Schizophrenia is a chronic illness often accompanied by metabolic disorders, diabetes, obesity and cardiovascular problems often associated with unhealthy lifestyles, as well as neuroendocrine problems caused by the disease itself. Lifestyle changes, such as regular physical exercise, have a positive effect on metabolic disorders and mental health, although the molecular changes that occur in this type of patient and how they explain the changes in their response are unknown. This study wants to analyze in a novel way the proteins and molecular pathways involved in critical plasmatic proteins in plasma to reveal the pathways involved in the implementation of physical exercise and the changes that occur among patients who participate in such programs with those who leave.
MethodsTwenty-one patients with chronic schizophrenia underwent a daily, 6-month aerobic training program. We divided them into a group that completed the program (12 patients) and a second group that left the training program (9 patients). The biochemical and clinical data of each patient were analyzed and the proteomic profile of the plasma was studied using ESI-LC–MS/MS.
ResultsProteomic analysis recognizes 21.165 proteins and peptides in each patient, of which we identified 1.812 proteins that varied between both groups linked to the metabolic and biological regulation pathways. After clinical analysis of each patient we found significant differences in weight, BMI, abdominal perimeter, diastolic blood pressure, and HDL cholesterol levels. The main change that vertebrates both groups is the Self-Assessment Anhedonia Scale, where we detected higher levels in the dropout group (no physical activity) compared to the active group.
ConclusionThe benefits of physical exercise are clear in chronic patients with schizophrenia, as it substantially improves their BMI, as well as their clinical and biochemical parameters. However, our study reveals the biological and molecular pathways that affect physical exercise in schizophrenia, such as important metabolic proteins such as ApoE and ApoC, proteins involved in neuronal regulation such as tenascin and neurotrophins, neuroinflammatory regulatory pathways such as lipocalin-2 and protein 14-3-3, as well as cytoskeleton proteins of cells such as spectrins and annexines. Understanding these molecular mechanisms opens the door to future therapies in the chronicity of schizophrenia.
La esquizofrenia es una enfermedad crónica que suele ir acompañada de trastornos metabólicos como la diabetes, la obesidad y problemas cardiovasculares asociados muchas veces a estilos de vida poco saludables, así como a problemas neuroendocrinos ocasionados por la propia enfermedad. Los cambios en el estilo de vida, como la práctica de ejercicio físico regular, tienen un efecto positivo sobre los trastornos metabólicos y la salud mental. Sin embargo, se desconocen los cambios moleculares y su consecuente repercusión en los pacientes diagnosticados con esquizofrenia. Con este estudio se pretenden analizar los cambios moleculares inducidos por el ejercicio físico en pacientes crónicos con esquizofrenia.
MétodosVeintiún pacientes con esquizofrenia crónica fueron sometidos a un programa de entrenamiento aeróbico diario durante 6 meses. El grupo de pacientes se dividió en 2 subgrupos: un subgrupo que completó en su totalidad el programa de entrenamiento (12 pacientes) y un segundo subgrupo que abandonó el programa el primer día (9 pacientes). Se analizaron los datos bioquímicos y clínicos de cada paciente y se estudió el perfil proteómico del plasma mediante ESI-LC–MS/MS de tipo shotgun.
ResultadosEl análisis proteómico reconoció 21.165 proteínas y péptidos diferentes en el plasma de los pacientes. Concretamente, 4.657 proteínas sufrieron variaciones significativas, de las cuales fueron identificadas 1.812 proteínas relacionadas con las vías metabólicas y de regulación biológica. Tras el análisis de los parámetros clínicos en estos pacientes, se encontraron diferencias significativas en el peso, el IMC, el perímetro abdominal, la presión arterial diastólica y los niveles de colesterol HDL. La puntuación en la Escala de Autoevaluación de Anhedonia fue el cambio más significativo, siendo más elevada en el subgrupo que abandonó el programa de entrenamiento en comparación con el subgrupo activo.
ConclusiónLos beneficios del ejercicio físico son claros en pacientes crónicos con esquizofrenia, ya que mejoran sustancialmente su IMC, así como sus parámetros clínicos y bioquímicos. Además, este estudio desvela las vías biológicas y moleculares afectadas por el ejercicio físico en la esquizofrenia. A nivel molecular, se identificaron las proteínas ApoE y ApoC, que están implicadas en vías metabólicas; la tenascina y las neurotrofinas, asociadas a la regulación neuronal; la lipocalina 2 y la proteína 14-3-3, involucradas en las vías de regulación neuroinflamatorias, y las proteínas espectrinas y anexinas del citoesqueleto de las células. La comprensión de estos mecanismos moleculares abre la puerta al estudio de estas proteínas asociadas a la cronicidad de la esquizofrenia.
Schizophrenia is a serious mental illness characterised by disorders of perception and thought.1 Patients diagnosed with schizophrenia have a lower life expectancy due to premature mortality associated with the intrinsic somatic comorbidities of this disorder.2,3 Cognitive impairment is also highly significant among these patients, involving several domains, such as executive function, language, social cognition, memory and attention.1 Dynamic interventions, such as regular physical activity, have a positive effect on metabolic disorders (metabolic syndrome, cardiovascular risk, obesity and diabetes) and mental health (depression and social and cognitive functioning).4 However, chronic patients with schizophrenia tend to have very sedentary lifestyles, often due to their lack of self-confidence, their mood changes, comorbidities and lack of social support,5,6 in addition to the actual underlying neuronal changes to the schizophrenia. Furthermore, antipsychotic drugs, the benchmark schizophrenia treatment is often associated with a variety of mechanism-of-action-associated metabolic side effects. In other words, the blocking of dopamine receptors.7,8
In patients with schizophrenia, physical exercise leads to minor improvements in the working memory and social cognition, although there are no significant improvements in other affected functions, such as the speed of processing and verbal and visual memory.9 The success of these exercise programmes applied to chronic patients with schizophrenia is achieved by sticking to a routine, ensuring that the patients enjoy the activity and stimulating their motivation.10 Regular physical activity induces functional, structural and neurobiological changes in the brain which improve not just social cognition but also negative symptoms (anhedonia, social isolation, apathy and abolition).11–13 Irisin has recently been identified, which is a molecule released in muscle during physical activity and which correlates with benefits in the brain.14 The release of irisin is produced after extracellular proteolytic cleaving of the protein FNDC5. This means that the proteic profile found in the extracellular medium is vital for obtaining beneficial effects on the brain, justifying the differences to be found between patients when they apply the same neurorehabilitation programme based on physical activity. Although it is very difficult to relate plasmatic level changes with brain functions, given the structural complexity and selective permeability of the haematoencephalic barrier, this is a line of research which is acquiring great relevance. Also, to determine whether changes in plasmatic proteomic profiles evoke different molecular responses would lead to an understanding of the different response of patients to similar progammes.15,16
Chronic schizophrenia patients present with metabolic and molecular changes which are characteristic of and adjunct to the disease itself. These molecular changes are often responsible for many cardio-metabolic problems which have been observed over the years in this patient population, including the reduction of their mean life expectancy by 10 years and the high percentages of obesity, diabetes and serious heart problems. For this reason, based on analysis using liquid chromatography coupled to tandem shotgun type mass spectrometry (LC-ESI-MS/MS), we wished to analyse the overall expression of plasma proteins in chronic schizophrenic patients,17,18 and by doing so, establish what the biological and molecular pathways affected by physical activity in these patients were.
In this context, the main aim of the study was to characterise the molecular and metabolic dynamic of chronic schizophrenia patients undergoing a physical exercise programme. The study was conducted based on a novel technique of mass proteome analysis (LC-ESI-MS/MS) combined with an exhaustive clinical study, aimed at providing comprehensive information on the effect of physical activity on the biology of the schizophrenia. It should be noted that combining proteomic study with a 6-month physical activity programme in chronic schizophrenic patients is highly complex, and this had an impact on the sample size (21 participants in total).
Since 9 out of the 21 patients with schizophrenia selected for the study did not present at any training session, they were treated as the control group.
Anhedonia was assessed in both the study group and the control group with the Self-assessment Anhedonia Scale (SAAS). We added a secondary objective which was to compare the molecular profile of the 12 patients of the programme with the 9 control group patients.
Material and methodsA longitudinal study was conducted with 21 chronic patients diagnosed with schizophrenia. Twelve completed the training programme and 9 comprised the control group. During the physical training programme the participants had to walk for one hour per day for 5 days per week, progressively increasing the distance and rhythm to reach a daily distance of 4 km, maintaining this routine of training until the sixth month (end of the programme). The other 9 patients, with no physical training, comprised the control group.
All participants were selected in the Hospital Nicolás Peña (Vigo, Spain) and were diagnosed with schizophrenia according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders. According to the inclusion criteria of the study, patients over 18 years of age who had signed an informed consent form were included. The study complied with the Declaration of Helsinki and with the approval of the Ethics Committee of Galicia. The people with other psychiatric or neurological disorders, or with a traumatic head injury were excluded from the study. Pregnant women or those who were breast-feeding were also excluded. A demographic questionnaire was administered to all participants and the SAAS was given to them, which assesses the ability of schizophrenic patients to experience pleasure or interest.
Preparation of the plasma sampleVenous blood was collected in 2 empty tubes which contained K2EDTA (BD Vacutainer®; Becton, Dickinson & Co., NJ, U.S.A.) between 7:00 and 9:00 h in the Hospital Nicolás Peña and immediately centrifuged at 1000 rpm for 10 min to separate the blood plasma. The aliquots were then stored at −80 °C. For proteome analysis, an aliquot of 450 μl of plasma was centrifuged at 16,000 rpm for 15 min and at 4 °C, and the resultant supernatant was collected and stored at −80 °C. The total proteins were measured using a bicinchoninic acid assay (Pierce Chemicals, Rockford, IL, U.S.A.). A protein-enriching commercial kit was also used (ProteoMiner®, Bio-Rad, Hercules, CA, U.S.A.) in keeping with the manufacturer’s instructions, to equalise proteins in a sample with a total content of 10 μg. The eluted fractions (10 μl) prepared with Laemmli 2× (10 μl of sample + 10 μl of loading buffer 2×) were loaded in a polyacrylamide gel (10×). Electrophoresis began and was stopped once the samples entered in a separator gel, which allowed them to be piled up and enter into the resolution gel, but not be become separated. After this, the pieces of gel selected were submitted for in-gel digestion which consisted of washing the pieces of gel sequentially with 25 mM ammonium bicarbonate and 50% acetonitrile/25 mM ammonium bicarbonate in an ultrasonic bath. After this, the proteins were reduced with 10 mM ditiotreitol for one hour and were alcalinised with 55 mM indolacetic acid for 30 min. Finally, the proteins were digested overnight with 40 ng of trypsin at 37 °C, and the tryptic peptides were extracted from the matrix of the gel in 2 steps with .5% of trifluoroacetic acid and 100% of acetonitrile bicarbonate.
Liquid chromatography tandem mass spectrometryThe samples were analysed triply (3 fractions). The tryptic peptides were dried in a speed vacuum concentrator at 45 °C (Concentrator plus, Eppendorf, Hamburgo, Germany), reconstituted in LC/MC grade water with a .1% (v/v) formic acid concentrate and analysed with electrospray ionization mass spectrometry simultaneously in a high resolution LTQ-Orbitrap ELITE® hybrid spectrometer (Thermo Fisher Scientific, Waltham, MA, U.S.A.) attached to a Proxeon EASY-nLC 1000® UHPLC (Thermo Fisher Scientific, Waltham, MA, U.S.A.) system. After this they were transferred to an reverse-phase column (PepMap® RSLC C18, 2 μm, 100 Å, 75 μm × 500 mm, Thermo Fisher Scientific, Waltham, MA, U.S.A.) and were eluted with 5%–30% acetonitrile gradient with a formic acid content of .1% for 20 min and a .3 μl/min flow. The resulting eluted analysis findings were transferred directly to the mass spectrometer which was adapted to a positive ion setting in data-dependent mode. Complete mass spectrometry scan was performed, with a mass/load range of 350–1.600 m/z and a resolution of 120,000. Finally, a mass spectrometry scanning was performed in tandem with the 15 best at 18% of normalized collision energy, with a dynamic exclusion time of 30 s, a minimum signal threshold of 1000, a resolution of 30,000 and an insulation width of 1.50 Dia.
Data analysisTo identify the proteins we used Proteome Discoverer 2.1.0.81 (Thermo Fisher Scientific, Waltham, MA, U.S.A.), y PEAKS® Studio v7.0 (Bioinformatics Solutions Inc., Waterloo, ON, Canada) software programmes for a search for “human” proteins in the database of the National Centre for Biotechnology Information, which is not redundant, and for the relative label-free quantification. We also started the search for proteins comparing this with the UniProt database. A decoy sequence database in the analysis was used to calculate the false discovery rates (FDR). Positive identifications of proteins were only accepted if the number of paired peptide sequences was >2, the only coincidences of peptide were >1, the coincidences of the peptide spectrum were <.5% of the FDR and the maximum score for protein identification was >20 (FDR: .0%). For relative label-free quantification, mass error tolerance and retention time was adjusted to 20.0 ppm and 20.0 min, respectively.
ELISA quantificationTo study neuroendocrine changes we measured the plasmatic level of leptin (ab100581, Abcam, Cambridge, United Kingdom) and adiponectin (ab99968, Abcam, Cambridge, United Kingdom), through a sandwich immunosorbent assay. These are neurohormones closely related to obesity and the metabolic syndrome, and also the changes in the actual central nervous system. We also measured the melanin hormone concentrator (MBS9328009, MyBiosource), according to the manufacturer’s instructions. Duplicate tests were performed and an automatic microplate reader (Biochrom Asys UVM 340, Cambridge, United Kingdom) measured the optical density at 450 nm with the MikroWin 2000 (Berthold Technologies, Bad Wildbad, Germany) software.
Statistical analysisThe GraphPadPrism 7 (GraphPad Software Inc., San Diego, CA, U.S.A.) software programme was used to manage the resulting data and to perform statistical analysis. The mean age of both groups was compared with the Mann–Whitney U test and the differences between the proportions of genders were analysed with the exact Fisher test. The student’s t-test was used for paired samples to determine the differences of means between the measurement of the variable (baseline and at 5 months) and a student t-test was used for non-paired samples to analyse the differences between the baseline data (group of participants) and the anhedonia data (patients who had left the programme). Statistical significance of results we set at a p value of ≤.05.
ResultsA longitudinal study was performed which compared how an aerobic programme of physical activity can improve the general health of chronic patients with schizophrenia. The clinical and demographic characteristics of the study participants are contained in Table 1.
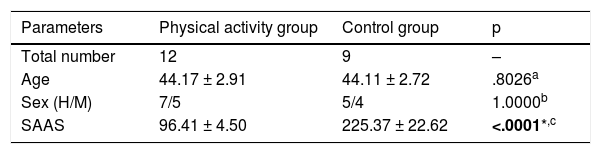
Demographic and clinical characteristics of the participants.
| Parameters | Physical activity group | Control group | p |
|---|---|---|---|
| Total number | 12 | 9 | – |
| Age | 44.17 ± 2.91 | 44.11 ± 2.72 | .8026a |
| Sex (H/M) | 7/5 | 5/4 | 1.0000b |
| SAAS | 96.41 ± 4.50 | 225.37 ± 22.62 | <.0001*,c |
SAAS scale range:0–810.
Value with statistical significance is in bold.
Comparison of demographic data of 12 patients from the physical activity group and 9 patients from the group with no physical activity (control group) led to no significant differences between the groups in terms of age (p = .8026, Mann–Whitney U test) and sex (p = 1.0000, Fisher test) (Table 1). On the contrary, the anhedonia scores according to the SAAS scale differed considerably, since the control group presented significantly higher scores than the patients who participated in the training programme (225.37 ± 22.62 vs. 96.41 ± 4.50; p < .0001) (Table 1). However, Table 2 shows the measurements of the different clinical parameters which comprise the metabolic profile of the patients at the onset and after finalizing the 6 months training programme. All the patients were treated with antipsychotics (for example, olanzapine, clozapine or risperidone).
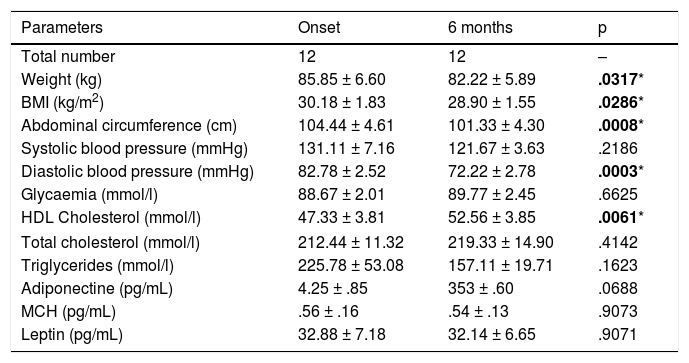
Description of the clinical parameters of the patients who participated in the study before and after the physical activity programme.
| Parameters | Onset | 6 months | p |
|---|---|---|---|
| Total number | 12 | 12 | – |
| Weight (kg) | 85.85 ± 6.60 | 82.22 ± 5.89 | .0317* |
| BMI (kg/m2) | 30.18 ± 1.83 | 28.90 ± 1.55 | .0286* |
| Abdominal circumference (cm) | 104.44 ± 4.61 | 101.33 ± 4.30 | .0008* |
| Systolic blood pressure (mmHg) | 131.11 ± 7.16 | 121.67 ± 3.63 | .2186 |
| Diastolic blood pressure (mmHg) | 82.78 ± 2.52 | 72.22 ± 2.78 | .0003* |
| Glycaemia (mmol/l) | 88.67 ± 2.01 | 89.77 ± 2.45 | .6625 |
| HDL Cholesterol (mmol/l) | 47.33 ± 3.81 | 52.56 ± 3.85 | .0061* |
| Total cholesterol (mmol/l) | 212.44 ± 11.32 | 219.33 ± 14.90 | .4142 |
| Triglycerides (mmol/l) | 225.78 ± 53.08 | 157.11 ± 19.71 | .1623 |
| Adiponectine (pg/mL) | 4.25 ± .85 | 353 ± .60 | .0688 |
| MCH (pg/mL) | .56 ± .16 | .54 ± .13 | .9073 |
| Leptin (pg/mL) | 32.88 ± 7.18 | 32.14 ± 6.65 | .9071 |
Values of statistical significance are in bold.
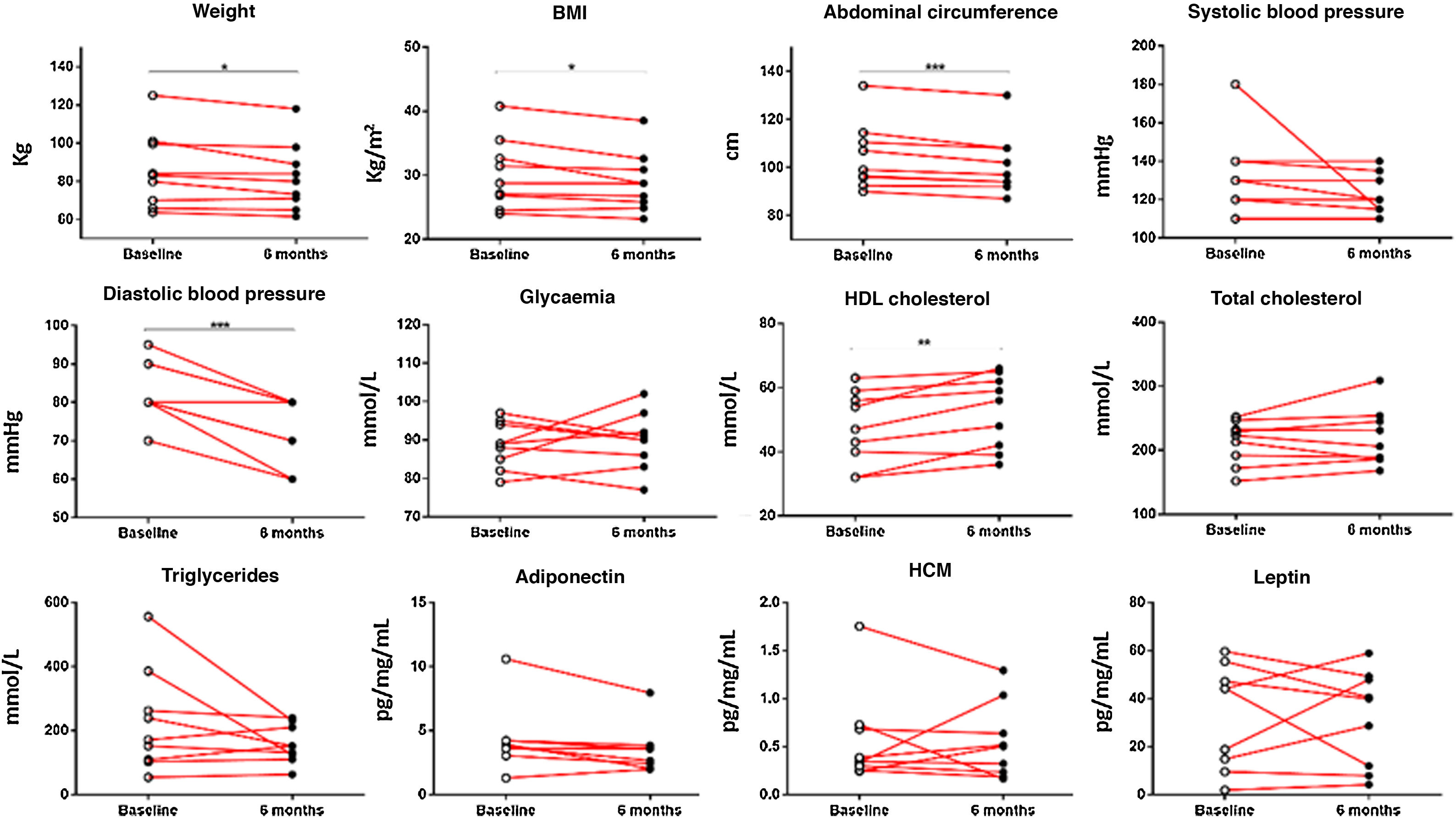
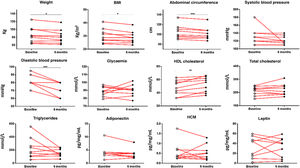
When the clinical data between the control group and the physical activity group were analysed, significant results were observed between both groups relating to weight (p < .05), BMI (p < .05), abdominal circumference (p < .001), diastolic blood pressure (p < .001) and HDL cholesterol (p < .001). Analysis of the changes which occurred within the physical activity group before and after 6 months revealed significant results regarding patients’ weight reduction (85.85 ± 6.60 vs. 82.22 ± 5.89 kg; p < .05), BMI (30.18 ± 1.83 vs. 28.90 ± 1.55; p < .05), abdominal circumference (104.44 ± 4.61 vs. 101.33 ± 4.30 cm; p < 0001), diastolic blood pressure (82.78 ± 2.52 vs. 72.22 ± 2.78 mmHg; p < .001) and HDL cholesterol levels (47.33 ± 3.81 vs. 52.56 ± 3.85 mm/dL; p < .001) (Fig. 1).
Clinical data of chronic schizophrenia patients measured at the beginning of the physical activity programme and on completing it after 6 months. Significant results were found in patients’ weight (p = .0317), BMI (p = .0317), abdominal circumference (p = .0008), diastolic blood pressure (p = .0003) and HDL cholesterol (p = .0061). *p ≤ .05; **p ≤ .01; ***p ≤ .001.
To confirm the homogeneity of both groups, we studied where differences existed between the control group (which did not perform physical activity) and the initial data of the group who did the training programme (Appendix B Supplementary Table 1). No significant differences were observed in any parameter.
We were interested in finding out whether there was any clinical difference between both groups which could explain why one group of patients accepted and were committed to the physical activity programme whilst another group agreed to participate but withdrew before it had begun. The aim was to find a way of predicting whether a patient would be a good candidate or not for this type of programme in the future. As a result, we measured the levels of anhedonia with the SAAS scale. It confirmed that the control group had a score of 217 (SAAS score >150), which means these patients had marked anhedonia. Anhedonia is one of the negative schizophrenia-associated symptoms. It is a withdrawal from or reduction of normal behaviour, a lack of energy and interest and a flattening or absence of affection. This could mean that the molecular changes to be found among both groups would be due to anhedonia in schizophrenia, although determining this causality would require studies with a larger number of patients. The clinical data of both groups are specified in Appendix B, Supplementary Table 1.
Proteomic profilesTo determine and create a molecular profile of the changes caused by physical activity in chronic schizophrenia patients, plasma samples obtained from the participants of both groups were analysed using a shotgun type LC-ESI-MS/MS. Within the group who carried out the physical activity programme, changes in the levels at the beginning of the programme were compared with those after 6 months of training. Also studied were the differences between the group who completed the physical activity programme and the control group.
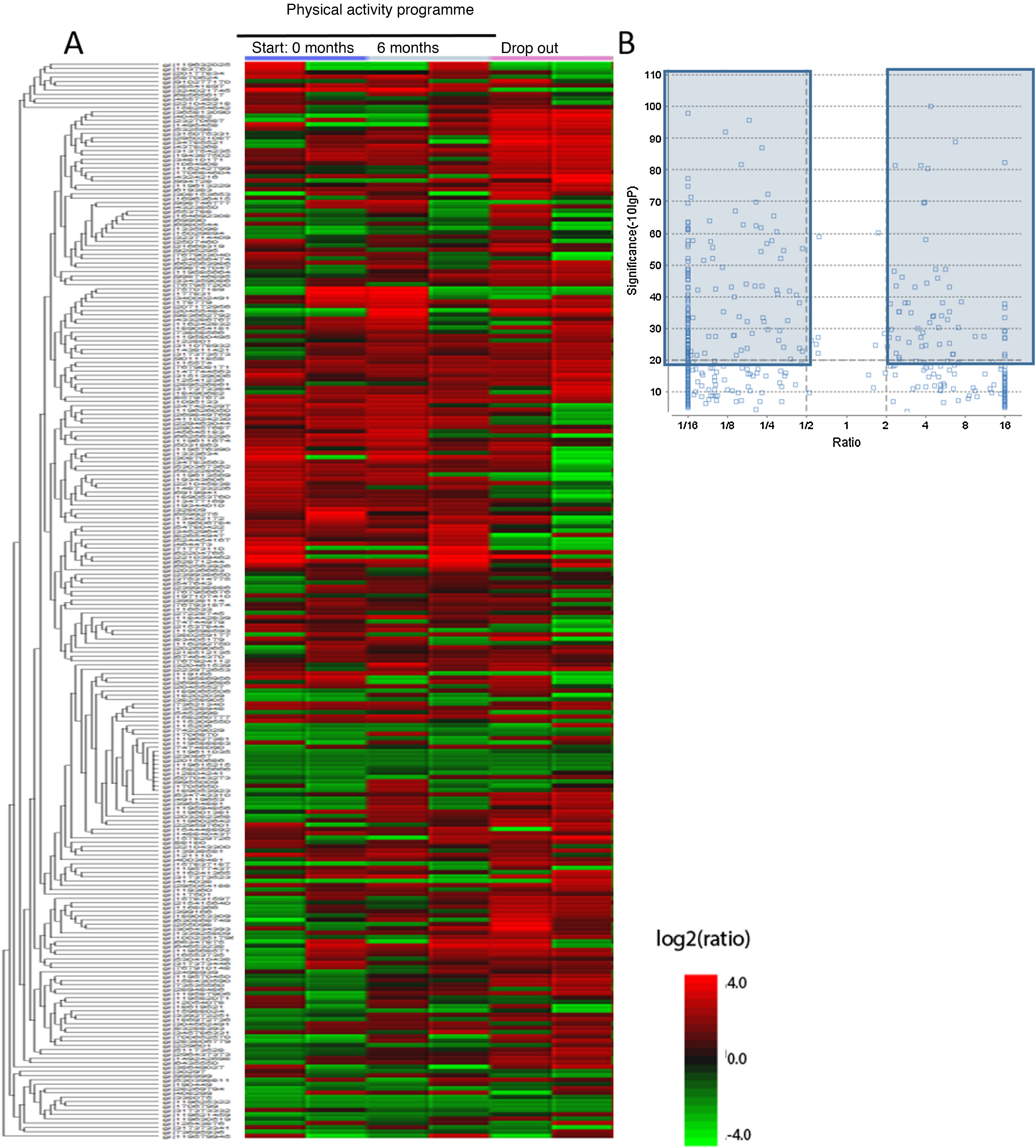
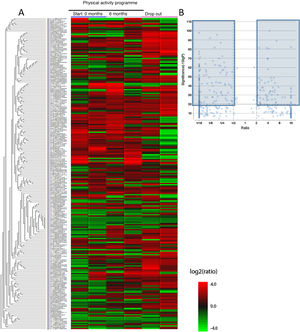
Firstly, plasma samples were analysed to achieve compete plasmatic profiles of 21,165 different proteins and peptides. These were compared with the databases from the National Centre for Biotechnology Information and after performing the bio-computerised analysis using the MaxQuant and the PEAKS, 4657 candidate proteins were identified, called master proteins, which differed significantly between the study groups and which can be observed in the heatmap type graph (Fig. 2A). As it is difficult to analyse the changes of so many proteins, from the 4657 proteins of the heatmap a seconds analysis was performed which increased the power of significance and obtained the volcano plot graph. In this graph we observed 194 proteins identified from the heatmap which varied significantly between the 3 sample groups (initial group, 6 month group and control group). These proteins are marked in dark-coloured charts, the proteins with an FDR > 2 or an FDR < .5 (Fig. 2B).
Differentiated proteins obtained after LC-ESI-MS/MS analysis in the different chronic schizophrenia patient groups: 1) group which participated in the physical activity programme, baseline score; 2) group which participated in the physical activity programme, score after 6 months and 3) group which dropped out of the physical activity programme. A) Heatmap type graph of the proteomic profile in the 3 groups. B) Volcano plot with selected proteins as significant from the heatmap data. The significant differentiated proteins are marked in blue.
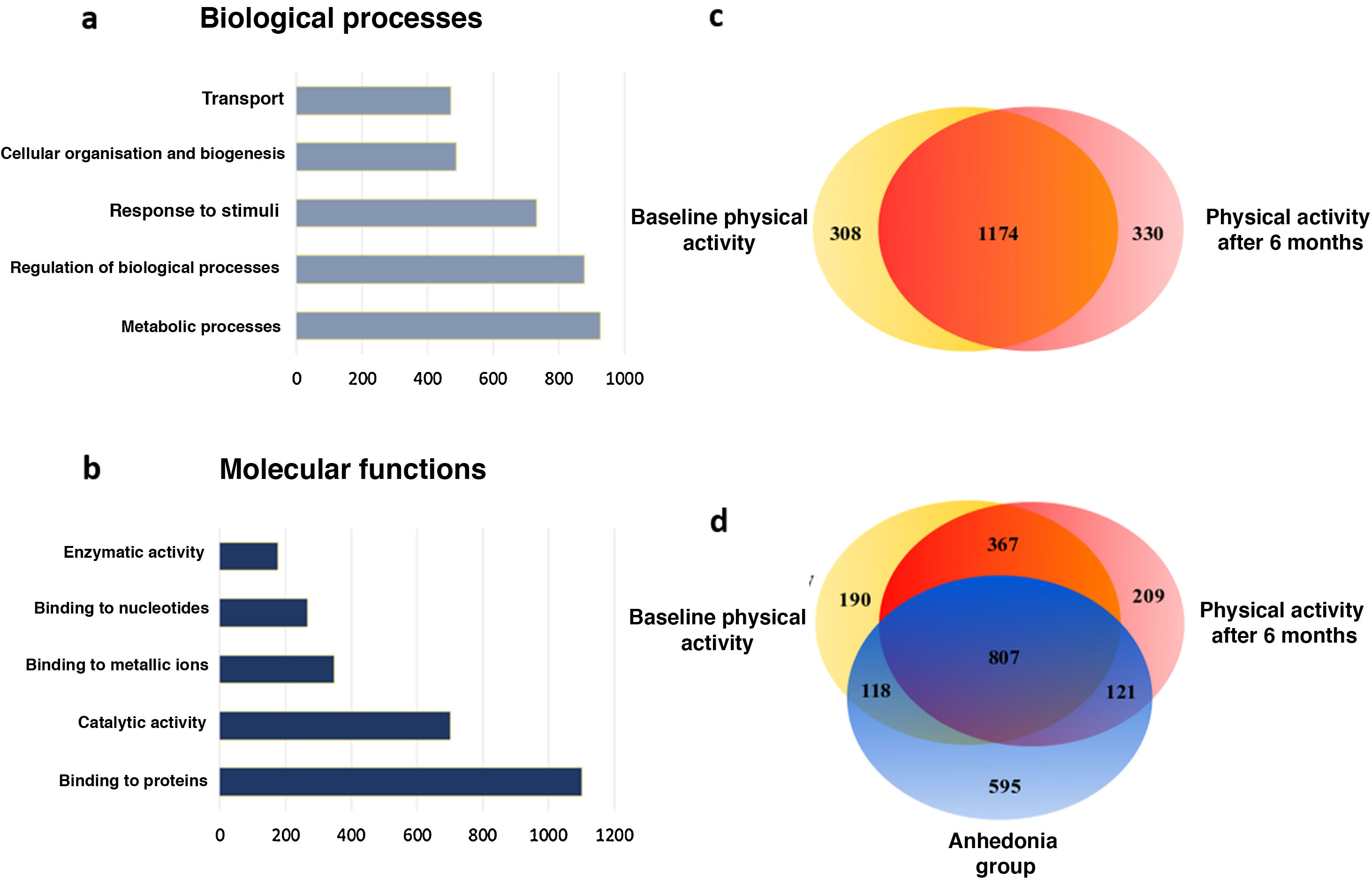
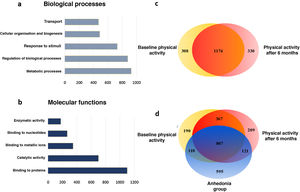
Following this, from the proteins selected through the heatmap a hierarchical ranking analysis was performed and they were classified by categories of biological processes based on gene ontology (Fig. 3). With this analysis we observed how the physical activity programme in patients with schizophrenia mostly affects the proteins involved in the metabolic processes and in the biological regulation processes (21.77% and 20.59%, respectively), followed by the response to stimuli (Fig. 3A). When we classify these data according to molecular processes we observe that the physical activity in patients with schizophrenia mostly modifies the binding proteins (34.13%) and the enzymes linked to catalytic activity (21.74%) (Fig. 3B).
Main biological and molecular processes induced by the physical activity programme in chronic schizophrenia patients. A) Bar chart of the main changes in the biological processes. B) Bar chart of the main changes in molecular functions. C) Venn diagram of total proteins identified and modulated by physical activity. The diagram shows the distribution of the proteins and that 64.79% of the proteins are common to both conditions. D) Venn diagram of total proteins identified and modulated by physical activity of the group which dropped out. The diagram shows the distribution of the proteins and the 33.51% of the proteins which are common to the 3 conditions.
Analyses were then performed based on set theories through the Venn diagrams, analysing the plasma proteins of each group. When the changes which occurred in the chronic schizophrenic patients were analysed from the start of the programme to its conclusion, it was observed that in the final stage 330 unique proteins appeared and in contrast the expression of the other 308 proteins disappeared (Fig. 3C). When this was compared with the control group, which were patients with anhedonia, it was confirmed that their molecular profiles in the Venn diagram significantly differed to those of the active programme group. Although the change in the number of proteins was similar, their class changed considerably, since 595 unique proteins appeared exclusively among the group of patients of the physical activity programme, whilst the expression of 766 proteins disappeared from the control patients plasma (Fig. 3D). These molecular changes mainly occurred in proteins involved in the metabolic processes, which suggests major molecular differences between these patient groups and that they have a direct effect on the observable clinical differences and response to the neurorehabilitation programmes.
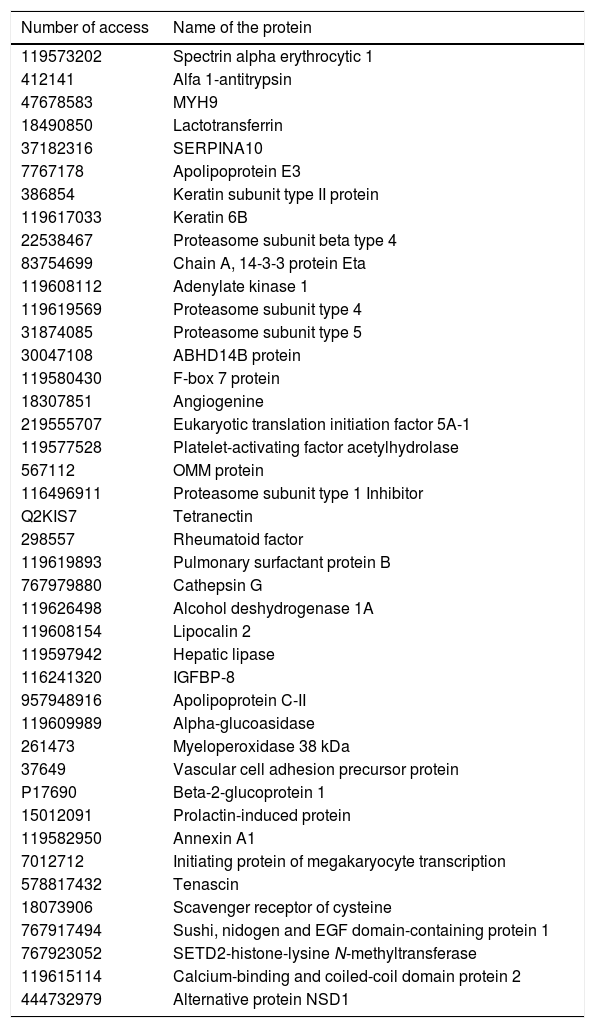
A STRING analysis followed, to select the interactions between master proteins whose presence or absence in plasma altered the patients’ molecular profiles of those engaging in physical activity. From this analysis a total of 87 candidate master proteins were identified, of which 42 were selected which related to different metabolic processes (Table 3).
List of proteins selected as candidates which modulate the physical activity at a molecular level of chronic schizophrenic patients.
| Number of access | Name of the protein |
|---|---|
| 119573202 | Spectrin alpha erythrocytic 1 |
| 412141 | Alfa 1-antitrypsin |
| 47678583 | MYH9 |
| 18490850 | Lactotransferrin |
| 37182316 | SERPINA10 |
| 7767178 | Apolipoprotein E3 |
| 386854 | Keratin subunit type II protein |
| 119617033 | Keratin 6B |
| 22538467 | Proteasome subunit beta type 4 |
| 83754699 | Chain A, 14-3-3 protein Eta |
| 119608112 | Adenylate kinase 1 |
| 119619569 | Proteasome subunit type 4 |
| 31874085 | Proteasome subunit type 5 |
| 30047108 | ABHD14B protein |
| 119580430 | F-box 7 protein |
| 18307851 | Angiogenine |
| 219555707 | Eukaryotic translation initiation factor 5A-1 |
| 119577528 | Platelet-activating factor acetylhydrolase |
| 567112 | OMM protein |
| 116496911 | Proteasome subunit type 1 Inhibitor |
| Q2KIS7 | Tetranectin |
| 298557 | Rheumatoid factor |
| 119619893 | Pulmonary surfactant protein B |
| 767979880 | Cathepsin G |
| 119626498 | Alcohol deshydrogenase 1A |
| 119608154 | Lipocalin 2 |
| 119597942 | Hepatic lipase |
| 116241320 | IGFBP-8 |
| 957948916 | Apolipoprotein C-II |
| 119609989 | Alpha-glucoasidase |
| 261473 | Myeloperoxidase 38 kDa |
| 37649 | Vascular cell adhesion precursor protein |
| P17690 | Beta-2-glucoprotein 1 |
| 15012091 | Prolactin-induced protein |
| 119582950 | Annexin A1 |
| 7012712 | Initiating protein of megakaryocyte transcription |
| 578817432 | Tenascin |
| 18073906 | Scavenger receptor of cysteine |
| 767917494 | Sushi, nidogen and EGF domain-containing protein 1 |
| 767923052 | SETD2-histone-lysine N-methyltransferase |
| 119615114 | Calcium-binding and coiled-coil domain protein 2 |
| 444732979 | Alternative protein NSD1 |
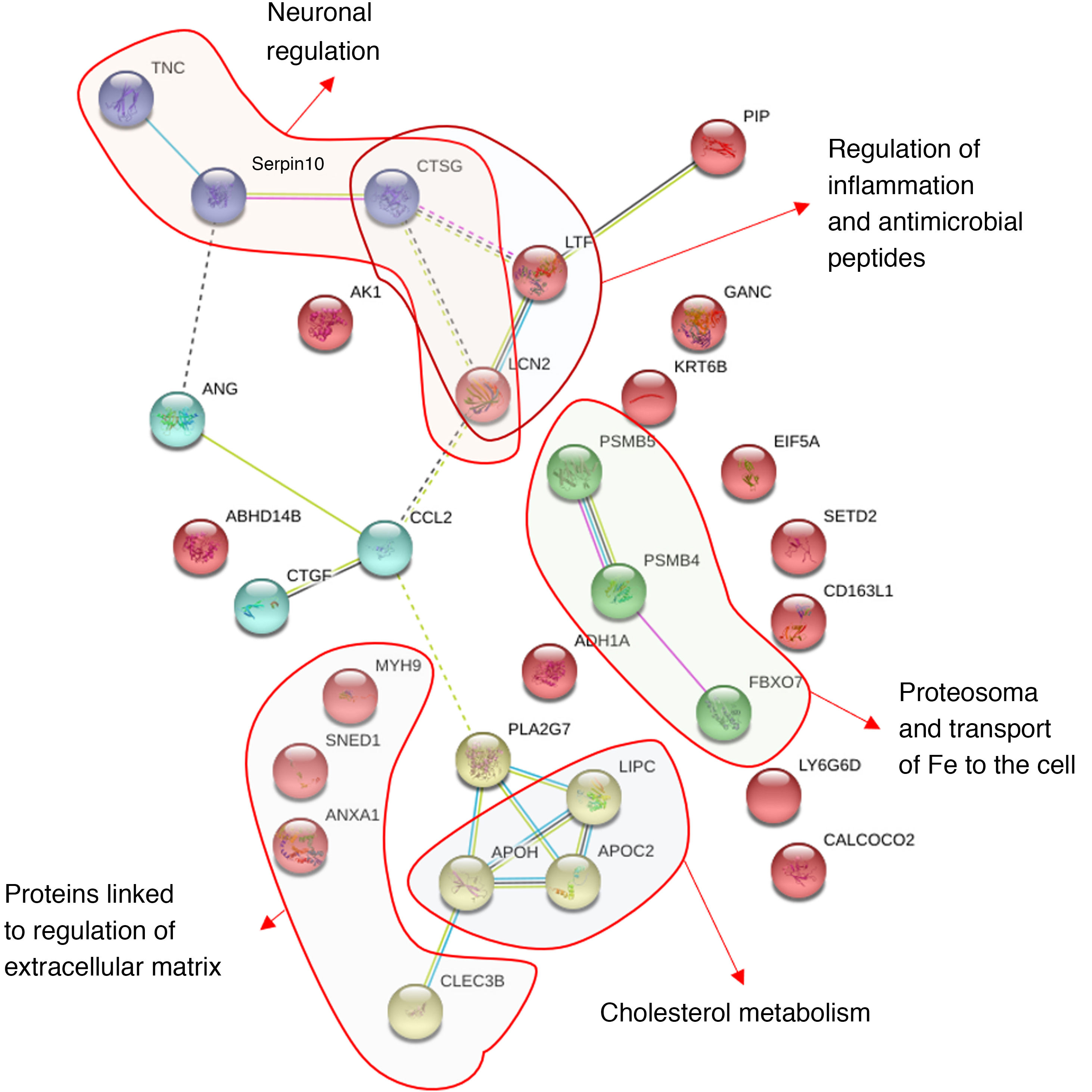
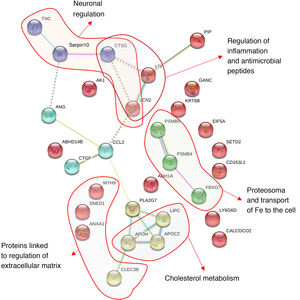
In Fig. 4 we many observe how these proteins interact with one another obtaining 30 significant nodes (p < .0001). This analysis confirms the importance of neurometabolic proteins, such as apolipoprotines E, C and H, where the largest part of the significant nodes are made in the regulation of the lipoproteins or the proteins involved in neuronal regulation, as is the case of tenascin or PAFAH1B3; of neuroinflammatory proteins, such as lipocalin 2 and 14-3-3; of regulatory cytoskeleton proteins, such as spectrin, lactotransferrin and annexin A1; of the neuroendocrine regulatory proteins, such as the prolactin-induced protein (Fig. 4). In Fig. 4 we may observe how the proteins are grouped together based on the interactions described in databases after applying a data-mining programme (k-clustering). It is observed that they are significantly grouped into 6 clusters, linked to neuronal regulation, inflammation regulation and antimicrobial peptides, to proteasome and the transport of iron, to cholesterol metabolism and the regulation of the extracellular matrix (Appendix B Table Supplementary 2).
DiscussionSchizophrenia is one of the most disabling diseases in existence today, with life expectancy of chronic patients being between 12 and 15 years lower than that of the general population.19,20 The loss of quality of life this disease engenders is combined with a high rate of comorbidity, such as cardiovascular disease which is the primary cause of death among schizophrenia patients.21 On a general level, the physical status of these patients is particularly deficient.12 In order to improve the quality of life of these patients, mental health units promote healthy lifestyle programmes to prevent cardiovascular risk factors and reduce premature mortality.22 Similarly with the population in general, these healthy lifestyle programmes seek to improve the quality of the person’s diet and to promote physical activity programmes. The benefits of physical activity in schizophrenic patients are clear, resulting in an improvement of cognitive function and of general physical health.23 However, social support, symptoms and side effects relating to treatment (antipsychotic drugs) are complex barriers which have a great impact on the motivation of patients with schizophrenia and in compliance with a physical activity regime.19,22
Aerobic exercise maintained over time is able to regulate the connectome of different regions of the brain, such as the frontal, temporal and parietal cortexes and the cerebellar cortex.24 However, it is not known how these changes occur in the cortexes of patients with schizophrenia, and it may be that, on possession of a different connectome, the expected benefits will also be different. It is known that when this type of physical activity programme is introduced in patients with severe mental illness, response is varied. In general, response varies according to the type of disuse or type of treatment administered in addition to inter-individual genetic variability. Applying this type of programme to patients with schizophrenia is costly, both in the time required and the need for specialised personnel. An understanding of how these changes occur could therefore help to optimise the healthcare system, by offering suitable programmes to each patient and helping to better understand the biology of schizophrenia itself. For this reason we decided to conduct an in-depth study of the plasmatic proteomic profile of patients with schizophrenia and how physical activity impacted them. The aim is to obtain a common profile with clinical response associated with the molecular changes in plasma.
At first 21 volunteers were selected to participate in this 6-month duration physical activity programme and the following variables of all of them were clinically assessed: weight, BMI, abdominal circumference, blood pressure levels, glycaemia, triglycerides, total cholesterol and HDL cholesterol. The 21 participants were due to start the exercise programme but 9 of them dropped out before the training began and agreed to continue in the study as the control group.
A blood sample was taken from the 21 volunteers at the beginning of the programme and another at the end. From this sample plasma was analysed by LC-ESI-MS/MS and, for the first time, a complete profile was achieved of the plasmatic proteome comprised by 21,165 proteins and peptides. From this analysis, 4657 total proteins in plasma were recognized. In other studies similar numbers were obtained, ranging from 200 to 4500 plasma proteins.25–28 When the significant differences between the 2 groups were analysed we observed that, from the total of 4657 proteins, 1812 had undergone significant changes and over 50% of them were involved in metabolic functions and biologic processes.
Within the group which undertook the physical activity programme, changes in the protein expression were compared at the beginning and end of the programme and it was observed that this only changed for one very specific group of proteins. Of the 1812 proteins which change their expression significantly, 330 were expressed and 308 had their expression inhibited at the end of the physical exercise programme, with the expression of 1174 proteins being constant. Bearing in mind the clinical aspects of all patients who completed the programme, an improvement in their general condition was confirmed, improving their metabolic syndrome with the consequent lowering of the BMI and an optimization of their blood pressure levels, plus the lowering of the glycaemia levels, cholesterolaemia and lipidaemia. This clinical improvement was correlated with molecular aspects, since the greater part of proteins involved in these changes are correlated with the metabolism, the immune system and the changes linked to the extracellular matrix.
The main changes in molecular patterns were detected when comparison was made between the levels at the beginning of the group which completed the physical activity programme and those of the control group.
In this analysis significant changes in 2217 proteins were detected, of which 576 are specific to the group who completed the programme and 713 to the control group. In order to understand the intergroup differences, the clinical parameters of both groups were compared and it was observed that there were no differences regarding anthropometric and biochemical parameters (weight, BMI, glycaemia, cholesterolaemia or lipidaemia) but there were in anhedonia. The control group, comprised of patients who rejected the programme, presented with an SAAS score >150,29 which means they had marked anhedonia compared with the group which completed the programme (SAAS < 150). This finding may be interpreted two-fold. On the one hand, both groups of patients which are apparently similar from a biochemical and clinical viewpoint are molecularly very different. This could help to provide an understanding of the molecular and biological reasons of why many patients are unable to complete the neurorehabilitaiton programmes. The molecular changes detected not only affect the metabolic processes and the immune system, but also the regulation of the nervous system and the extracellular matrix proteins such as tenascin and serpins. However, this result opens the door to understanding the underlying molecular mechanisms to anhedonia.
From a clinical viewpoint, with no prior study, these chronic schizophrenic patients are indistinguishable. One fact that proves this is that when the plasma levels of leptin and adiponectin are measured – 2 neurohormones linked to the regulation of obesity and the metabolism of general intake – 30–32 no significant differences were found between the 2 groups. However, in order to establish individualised treatment when the patients start different neurorehabilitation programmes, it would be of great importance to know in advance what type of response they will have regarding adherence.33 However, this study proves that molecular differences exist on a plasma level between both groups. At present, studies through LC-ESI-MS/MS for each patient is unviable, but from the results of this study, a pattern of 4657 proteins were significantly expressed in plasma. Obviously, not all proteins detected require a comprehensive study, but from biocomputerised analysis a list of proteins was structured (Table 3) which was able to be measured in arrays in large population groups. This list included proteins of highly metabolic, neuronal, immunological or structural importance, as is the case of IGFBP-8, lipocalin 2, tenascin, the annexins, lactotransferrins, prolactin-induced protein and apolipoproteins, among others.
After molecular and biocomputerised analysis the selected proteins were compared within the group which participated in the physical activity programme before and after completing it. This comparison revealed 87 proteins which could explain the changes detected in these patients, including the apolipoproteins E and C, tenascin, spectrin, pulmonary surfactant protein B, annexin A1, prolactin-induced protein and the neruoinflammatory protein lipocalin 2. These proteins are specifically of extreme relevance in the development of different neurodegenerative and neurological diseases, as is the case of the apolipoproteins E and C in the biology of Alzheimer’s disease,34,35 or the case of tenascin, the proteins involved in the regulation of the extracellular matrix (spectrins, serpins), the precursor protein of vascular adhesion and annexing, which are vital for the mechanism of glia-neuron cellular communication.36,37
One interesting fact we found in our study is that the physical activity programme in chronic schizophrenic patients regulates the ubiquitin proteasome system. This system is a major regulator of protein processing, trafficking and degradation.38 It has been proven that this system is de-regulated in patients with schizophrenia,39,40 and these changes may be detected both in the blood and the brain.41,42 Our findings prove that this ubiquitin proteasome system is regulated in patients with schizophrenia who adhere to a physical activity programme. Similar results were obtained in an animal model (rat), where we confirmed that aerobic physical exercise improved oxidation models and reduced protein ubiquitination levels.43
Although it is imperative to carry out further research to determine what these changes mean, physical activity leads to a regulation of molecular systems.
The proteomic profiles of active patients demonstrated they were very different from patients who rejected the programme and formed the control group.
Another major aspect is to confirm how aerobic physical training programmes have an effect, compared with pharmacological therapy with antipsychotics. Achieving molecular profiles which are able to explain the profound changes observed from medical symptoms would be one of the first steps to being able to establish individualised medical treatment in psychiatry.
Study limitationsThe main limitation of the study was the small sample size. This reduced number of participants is due to the inherent difficulty in maintaining a daily training programme for 6 months in patients with schizophrenia, at the high cost of proteomic analysis to obtain complete profiles of the plasma proteome and the inter-individual variability, which requires working with highly homogenous groups. Despite this, the final aim of this project was to start from this small pilot study which in the future may be increased into molecular study using a larger number of patients. As a result, molecular profiles which are extended and validated with other techniques will be obtained, and patients with schizophrenia may be classified in accordance with these profiles.
Another study limitation is that of not analysing the effects of the antipsychotics. All the patients were in treatment with olanzapine, clozapine or risperidone, which may be a limitation that should be studied to identify the potential effect of antipsychotics on molecular changes.
ConclusionWith this study we obtained plasmatic proteomic profiling comprising 21,165 proteins and peptides, of which 4657 were proteins. From these profiles we proposed to distinguish 2 patient groups of chronic schizophrenic patients which are indistinguishable at a clinical level: the first group, which responded and improved with an aerobic physical activity programme and the second group which rejected the physical activity programme. For this study methodology was established which combined daily work with chronic patients for 6 months with advanced technology for shotgun LC-ESI-MS/MS type proteomic analysis, which is highly complex and costly, and this limited the sample size.
Despite this, it was possible to cover different aspects. Firstly, the existing major molecular difference between the patients from both groups before starting the programme. It should be noted that the first samples were taken from the 21 participants prior to the group of 12 starting the aerobic training programme and therefore, at that time, the 21 participants were indistinguishable at a clinical level. This means that a panel of plasmatic proteins capable of predicting whether a patient will adhere to the neurorehabilitation programme or not could be developed. In turn this would lead to financial cost-saving for the healthcare system and redirection of the patient towards other types of interventions.
Secondly, this study probed into the molecular changes occurring to chronic schizophrenic patients when they undertake an aerobic training programme. We are familiar with the beneficial effects of physical activity on a health brain and we are increasingly discovering more about the molecular mechanism of how physical activity regulates the brain. However, little is known about how aerobic training can affect the brain of a patient with schizophrenia, and whose main neurobiological problem is an incorrect connectome. Our study suggests that the main pathways to be modified in these patients are metabolic, immune and the biological regulation of the extracellular matrix. It would be interesting to study in greater depth how these mechanisms may be modulated, so that the efficaciousness of neurorehabiliation and social therapies could be more efficient.
Despite the intrinsic difficulties associated with the practice of physical activity by individuals with severe mental illnesses, our study revealed a dynamic metabolic and proteomic profile which is characteristic of patients with chronic schizophrenia, and increased our current knowledge on physiopathological pathways to schizophrenia.
FinancingThis study was financed by the Foundation for Science and Technology, Portugal (FCT SFRH/BD/135623/2018); the ISCIII, Spain (P16/00405); the Ministry of Health, Equality and Social Policy – Government Delegation for National Plan on Drugs (2017I054), and the Agency for Innovation (GAIN) of the Regional Government of Galicia (IN607C-2017/02, IN607B 2018/17).
Conflict of interestAll the authors have no conflict of interest to declare.
The authors wish to thank the Institute for Health Research Galicia Sur yond to Paula Álvarez Chaver, form the Proteomic Service of the CACTI from the University of Vigo for its support. The authors would also especially like to thank the help offered by the Nursing services from the Hospital Álvaro Cunqueiro and the Hospital Nicolás Peña, in addition to the Psychiatry Service of the EOXI-Vigo.
Coauthors.
Please cite this article as: Vallejo-Curto MC, Rodrigues-Amorim D, Jardón-Golmar L, Blanco-Formoso M, Rivera-Baltanás T, Rodriguez-Jamardo C, et al. Perfil proteómico y metabólico de pacientes crónicos con esquizofrenia tras un programa de actividad física: estudio piloto. Rev Psiquiatr Salud Ment (Barc.). 2021;14:125–138.