with some psychiatric alterations, above all in females.174 Greater rates of hostility, anxiety, depression and dysthymia have been reported in patients with hyperprolactinaemia175,176 (LE: III). The mechanism by which they would originate is unclear and is probably related to hypogonadism.69,177
Summary of levels of evidenceHPRL is an adverse effect that is unknown or underestimated by those prescribing APs. The short-, medium- and long-term consequences of HPRL threaten therapy adherence, and can even be severe.
- 1.
PRL after the administration of some APs. The ones producing the highest levels are the typical APs, amisulpride, risperidone and paliperidone; those that produce lower levels are clozapine, quetiapine and aripiprazol (which even lower the prior levels of PRL) (LE: I).
- 2.
There is evidence that HPRL is clearly related to adverse effects on sexuality, fertility, breasts and bone (LE: I and II). There are data that indicate there is a long-term association with increased cardiovascular risk, as well as with some types of cancer, especially in the breast and endometrium (LE: IIb and III).
- 3.
The populations of the elderly and children are more sensitive to the consequences of HPRL. These affect bone formation in the youngest individuals, in whom they should be avoided due to the great risk of low bone mass and, consequently, of osteoporosis and fractures in later stages (LE: II).
Unfortunately, HPRL can frequently go unnoticed by clinicians, giving rise to a lengthy patient exposure to PRL that can have serious health consequences for the patients in the short-, medium- and long-term.4,178
Method of establishing prolactinaemia levelsThe guidelines of the Endocrine Society,21 the Pituitary Society,63 the British Association for Psychopharmacology178 and the Spanish Society of Endocrinology and Nutrition (Spanish acronym: SEEN)68 coincide in that establishing the PRL level in a single extraction is sufficient for diagnosis if the venipuncture is not traumatic. This can be done at any time during the day, with the patient lying down and at least an hour after waking up or having eaten. If the PRL levels are high in this first determination, above all if the increase is slight, they should be confirmed by repeating the extraction on another day, placing a catheter first and with 2 or 3 samples obtained at 15/20-min intervals to minimise the distress and pulsatile effects. If HPRL is confirmed, it is necessary to rule out that it is secondary to the presence of high circulating levels of macroprolactin (larger-sized PRL molecules, generally from the union of PRL to an IgG antibody, or from dimerisation or glycosylation of monomeric PRL). Macroprolactin has a low biological activity and is accumulated by a decrease in its clearance, giving rise to a pseudo-HPRL. It can be present in 20–40% of the patients with HPRL.21,63
Nevertheless, in the interest of a realistic, effective clinical practice for patients in treatment with APs, based on recent evidence, a single collection is sufficient. In a comparative study in patients with schizophrenia treated with APs, several PRL measurements with 2 different techniques (single collection early in the morning vs 3 sequential collections separated by 20min) were performed, and only insignificant variations from the clinical point of view were found (1–3ng/ml).59
The Endocrine Society21 and the SEEN68 recommend that once HPRL has been confirmed and macroprolactin has been ruled out as a cause, the suspicious drug should be suspended if possible for 72h or should be substituted for another of similar efficacy for the underlying patient disease and that does not increase PRL, and the test should be repeated. If the HPRL persists or if it is impossible to suspend the drug, they recommend carrying out a magnetic resonance imaging (MRI) scan on the pituitary to rule out prolactinoma. If performing an MRI scan is impossible, high-definition computed tomography should be carried out.
For early detection of HPRL and its possible associated symptoms, after the consensus meeting, our group proposed a series of recommendations to be borne in mind when dealing with patients with need for prolonged AP treatment, besides informing the patients adequately about treatment safety and tolerability of the drug prescribed for them. The objective of this is to improve their well-being and avoid possible abandonment in the face of adverse effects about which they have not been appropriately informed (for example, SD and infertility). Another point is that the lack of adequate information might have clinical and ethical consequences in future.
Recommendations for the diagnosis of hyperprolactinaemia- 1.
Carry out a detailed initial anamnesis, including the presence of risk factors (principally for osteoporosis and cancer) in personal and family antecedents; presence of prior sexual dysfunction and menstrual/menopausal history; possibility of or wish for current or future pregnancy, history of galactorrhea or gynecomastia; personal and family antecedents of breast cancer or prolactinoma; and antecedents of diagnosed fractures or osteoporosis. All this information should be included in the standard clinical history so that clear confirmation that the patient has been informed in this regard.
- 2.
Patients should be informed of HPRL-associated effects when the condition is detected (LE: I; degree of recommendation [GR]: A); decide together with the patients what the best strategy to maintain efficacy and preserve safety and adherence is.
- 3.
Systematic PRL level determinations are recommended in all the patients with routine, continued AP treatment, as a set of secondary effects are involved that are often silent and seldom communicated spontaneously; these determinations should be performed at basal level and 3 months after beginning (GR: A). In the cases in which HPRL is detected (whether it is asymptomatic or signs-symptoms indicative of HPRL appear), periodic follow-up of serum levels is recommended, depending on the severity of the HPRL. Lack of amenorrhea or galactorrhea is insufficient for considering that there is no clinical manifestation of HPRL, and SD should be explored as often as possible as a marker in sexually active patients. Mild HPRL levels (25–50ng/ml) should be controlled periodically (every 6 months); if there is amenorrhea lasting >3 months, a change in APs should be evaluated due to the risk of osteoporosis (LE: II; GR: B). For PRL levels >150ng/ml, prolactinoma should be ruled out and appropriate imaging tests (MRI scan) performed or the patient should be referred to Endocrinology (GR: B).
- 4.
If an increase in dosage or change in AP is prescribed, it is a good idea to reassess PRL 3 months after the treatment change, especially in the patients treated with an AP related to HPRL (GR: A).
- 5.
If there is HPRL, gonadal hormone status should be assessed jointly, with determinations of FSH, LH and testosterone together with PRL, at basal level and every 6 months to rule out hypogonadism (GR: B).
- 6.
Routinely explore the adverse effects most frequently associated with HPRL, giving special emphasis to those that patients do not share spontaneously such as SD (GR: A). Patients having an active sexual life and those planning pregnancy should be especially chosen to avoid HPRL. Many patients can desire maternity and this should always be remembered, discussed and appropriately assessed together with the clinical reality of each case (GR: B).
- 7.
Possible alterations in the breast and the menstrual cycle in females should be explored both at the beginning of treatment and during follow-up, at least once a trimester. Filling in a specific form for follow-up of all these possible symptoms secondary to HPRL would be of great help (GR: B).
- 8.
In patients in whom HPRL is detected and who have had lengthy exposure to AP treatment (>5 years), perform a bone density examination to assess the risk of fracture and osteoporosis using BMD measurement, preferably with a DXA of the lumbar area and of the area around the femur because of their greater cost-effectiveness (GR: B).
- 9.
Males >50 years and postmenopausal females should be considered to be in a situation of high alto risk of fracture if there is a history of densitometric fragility or osteoporosis (T-score <−2.5). Scales of fracture risk can be used to calculate the absolute risk of osteoporotic fracture calculated by FRAX. Consider a situation to be of high risk for hip fracture if it is >3% or for major fracture if it is >10% from the age of 50 years on (GR: B). As for increased risk of osteoporosis, it is also a good idea to measure vitamin D levels at basal level and every 6 months if there is HPRL (GR: B).
- 10.
Consider the HPRL treatment options based on the type of secondary effect detected, its impact on the patient, careful weighing of the benefits and disadvantages of continuing with the current medication or initiating a new therapeutic strategy (GR: B).
- 1.
Levels above 50ng/ml or with clinical repercussion (including systematic exploration of poorly tolerated SD) require treatment intervention adapted to each specific case, such as lowering the dose, changing AP or adding drugs with known ability to decrease PRL levels (aripiprazole) (LE: I; GR: A).
- 2.
In cases of severe HPRL (>100ng/ml), there should always be an intervention, even if there is no amenorrhea or galactorrhea because of the medium-/long-term risk of osteoporosis, cardiovascular disease and possible increase in the risk factors or breast cancer or endometrial cancer (LE: II; GR: B).
It is a good idea to construct a new intervention model for the problems related to safety, quality of life and treatment adherence that includes in-depth assessment of the secondary effects associated with AP use, effects often not revealed by the patient or minimised by the evaluating physician, but that compromise treatment continuity and patient safety.
FundingThe consensus was funded by the Lundbeck and Otsuka laboratories.
Conflict of interests1. Dr. Montejo has been a consultant or has received fees or research funding from Eli Lilly, Forum Pharmaceuticals, Rovi, Servier, Lundbeck, Otsuka, Janssen Cilag, Pfizer, Roche, Instituto de Salud Carlos III, Junta de Castilla y León and Osakidetxa.
2. Dr. Arango has been a consultant or has received fees or research funding from Abbot, AMGEN, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, the Alicia Koplowitz Foundation, Instituto de Salud Carlos III, Janssen Cilag, Lundbeck, Merck, Spanish Ministery of Science and Innovation, Ministery of Health and Ministery of Economy and Competitiveness, Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Takeda and Schering Plough.
3. Dr. Bernardo has been a consultant or has received fees or research funding from ABBiotics, Adamed, AMGEN, Eli Lilly, Ferrer, Forum Pharmaceuticals, Gedeon, Janssen-Cilag, Lundbeck, Otsuka, Pfizer and Roche.
4. Dr. Carrasco has been a consultant or has received fees or research funding from Lilly, Janssen-Cilag, Pfizer and Lundbeck. He has also participated as a speaker at Lilly, Janssen-Cilag, Lundbeck, Pfizer, Servier and Otsuka.
5. Dr. Crespo-Facorro has been a consultant or has received fees or research funding from Otsuka, Lundbeck and Johnson & Johnson.
6. Dr. Cruz Hernández has been a consultant or has received fees or research funding from Janssen-Cilag, Eisai, Roche Farma, Mundipharmaceuticals, Astra-Zeneca, Glaxosmithkline, Sanofi-Aventis, Celgen, Lundbeck España S.A., Bristol-Myers Squibb, Pfizer, Merck Sharp and Dohne Spain.
7. Dr. del Pino-Montes has been a consultant or has received fees or research funding from AMGEN, Lilly, Lundbeck, MSD, Otsuka and Pfizer.
8. Dr. García Escudero has been a consultant or has received fees or research funding from Janssen, Otsuka, Pfizer, Esteve and Servier.
9. Dr. García has been a consultant or has received fees or research funding from Ferrer, Otsuka, Bristol Myers Squibb, Janssen Cilag, Lundbeck and Eli Lilly.
10. Dr. Gonzalez-Pinto has been a consultant or has received fees or research funding from Almirall, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Servier, Shering-Plough, Solvay, CIBERSAM, Instituto Carlos III, the Basque Government, Stanley Medical Research Institute and Wyeth.
11. Dr. Ana I. Hernández has no conflicts of interest to declare.
12. Dr. Martin-Carrasco has been a consultant or has received fees or research funding from Esteve, Eli Lilly, Glaxo-Smith-Kline, Janssen, Lundbeck, Novartis, Otsuka, Pfizer, Rovi, Servier and CIBERSAM.
13. Dr. Mayoral has been a consultant or has received fees or research funding from Roche, Lundbeck and Jansen.
14. Dr. Mayoral van Son has no conflicts of interest to declare.
15. Dr. Mories has been a consultant or has received fees or research funding from Eli Lilly, Lundbeck, Novo Nordisk and Weber Economía y Salud S.L.
16. Dr. Ros has been a consultant or has received fees or research funding from Otsuka, Lundbeck, Servier, Ferrer, Pfizer and Esteve.
17. Dr. Vieta has been a consultant or has received fees or research funding from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Solvay, Sunovion, Takeda, Teva, CIBERSAM, the Seventh European Framework Programme (ENBREC) and from the Stanley Medical Research Institute.
18. Dr. Isabella Pacchiarotti has no conflicts of interest to declare.
Please cite this article as: Montejo ÁL, Arango C, Bernardo M, Carrasco JL, Crespo-Facorro B, Cruz JJ, et al. Consenso español sobre los riesgos y detección de la hiperprolactinemia iatrogénica por antipsicóticos. Rev Psiquiatr Salud Ment (Barc). 2016;9:158–173.