To cultivate the button mushroom Agaricus bisporus in warm countries or during summer in temperate countries, while saving energy, is a challenge that could be addressed by using the biological diversity of the species.
AimsThe objective was to evaluate the yield potential of eight wild strains previously selected in small scale experiments for their ability to produce mature fruiting bodies at 25°C and above.
MethodsCulture units of 8kg of compost were used. The yield expressed as weight or number per surface unit and earliness of fruiting were recorded during cultivation in climatic rooms at 17, 25 or 30°C.
ResultsOnly strains of A. bisporus var. burnettii were able to fruit at 30°C. At 25°C they produced the highest yields (27kgm−2) and had best earliness. The yields at 25°C for the strains of A. bisporus var. bisporus ranged from 12 to 16kgm−2. The yield ratios 25°C/17°C ranged from 0.8 to 1.2.
ConclusionsThe variety burnettii originated in the Sonoran Desert in California showed adaptation for quickly producing fruiting bodies at high temperature when humidity conditions were favorable. Strains of the variety bisporus showed interesting potentials for their ability to produce mature fruiting bodies at higher temperature than present cultivars and might be used in breeding programs.
El cultivo del champiñón (Agaricus bisporus) en países de clima tropical, o durante el verano en países de clima templado, además del ahorro energético que supone, es un reto que podría abordarse con el uso adecuado de la diversidad biológica de la especie.
ObjetivosEl objetivo del presente estudio fue examinar el rendimiento potencial de ocho cepas silvestres, previamente seleccionadas en experimentos a pequeña escala, para determinar su capacidad para producir frutos maduros a temperaturas ≥25°C.
MétodosSe utilizaron unidades experimentales de cultivo con 8kg de compost. El rendimiento se expresó como peso o número de frutos producidos por unidad de superficie; en naves climatizadas, a una temperatura de 17, 25 o 30°C, durante todo el cultivo, se registró el momento de fructificación.
ResultadosSólo las cepas de A. bisporus var. burnettii fueron capaces de fructificar a 30°C. Éstas produjeron un alto rendimiento a 25°C (27kg/m2) y la fructificación aconteció más temprano. El rendimiento a 25°C para las cepas de A. bisporus var. bisporus varió de 12 a 16kg/m2, con una proporción 25°C/17°C de 0,8 a 1,2.
ConclusionesLa variedad burnettii, originaria del desierto de Sonora en California, está adaptada para producir cuerpos fructíferos a temperaturas elevadas cuando las condiciones de humedad son favorables. Para las cepas de la variedad bisporus se demostró un interesante potencial para producir cuerpos fructíferos maduros a una mayor temperatura que la utilizada en los cultivares actuales y puede ser utilizada en los programas de mejora genética.
The button mushroom Agaricus bisporus (J.E. Lange) Imbach has been cultivated in France since the 18th century and expanded to other continents later. It is now the dominant cultivated mushroom in Europe and the Western hemisphere. Unfortunately it requires temperature below 20°C for producing fruiting bodies, which is currently limiting the development of its cultivation in some warm areas of the world. The pavement mushroom Agaricus bitorquis (Quelet) Saccardo has been proposed as an alternative because it prefers higher temperatures (25°C) than A. bisporus during harvesting.6,13 However, the yields of commercial strains are lower than those obtained with A. bisporus under optimal conditions. In middle scale experimental cultures, yields from 9.313 to 22kgm−212 had been reported whereas for cultivated strains of A. bisporus we observed average yields between 22kgm−2 and 30kgm−2.4,9 Comparing two efficient composts, Kariaga et al.7 observed yields of A. bitorquis being 51 or 74% that of A. bisporus. Consequently it is a challenge to find strains of A. bisporus able to produce high yields of good quality mushrooms at 25°C for saving energy used in cooling systems during cultivation in warm countries.
Crop wild relatives and local varieties are the elements of agricultural biodiversity most likely to contain the necessary novel genetic diversity which is needed to sustain innovations in breeding programs. This assertion is also true for cultivated mushrooms. Since the end of the 1980s, A. bisporus germplasm has been built with hundreds of wild isolates originating from various habitats and numerous geographic origins.1,8 In a previous study, we screened 114 wild strains from the major geographical populations of A. bisporus for their ability to produce mature fruiting bodies at the normal temperature used for cultivation (around 17°C) and at a higher temperature, set around 24°C.10 One quarter of the strains of A. bisporus var. bisporus were able to fruit at this higher temperature and were called FHT+. They belonged to all the studied populations. The experiments had been performed in small crates containing 500g of commercial compost, which are too small for an efficient evaluation of yield potentials at both temperatures. The objective of the present work was to better understand the adaptation of A. bisporus to temperature by analyzing the yield related traits of a selected sample of FHT+ strains cultivated both at 17, 25 and 30°C.
Eight wild strains of A. bisporus with the highest fructification level at 25°C expressed as the percentage of the yield at 17°C were selected from the data of Largeteau et al.10 Bs0094 and Bs0738 were A. bisporus var. burnettii originated from the population in Sonoran Desert of California and isolated by J. Burnett in 1991.2 The others were A. bisporus var. bisporus: Bs0190, Bs0419B, Bs0483 and Bs0739 that had been isolated under Cupressus macrocarpa by P. Callac. Bs0190 was originated in the west of France (Lorient) in 1992; Bs0419B was originated in the southwest of France (Capbreton) in 1994. Bs0483 was originated in Canada (Alberta) in 1996, and was supplied by R. Kerrigan. Bs0739 was originated in Mexico (Tlaxcala) in 2007 (isolated by G. Mata). The strain Bs0571 had been isolated on cow manure in Greece (Larissa) in 1998, and the last one, Bs0705, grew in a mule ring fence in Portugal (Alentejo) and had been isolated in 1998 by P. Callac. The cultivated hybrid HU1 that entered in collection in 1990 with the reference number Bs0026 was unable to produce fruiting bodies at 25°C.10 It was used as FHT− control. All the strains are stored under liquid nitrogen in CGAB (Collection du Germoplasme des Agarics à Bordeaux), at INRA-Bordeaux, France.
Mushrooms were grown on commercial mushroom compost based on wheat straw and horse manure supplied by a local mushroom grower using an indoor composting technology (SARL Renaud, France). Compost was spawned at 0.8% and placed in 0.09m2 trays filled with 8kg of spawned compost. Spawn was obtained as axenic cultures on processed rye grain used by spawn makers (Euromycel, France). Twelve trays were spawned with each A. bisporus strain. Incubation was performed in a mushroom growing room equipped with controlled environment (temperature, air speed, and humidity) at 25°C (compost temperature) and 92% relative humidity in air for 13 days, after which a conventional casing layer (SARL Renaud, France) was added on top of the compost. Nine days after casing, the trays were distributed in three different mushroom growing rooms. In each 19m3 room, CO2 concentration was lowered by air flow (38m3h−1). A room was set at 17°C and the air and relative humidity was maintained at 87–90%. Another room was set at 25°C and the last one at 30°C, both with 92–95% of relative humidity. Mushrooms were harvested for 5 weeks at developmental stage 3, with a closed veil and the weights of and numbers of mushrooms were recorded daily for each tray. The yield (kgm−2) and the number of fruiting bodies (nbm−2) per surface unit were used as parameters. The earliness was defined as the time, in days, from casing until the first harvest.4 Pearson correlation coefficient and their Bonferroni probabilities were used to show the relationships between the parameters. Two ways variance analysis was used for the significance of the effect of the strains and temperature on each parameter. The analyses were performed with Systat 8.0.
The FHT− control (strain Bs0026) did not produce mature fruiting bodies at 25°C and the mean yield at 17°C was 23.2kgm−2 which was in the mean values usually recorded in our experimental facilities for this strain. These data stress that the compost and cultivation conditions were suitable for the present experiment. For the FHT+ strains of A. bisporus var. bisporus, the distribution of the data as shown with the box-plot representations3 was wider at 25°C than at 17°C for all the three parameters (Fig. 1). The strains Bs0705 produced few mushrooms, and only on half of the trays at 25°C. Consequently it was removed for the following ANOVA. For both yield and mushroom number, the effects of the strains were significant at p<0.01 and there were no significant effects of temperature. The effect of the interaction strain×temperature was significant at p<0.1 for the yield and p<0.05 for the number. Both strains, temperature and their interaction affected significantly earliness (p<0.05). The absence of significant effect of temperature in ANOVA was the result of some opposite behavior among the strains. They were differently affected by the temperature.
Box-plot representation of data distribution for yield and earliness measured at 17°C or 25°C on 8 FHT+ strains of A. bisporus. (A) Yield as kgmushroomm−2 obtained at 17°C; (B) yield obtained at 25°C; (C) number of mushrooms m−2 at 17°C; (D) number of mushrooms m−2 at 25°C; (E) earliness (delay for the first harvest of mushrooms, in days) at 17°C and (F) earliness at 25°C.
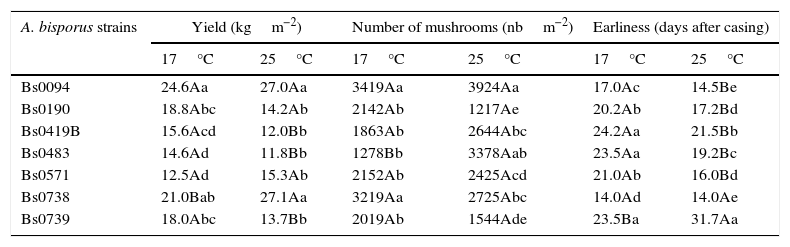
Only the two strains of A. bisporus var. burnettii produced fruiting bodies at 30°C, with 12.8 and 34.9kgm−2 for Bs0094 and Bs0738, respectively. Both the weight and number of mushrooms were not significantly different at 25°C and 30°C for Bs0738, whereas Bs0094 was affected by temperature increase from 25 to 30°C. Bonferonni tests at p<0.05 for comparing the individuals after ANOVA (Table 1) show that Bs0094 and Bs0738 were also the strains with the highest yields at 25°C (Fig. 1B), the highest mushroom numbers at 17°C (Fig. 1C) and the best earliness at both temperatures (Fig. 1E and F). The differences between the strains of the variety bisporus were not significant for the yield at 25°C and mushroom numbers at 17°C. At 17°C (Fig. 1A), Bs0190 and Bs0739 equaled the yield of Bs0738, but not of Bs0094. At 25°C the number of mushrooms (Fig. 1D) produced by Bs0419B and Bs0571 were as high as that produced by Bs0738, and Bs0483 equaled the highest value of Bs0094. The comparison of means at 17°C and 25°C for each strain showed no significant differences in yield for Bs0094, Bs0190 and Bs0571 (Table 1) whereas the yield of Bs0738 was significantly higher at 25°C than at 17°C, and it was significantly lower for the other strains. The only significant difference in number of mushrooms was a higher value at 25°C for Bs0483. Earliness was significantly different for all the strains but Bs0738. All the numbers of days after casing were higher at 17°C than 25°C, except for Bs0739 with a lesser earliness at 25°C.
Yield and earliness of FHT+ strains of A. bisporus cultivated at 17°C or 25°C.
| A. bisporus strains | Yield (kgm−2) | Number of mushrooms (nbm−2) | Earliness (days after casing) | |||
|---|---|---|---|---|---|---|
| 17°C | 25°C | 17°C | 25°C | 17°C | 25°C | |
| Bs0094 | 24.6Aa | 27.0Aa | 3419Aa | 3924Aa | 17.0Ac | 14.5Be |
| Bs0190 | 18.8Abc | 14.2Ab | 2142Ab | 1217Ae | 20.2Ab | 17.2Bd |
| Bs0419B | 15.6Acd | 12.0Bb | 1863Ab | 2644Abc | 24.2Aa | 21.5Bb |
| Bs0483 | 14.6Ad | 11.8Bb | 1278Bb | 3378Aab | 23.5Aa | 19.2Bc |
| Bs0571 | 12.5Ad | 15.3Ab | 2152Ab | 2425Acd | 21.0Ab | 16.0Bd |
| Bs0738 | 21.0Bab | 27.1Aa | 3219Aa | 2725Abc | 14.0Ad | 14.0Ae |
| Bs0739 | 18.0Abc | 13.7Bb | 2019Ab | 1544Ade | 23.5Ba | 31.7Aa |
Within a column, values followed by different lower case letters differ significantly at p<0.05 with Bonferonni tests.
Within a line, values followed by different capital letters differ significantly at p<0.05 (Bonferonni test) between 17 and 25°C, for each parameter.
Pearson correlation matrix (Table 2) shows the significant positive correlation of the yields (both as weight or number) and earliness at 17°C with the yields and earliness at 25°C. Earliness at 17°C was significantly correlated with all the other parameters. The more productive strains expressed this ability at both temperatures and were those producing rapidly their first mushrooms at 17°C. Earliness at 25°C was only significantly correlated with the weight of mushrooms harvested at 25°C. This was a consequence of the specific behavior of Bs0739. These correlations show the fitness of the strains, or their ability to complete their reproductive cycle rapidly, abundantly and under various conditions.
Pearson correlation matrix of yield and earliness measured at 17°C or 25°C on seven FHT+ strains of A. bisporus.
| Yield 17°C | Yield 25°C | Mushroom number 17°C | Mushroom number 25°C | Earliness 17°C | Earliness 25°C | |
|---|---|---|---|---|---|---|
| Yield 17°C | ||||||
| Yield 25°C | 0.697*** | |||||
| Mushroom number 17°C | 0.513* | 0.605*** | ||||
| Mushroom number 25°C | 0.367 | 0.552** | 0.190 | |||
| Earliness 17°C | −0.635*** | −0.802*** | −0.657*** | −0.334 | ||
| Earliness 25°C | −0.254 | −0.504* | −0.377 | −0.360 | 0.660*** |
All the present data are in agreement with our previous observation of a relationship between the FHT+ trait and the infraspecific taxa. The FHT+ trait appeared to be fixed in the isolated population of A. bisporus var. burnettii from the Sonoran Desert in California.10 This variety tolerates a wide range of temperature (17–30°C) for fruiting body development with a low delay for fruiting, even if some diversity between the scores of the strains might be observed. However, the mushrooms are small and they open rapidly. The combination of these traits contributes to the adaptation of A. bisporus var. burnettii for completing rapidly its life cycle in an environment where the favorable period with enough humidity is short. This variety is probably an interesting source of genetic background for introducing the ability to fruit with high yields under various temperature conditions. However, as it is far from the standard of commercialization in Europe, it might introduce undesirable traits hampered its use for breeding,4 even if its life cycle is an advantage for the selection of homokaryons.2
We confirmed here the potential of some wild of strains of A. bisporus var. bisporus as a good genetic basis to start breeding programs in view to produce FHT+ commercial strains. For instance the strains Bs0190 and Bs0571 combined interesting scores for both yield and earliness at 25°C, and they came from different populations isolated on different substrates. One of the limits in the use of wild strains or hybrids adapted to fruit at 25°C is the fact that at this fruiting temperature, the mushrooms develop faster, rapidly lost firmness and are susceptible to bruising leading to discoloration. These traits might affect the quality during shelf-life and should be counter-selected. Works are in progress in order to study the mechanism behind discoloration and to breed for bruise-related browning resistant strains.5,14 The combined selection of FHT+ and bruising sensitivity could be promising. On the other hand, the improvement of the strains able to fruit at 25°C will benefit of the genomic data that are now available for A. bisporus.11
P. Navarro was supported by a fellowship from CONACYT, Mexico. This work was partly funded by the ECOS-Nord-ANUIS Committee (M06A01). We thank L. Devaux for mushroom cultivation.