Paracoccidioidomycosis (PCM) is an endemic disease in Latin America. In immunocompetent hosts, PCM occurs in two main clinical forms: acute and chronic. However, in HIV-infected patients PCM may show up simultaneous manifestations of acute and chronic forms.
Case reportWe present the case of a patient diagnosed with HIV who had disseminated skin lesions and generalized lymphadenopathy, as well as respiratory and central nervous system involvement. The PCM diagnosis was confirmed by direct KOH examination, double immunodiffusion and the isolation of the fungus in samples of an abscess in the subcostal region. The isolate was identified as Paracoccidioides brasiliensis S1 by species-specific PCR using primers for protein-coding gene GP43 (exon 2) followed by PCR-RFLP of the alpha-tubulin gene.
ConclusionsThere are few data in literature reporting species-specific molecular identification of Paracoccidioides in HIV/PCM patients. Therefore, this case report may contribute to improve the knowledge about this severe disease, its causative cryptic species, and its consequences to patients.
La paracoccidioidomicosis (PCM) es una enfermedad endémica en Latinoamérica. En los pacientes inmunocompetentes, la PCM cursa con dos principales formas: aguda y crónica. Sin embargo, los pacientes infectados por el VIH pueden presentar manifestaciones simultáneas de las dos formas clínicas.
Caso clínicoSe presenta el caso de un paciente VIH-positivo, con lesiones cutáneas diseminadas, linfadenopatía generalizada y afectación del sistema nervioso central y respiratorio. El diagnóstico de PCM se confirmó mediante un examen directo con KOH, doble inmunodifusión y el aislamiento del hongo en cultivo, a partir de muestras de un absceso en la región subcostal. La cepa aislada se identificó como Paracoccidioides brasiliensis S1 mediante PCR especie-específica del gen codificador de la proteína GP43 (exón 2), seguida de PCR-RFLP del gen de la alfa-tubulina.
ConclusionesExisten pocos datos en la literatura que describan la identificación molecular especie-específica de Paracoccidioides en pacientes con VIH/PCM. Por lo tanto, la presentación de este caso clínico puede contribuir a mejorar el conocimiento sobre esta enfermedad grave, la especie críptica implicada y sus consecuencias para los pacientes.
Paracoccidioidomycosis (PCM) is an endemic mycosis in Latin America caused by a species of the genus Paracoccidioides and is the cause of approximately half of the total deaths due to systemic mycoses in Brazil.16 PCM in immunocompetent hosts may present with two clinical forms: acute/subacute and chronic. However, clinical findings related to PCM in HIV-infected patients show that both the acute and chronic presentations of the disease may occur simultaneously. Therefore, HIV/PCM co-infection can increase the clinical severity of patients.2,13,15
The development of molecular tools for identifying the species of the genus Paracoccidioides helped to improve our knowledge of PCM in the field of epidemiology, although in the clinical setting the need to identify the species is uncertain. It is within this context of expanded knowledge about PCM that it is relevant to study Paracoccidioides phylogenetic cryptic species in vulnerable populations, such as HIV-infected patients. Our study focused on describing the clinical-epidemiological and microbiological characteristics, as well as the evolution of the infection, in a HIV/PCM co-infected patient seen at the reference center for PCM care from May 2019 to March 2020, at the University Hospital Cassiano Antonio Moraes at the Federal University of Espirito Santo, Brazil. This study was approved by the Ethics Committee CAAE 30505420.0.0000.5060. All personal medical data from medical records were anonymized. The patient signed a consent form authorizing the case's description and the release of the photographs taken to him.
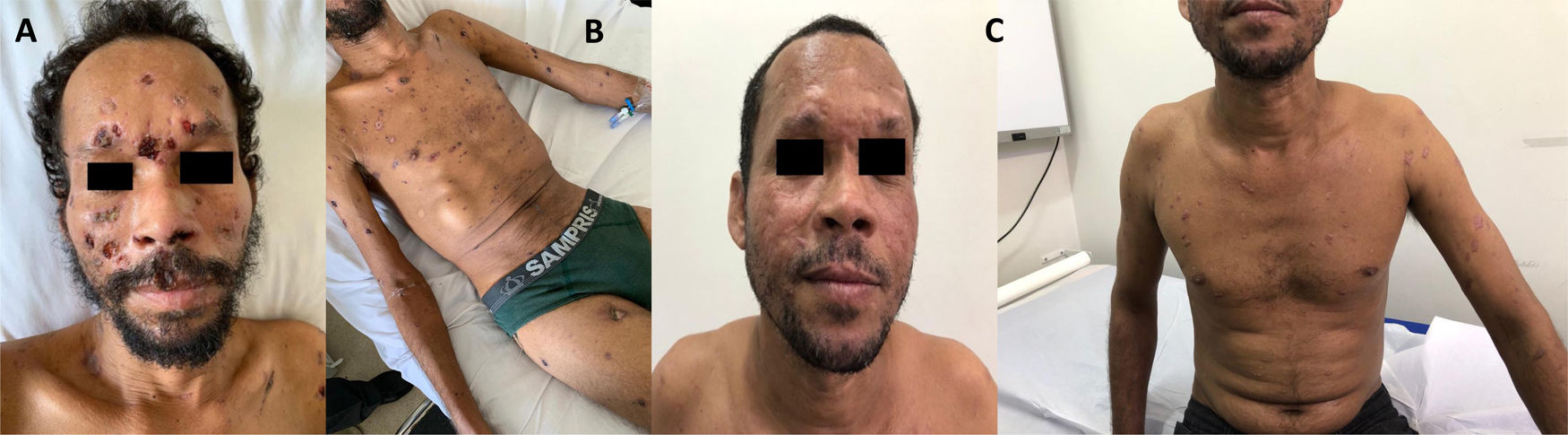
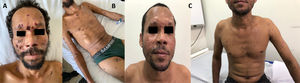
Case reportOn August 2018, a 37-year-old male from an urban area of São Mateus-ES was diagnosed of an HIV infection. The man was smoker, drinker, and reported having carried out rural activities working as coffee farmer for 24 years. There were no records of previous diseases and tenofovir, lamivudine and dolutegravir were prescribed for the HIV infection. In the same month the patient presented with disseminated lesions (Fig. 1A and B), as well as diffuse lymphadenopathy associated with asthenia, dyspnea on exertion and hyporexia. Nine months later, the patient's case evolved to a worsening asthenia, onset of productive cough and pleuritic chest pain, which led to his admission to the University Hospital (HUCAM). On physical examination, disseminated skin lesions, generalized lymph adenomegaly and bilateral basal crackles were observed. Additional tests were also conducted, showing an oxygen saturation of 92%, without signs of respiratory effort, C-reactive protein 33.3mg/l, CD4+ 402cells/mm3, and undetectable viral load. Important findings were noted in a chest computed tomography (CT), abdominal CT and brain magnetic resonance imaging (MRI) (Fig. 2). Samples of secretion discharge from an abcess in the subcostal region were collected, and direct mycological examination using Parker KOH stain showed yeast cells with multiple budding, consistent with Paracoccidioides species. Double immunodiffusion tests, conducted with an antigen prepared with the standard strain P. brasiliensis B-339, yielded positive results (1:32). The fungus was isolated in Sabouraud dextrose agar at 25°C and 37°C. The molecular identification of the species was performed by species-specific PCR of 43-kDa glycoprotein GP43 locus (exon 2) encoding gene, followed by PCR-RFLP of alpha-tubulin (TUB1) gene, as previously described.17 The patient received amphotericin B lipid complex (cumulative dose of 4600mg), evolving with regression of skin lesions and improvement of dyspnea, chest pain and oxygen saturation in ambient air. Outpatient treatment consisted of sulfamethoxazole-trimethoprim 1600+320mg/daily. After 10 months of regular treatment, the patient remains clinically well (Fig. 1C), without sequelae of PCM or adverse effects of the medication.
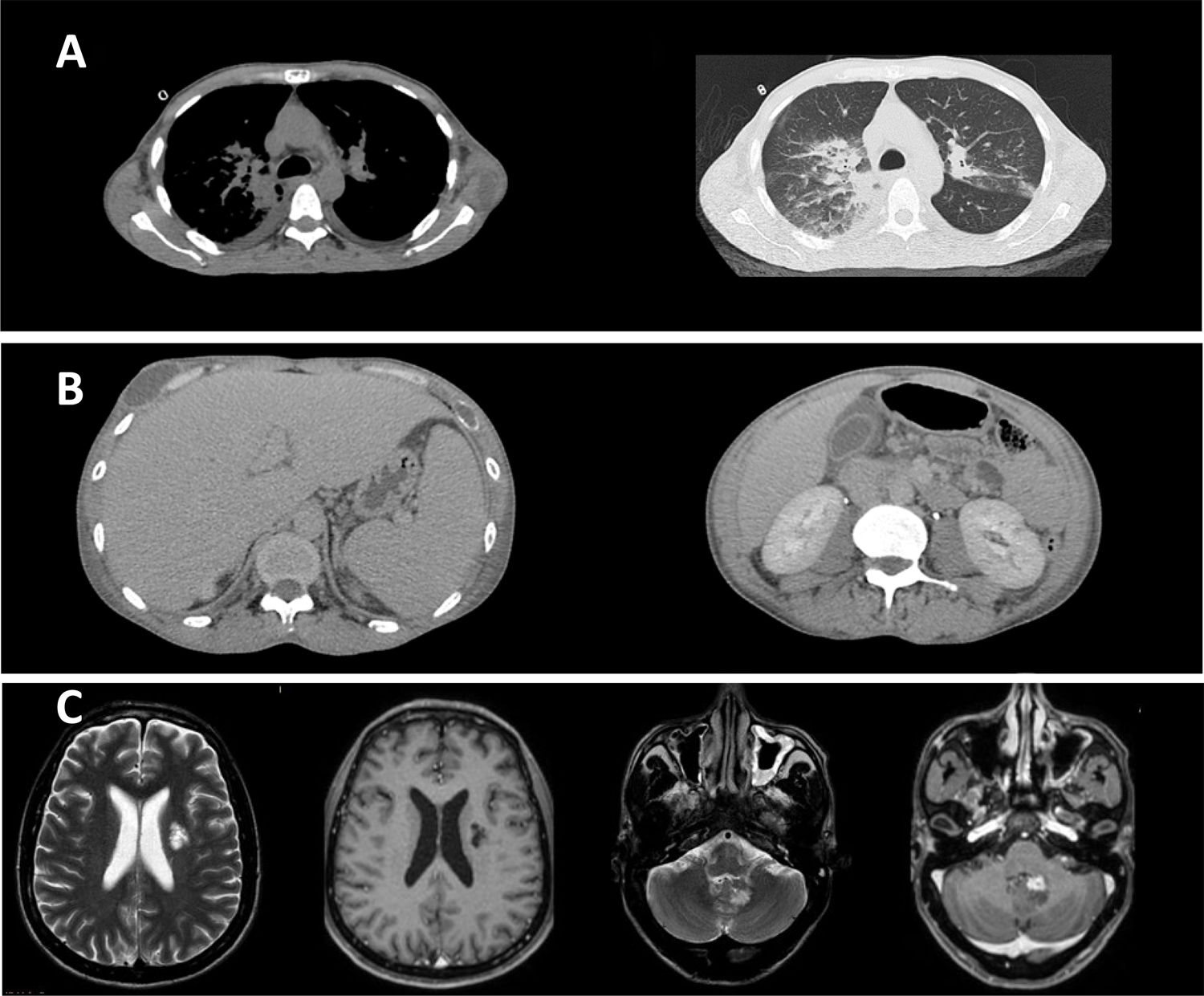
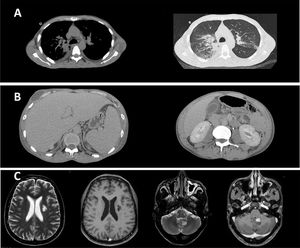
Paracoccidioidomycosis diagnostic by computed tomography (CT) and magnetic resonance imaging (MRI). (A) Chest CT showing: (1) moderate bilateral pleural effusion; (2) bilateral pleural effusion and ground-glass opacities predominating in posterior fields of both lungs, sometimes confluent (consolidations). (B) Abdominal CT showing mesenteric lymphadenopathy with necrotic center, sometimes clustered. (C) Brain MRI showing an area of signal change in corona radiate, characterized by hypersignal in T2-FLAIR and hyposignal in T1, without contrast enhancement. In addition, an intra-axial lesion, partially calcified and with pathological enhancement by venous contrast, was observed near the left lateral wall of the fourth ventricle, without mass effect or peripheral edema.
HIV/PCM co-infection is uncommon compared to other superficial and systemic mycoses as histoplasmosis, pneumocystosis, and cryptococcosis.10 The low frequency of this co-infection may be related to the fact that AIDS is still predominantly an urban disease, in contrast to PCM, that is more common in rural areas.18 In our case, the patient came from an urban area, but he worked in coffee harvesting for long time, where he probably was exposed to the mycotic infection. Diagnosis of PCM is established by a combination of clinical findings and agent identification in histopathological sections, culture of clinical specimen or direct microscopic examination. It is not possible to identify the microorganism at the species level only by morphological characteristics and yeast visualization in direct mycological examination. Thus, molecular tools have been used to accurately discriminate the species. Some molecular markers are applied for this purpose, among which the best choices are gp43, arf, α-tubulin, and hsp70.17,20 Despite the multiple resources listed here, the recovery rate of this fungus in culture is very low, making the study of its molecular properties extremely difficult, and consequently impairing a more detailed study of the species of Paracoccidioides and their clinical implications.5,21
There are four studies (8 cases) in the literature reporting HIV/PCM co-infection in which a molecular approach to identify the isolates has been carried out.4,6,11,12 However, only two of those studies successfully achieved a specific discrimination of the species. The first one to do it was a case-study from Argentina, which identified the species Paracoccidioides americana sp. nov. (PS2) by PCR-RFLP.12 This species has been identified in eastern Brazil and it has been described in a single event in Venezuela. Nevertheless, it is less frequently found when compared to P. brasiliensis.4 In the second study, five isolates recovered from Brazilian patients were identified by partial DNA sequencing of two protein-coding genes (arf and gp43) as P. brasiliensis S1.4P. brasiliensis is constituted by two phylogenetic cryptic species, S1a and S1b, and they are better discriminated by GP43-exon-2 region DNA sequencing.9 These cryptic species have a distinct geographic distribution; they are widely present in Argentina and Brazil.14 A great diversity of PCR-based molecular tools has been developed as viable options for the identification of Paracoccidioides.1,7,8,20 It is worth mentioning that PCR-RFLP-based screening assays are very effective for genotyping, and provide important advantages as inexpensiveness and high discriminatory power depending on the target gene.17 All HIV/PCM Brazilian cases involving P. brasiliensis S1 came from the Brazil's southeast region, including the states of Minas Gerais, São Paulo and Rio de Janeiro. Our case report is the first from the state of Espírito Santo. In five out of eight HIV/PCM cases previously described, there was no record of patients having carried out rural labor, differing from the usual epidemiology of the disease. As HIV infection is spreading in South America's countryside, the possibility of HIV being associated with endemic diseases prevalent in such countries must be considered in our clinical reasoning.11
HIV/PCM patients can present a severe and disseminated disease even with normal CD4+ cell count, as observed in this case report. Nevertheless, most HIV/PCM patients present CD4+ cell counts below 200cells/mm3, suggesting that Paracoccidioides may take advantage of the immunosuppression to shift from quiescent infection to systemic disease.3 Regarding therapeutics for PCM, a particular drug combination is an important strategy in patients HIV/PCM due to the severity and refractoriness in these cases, particularly when there is a low treatment adherence and CD4+ cell counts are lower than 200cells/mm3. Amphotericin B should be promptly administered, followed by itraconazole or sulfamethoxazole-trimethoprim. Treatment duration depends on the severity of the clinical presentation, site of infection, restoration of the host's immune response, as well as on the clinical response to therapy and laboratorial findings.19 In our case, the patient's treatment was based on the above recommendations using amphotericin B for 22 days, followed by sulfamethoxazole-trimethoprim.
Molecular tools are important for an accurate identification of the pathogen, which then allows choosing a successful therapy while also providing epidemiological data. Furthermore, there are no studies about the phylogenetic species of Paracoccidioides in Espírito Santo. Thus, this case report may contribute to the knowledge about this severe disease, its causative cryptic species, and their consequences to the patients. Subsequent studies with more patients should be carried out to assess clinical implications for this type of infection and other peculiarities related to HIV/PCM co-infection.
FundingAMR was supported by grants from the São Paulo Research Foundation (FAPESP 2017/27265-5).
Conflict of interestsNone declared.