Rotator cuff repairs have shown a high level of re-ruptures. It is hypothesised that the use of rhBMP-2 in a carrier could improve the biomechanical and histological properties of the repair.
Material and methodsControlled experimental study conducted on 40 rats with section and repair of the supraspinatus tendon and randomisation to one of five groups: Group 1 (control) only suture; Group 2 (double control), suture and alginate-chitin carrier; Group 3 (alginate-control), the rhBMP-2 was added to the alginate; Group 4 (chitin-control), application of the rhBMP-2 to the chitin; and Group 5 (double sample): the two components of the carrier (alginate and chitin) have rhBMP-2. A biomechanical and histological analysis was performed at 4 weeks.
ResultsA gap was observed in all cases 4 weeks after supraspinatus detachment. The re-rupture rate was 7.5%, with 20% of them in the control-alginate group. Histologically the best results were obtained in the double sample group: 4.5 (3.3–5.0). Double sample were also able to support higher loads to failure: 62.9N (59.8–69.4) with lower rigidity 12.7 (9.7–15.9).
ConclusionsThe use of alginate-chitin carrier with rhBMP-2 improves the biomechanical and histological properties of the repair site in a chronic rotator cuff tear.
La reparación del manguito rotador se acompaña de una elevada tasa de rerrotura. Nuestro objetivo es determinar si el empleo de rhBMP-2 vehiculizada en un transportador híbrido mejora el proceso de reparación en lesiones crónicas del manguito.
Material y métodosEstudio experimental en 62 ratas. A las 4 semanas de la lesión se llevó a cabo una sutura transósea y la asignación aleatoria a uno de los 5 grupos de estudio: 1) grupo control: solo sutura; 2) grupo doble-control: se aplicó además un transportador de alginato-quitina; 3) grupo control-alginato: se añadió rhBMP-2 al alginato; 4) grupo control-quitina: se añadió rhBMP-2 a la quitina, y 5) grupo doble-muestra: se añadió rhBMP-2 a ambos componentes. A los 4 meses se evaluaron los resultados mediante estudios biomecánicos e histológicos.
ResultadosEn todos los casos se observó una brecha osteotendinosa macroscópicamente a las 4 semanas. La tasa de rerrotura fue del 7,5%, ocurriendo el 20% de ellas en el grupo control-alginato. En la evaluación histológica los mejores resultados se obtuvieron en el grupo doble muestra: 4,5 (3,3-5). La carga máxima soportada fue mayor en el grupo doble muestra 62,9N (59,8-69,4) presentando además una menor rigidez 12,7 (9,7-15,9).
ConclusionesEl empleo de la rhBMP-2 vehiculizada en un transportador híbrido de alginato-quitina parece mejorar las características histológicas de la reparación e incrementar las propiedades biomecánicas del tendón en el contexto de una lesión crónica del manguito rotador.
Shoulder pain is the third most common cause of specialised medical consultation.1 Rotator cuff tears present in approximately 30% of the population over 60 and up to almost 60% of people over 80. In the United States alone approximately 450,000 operations per year are carried out, entailing direct medical costs of 7 billion dollars.2 Although several studies recommend conservative treatment for complete ruptures,3 the most standard treatment is surgical reconstruction. Notwithstanding, despite good clinical outcome, re-rupture percentages following repair range from between 16% and 94% depending on the series,4–10 and although clinical outcome after re-rupture are higher than non-suture of a damaged tendon, they are accompanied by a reduction in strength and lower joint balance if compared to a healthy tendon.11
Improved techniques have been developed to reduce the failure rate of repair. They aim to increase the tensile strength of suture, which is linked to both the implant and the suture properties. There are metallic clasps, of re-absorbable materials, thermoplastic polymers, compounds consisting only of sutures, transosseous sutures and all of them have different types of configurations: simple, modified Masson-Allentype, “double-row” configurations (theoretically biomechanically superior, but which have not yet been demonstrated to provide superior functional results).12 Despite these improvements both in implants and in the technique itself, the percentage of suture failure continues to be high, leading to the search for a different type of strategy to improve said repair. In this regard, tissue engineering may play an essential role.
There are many experimental studies which have used growth factors in the rotator cuff injury restoration process. The group of bone morphogenetic proteins (BMP) are growth factors with more powerful osteo-inductive capacities, and there are several experimental studies which recommend the benefits of these in the treatment of rotator cuff injuries, improving the biomechanical properties and promoting tendon differentiation13–16 in others, but this role is not as clear.17 There are therefore many biomaterials which have been tested in vitro, in test animals or in humans, where the aim is to promote injury repair. The carriers are not only used as passive materials, allowing the surrounding tissue to grow over the injury and acting as a scaffolding for it, but they also play an active part as carriers for growth or cell factors.18 In short, the biomaterial selection is critical to the success or failure of the tissue engineering procedure development. This study uses the combination of alginate and chitin as growth factor carriers. Both carriers have been extensively used in tissue engineering procedures.18–21 These are 2 biocompatible and biodegradable materials whose main advantage as a hybrid carrier is their mechanical superiority with regard to either of the 2 carriers in isolation. Furthermore, unlike the isolated alginate or chitin carriers, the hybrid carrier may be prepared with ph neutral solutions, offering the additional advantage of systematically incorporating proteins or drugs in the centre of their matrix with or without minimum denaturalisation.22
Our hypothesis is therefore as follows.
The application of rhBMP-2 loaded into the appropriate carrier, within the context of a chronic rotator cuff injury, may contribute to histological and biomechanical improvement in the regeneration process, following surgical intervention.
Material and methodsA total of 62 male Sprague-Dawley type rats 8 months in age and with weights between 480g and 850g were used for this research study. The study was approved by the Ethical Committee responsible for human experimentation in our centre and all regulations governing international legislation for animal experimentation (86/609/EU) were adhered to.
Obtainment of rhBMP-2s and their carriersThe rhBMP-2s were supplied by Noricum (batch B02B05) similarly to previous procedures.12
With regard to the carriers used, the samples introduced present the following code:
- -
Alginate plus chitin: double control (DC).
- -
Alginate-rhBMP-2 plus chitin: chitin control (CC).
- -
Alginate plus chitin-rhBMP-2: alginate control (AC).
- -
Alginate-rhBMP-2 plus chitin-rhBMP-2: double sample (DS).
The alginate was prepared (2% Alginic Acid, A28309 Sigma–Aldrich) in acetic acid (1%). It was left agitating for 2h at room temperature and filtered by 0.22μm in a sterile condition with the preparation of proportions of 200μl final volume in a vertical laminar flux bell.
The CC sample was prepared with alginate 160μl and 40μl of rhBMP-2 (1mg/ml) protein. It was left agitating at 4°C over night. The following day it was mixed with approximately 3mg of chitin (Sigma–Aldrich, Chitin, C3641) in a gamma radiation sterile powder. By constant pipetting it was mixed again and subsequently the film was made in sterile conditions. The solvent was left to dry for 24h (solvent evaporation technique).
The AC sample was prepared in the same way but the alginate did not contain the rhBMP-2 protein. 3mg of chitin was added to it. The chitin with protein (40μl) was agitated overnight at 4°C. The alginate was prepared the following day and mixed with the chitin and protein preparing films under the same conditions.
The DC sample was prepared the same day as the films, mixing 200μl of alginate (2%) with 3mg of chitin to later make the film. It was left to dry for 24h until the next day.
The DS sample contained protein in 2 carriers; 140μl of alginate (2%) and 40μl of rhBMP-2. It was agitated at a 4°C overnight together with another test tube which contained the 3mg of chitin and 40μl of protein. The following day the contents of both test tubes were mixed together and the film was prepared.
The films were implanted after being submerged in calcium chloride at 0.2M and previously filtered under sterile conditions.
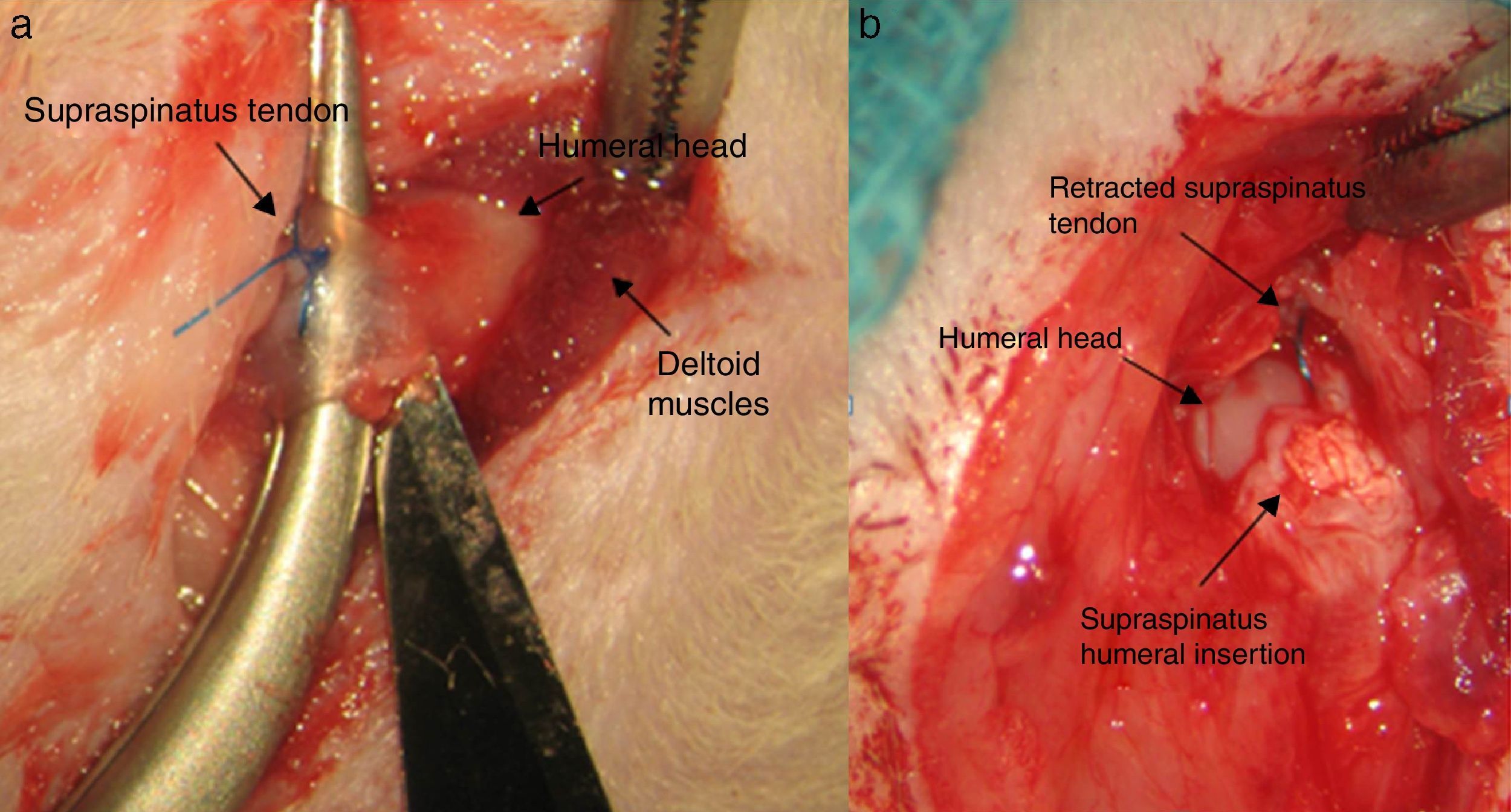
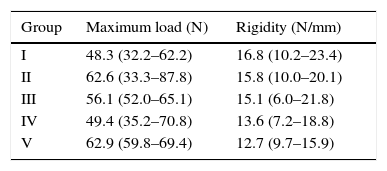
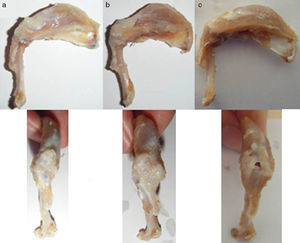
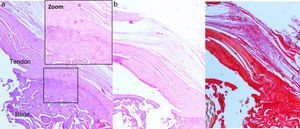
Surgical techniqueThe animals operated on underwent anaesthetic induction with 0.3mg/kg (Domtor®, Pfizer, Madrid, Spain) of medetomidine and 0.3mg/kg (Fentanest®, CERN Pharm, Barcelona, Spain) of phentanile, both intraperitoneal. Maintenance was carried out with isoflurane (2%) (Isoflo®, Esteve, Barcelona, Spain). The first dose of antibiotic therapy was administered on initiation of the anaesthesia (enrofloxacin 5mg/kg/24h s.c.) and on termination of surgery a dose of anti-inflammatory (meloxicam 0.2mg/kg/24h s.c.) and analgesic (buprenorphinea 0.1mg/kg/12h s.c) was administered. With the animal in a supine position and the limb to be operated on in abduction and internal rotation, a surgical incision of the skin in the anterior area of the shoulder was made using the MC1 Leica microsurgical microscope (Leica Microsystems, Schweiz). The subcutaneous plane was sectioned up to the deltoid muscle, which was opened with a “T” incision, de-inserting the anterior and middle abdomens, with the supraspinatus tendon remaining exposed; this was marked with Prolene® 5-0 (Ethicon, Johnson and Johnson, U.S.A.) and was then sectioned with the point of a scalpel number 11 perpendicular to its greater axis and at a distance of 4mm from its insertions, resecting the remaining sinewy stump to the troquiter and debriding, with the scalpel, the remains of this at insertion site, leading to its retraction (Fig. 1). During the surgical technique particular care was taken to maintain the remainder of the rotator cuff tendons intact and the scapula-humeral joint structures. Finally, the deltoid muscles were reinserted to the acromion, clavicle and trapezoid muscles with polyglactin 910 (Vicryl® 5-0) and the skin was closed with simple suture stitches.
During the first 3 days the animals received antibiotic, anti-inflammatory and analgesic treatment depending on the previous doses. During the whole post-operative period they were given free movement within the actual limitations of their cages. The surface area of each cage was 800cm2 and 14cm high, with a temperature maintained between 20 and 24°C, with relative humidity of 65% and periods of exposure to light for 12h. The animals were fed a standard diet (Panlab SL) and were given water as needed.
Random selection of the test animals was made using an IT programme (Excel, Microsoft, Redmond, VA, U.S.A.).
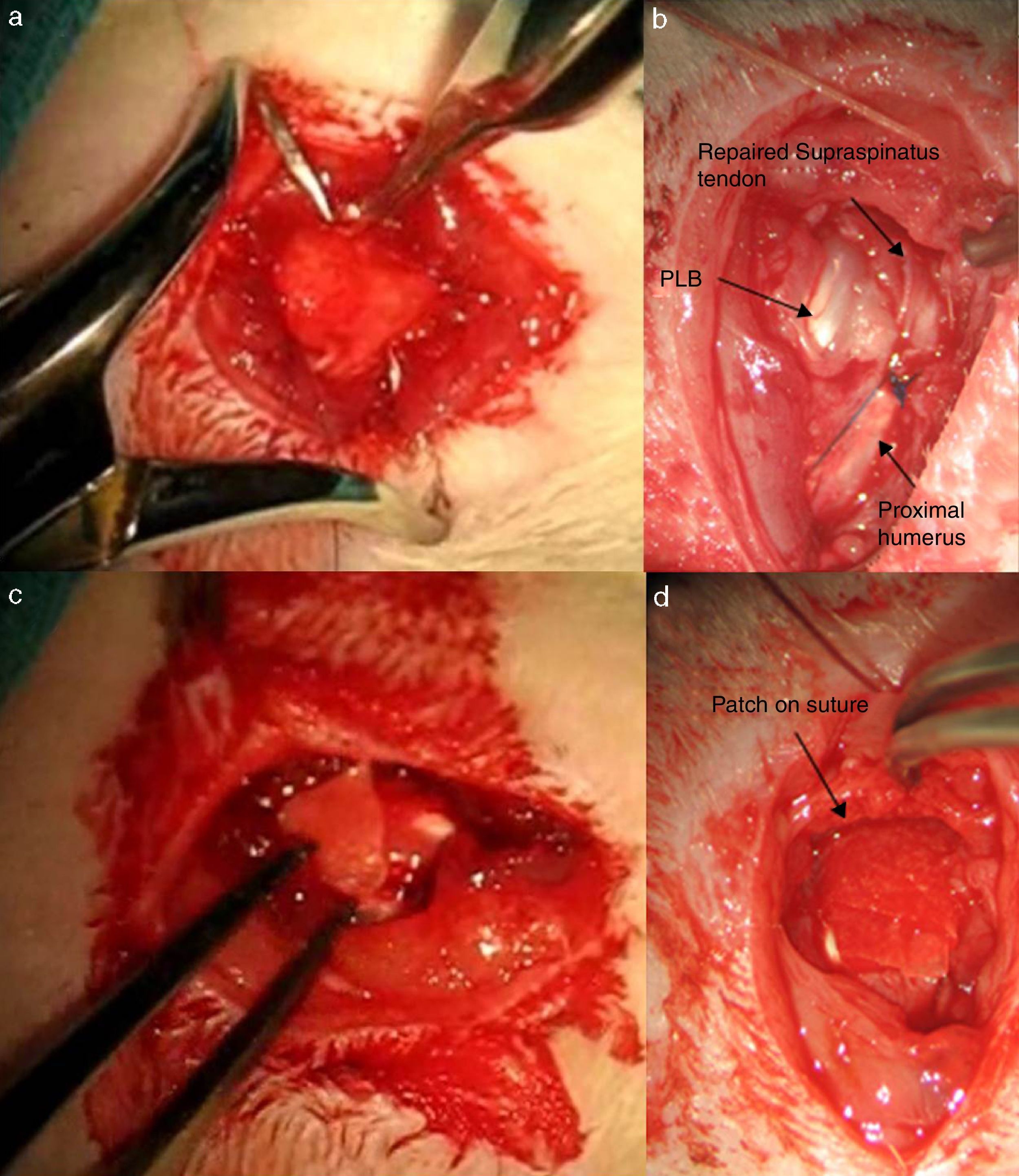
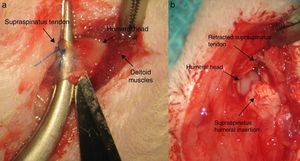
After 16 weeks of the previous process, with a chronic injury model having been generated, as reported in previous studies23,24 tendon repair was made with transosseous suture with Prolene® 6/0 (Ethicon, Johnson and Johnson, U.S.A.) (Fig. 2) either alone or with the additional use of a chitin/alginate with/without rhBMP-2 carrier.
The rats were divided into 5 groups according to the type of tendon repair made:
Group I: control group C. A total of 10 rats were included in this study group. After selection had been made repair of the tendon was performed using transosseous suture with Prolene® without any other types of materials as support.
Group II: double control group DC. A total of 10 rats were included in this study group. After tendon section had been performed, repair of the same lasted 4 weeks using the same suture as the previous group, with additional support from an alginate and chitin patch. Said patch was sutured with loose stitches around the tendon using non-re-absorbable polypropylene 7/0 material (Prolene® 7/0 Ethicon, New Jersey, U.S.A.). The results were assessed in the same way as the previous group with the same number of samples.
Group III: alginate control group AC. A total of 10 rats were included in this study group with a tendon repair using the previously described suture and a chitin patch with BMP-2 and alginate without BMP-2. Analysis of the results corresponds to that described in the previous groups.
Group IV: chitin control group CC. A total of 10 rats were included in this study group with supraspinatus tendon suture four weeks after section had been performed. A chitin patch without BMP-2 and alginate with BMP-2 was also used as support.
Group V: double sample group DS. For this part of the study 10 tendons of the previously sectioned supraspinatus were sutured. During surgery a patch with chitin with BMP-2 and alginate with BMP-2 was added to analyse the results both histologically (n=5) and biomechanically (n=5).
At the end of the observation period, the animals were sacrificed and the extraction of the specimens was performed consecutively by interscapulothoracic amputation. The samples were preserved at −20°C up to the performing of the biomechanical study, prior to which thawing took place at room temperature.
Macroscopic assessmentThe existence of the chronic injury was determined during the first phase of the procedure (absence of tendon continuity and presence of a gap between marked tendon and its insertion into the troquiter), and the presence of tendon repair (macroscopic continuity of the injured tendon), re-rupture (lack of continuity) and muscular atrophy after the sacrifice of the test animal.
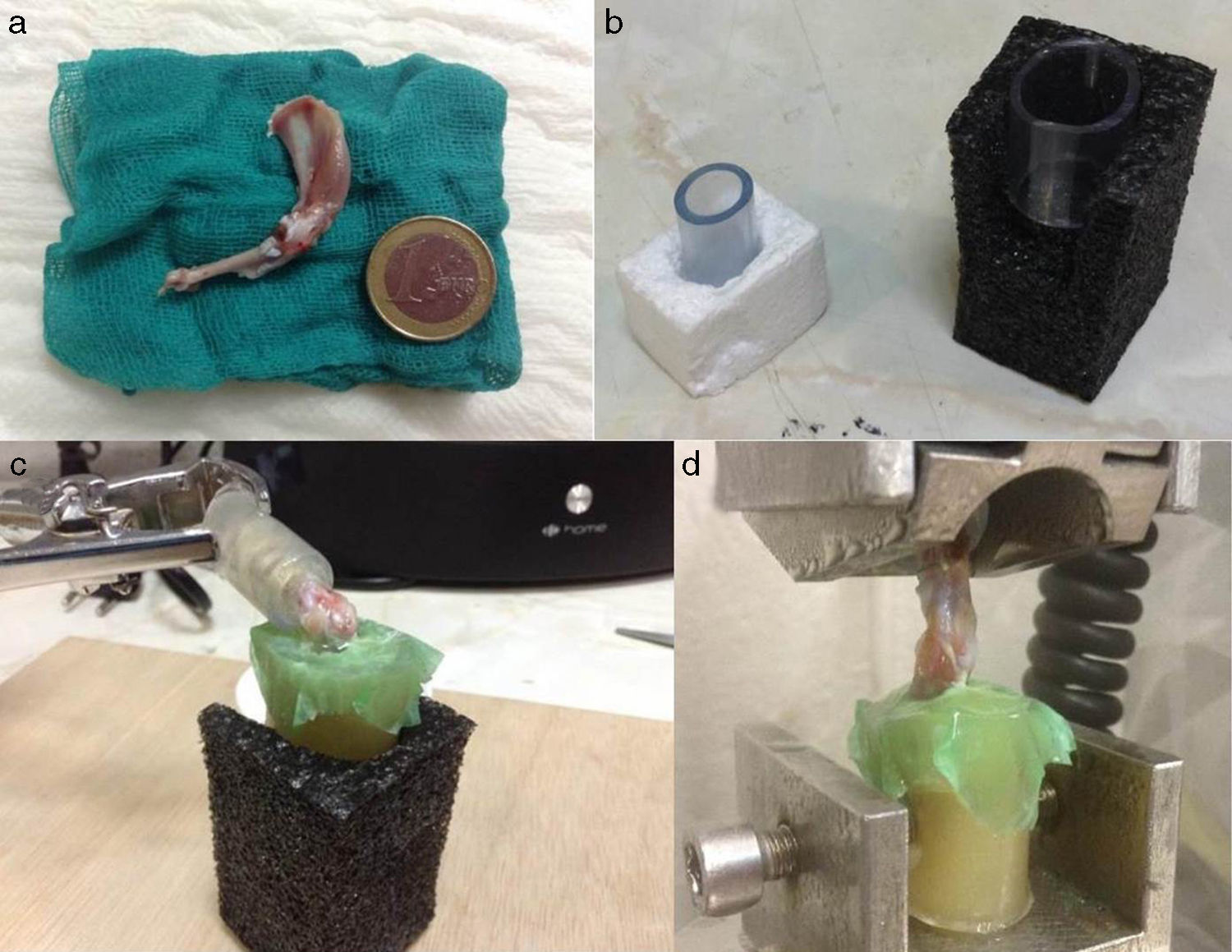
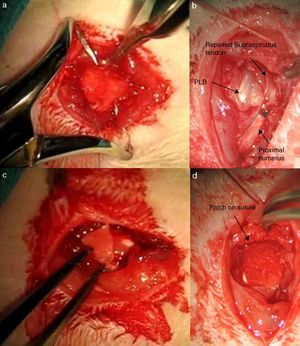
Biomechanical and histological studyThe preparation of samples for biomechanical study consisted of funnelling the limb on an epoxy type resin (Araldit Rápido-Ceys®) inside several plastic tubes which were fixed by clamps to the traction machine, thus enabling single axis traction. To increase the strength of the rubbing between limb and resin the scapula and humerus were wrapped in an elastic band and several drops of glue were deposited on the rubber-limb contact. It is important that the glue does not reach the joint area. Once the resin had cooled and acquired some resistance, we did the same at the scapula end. To maintain humidity throughout this time, we wrapped the joint with a damp dressing and we left a humidifier on to keep the atmosphere humid as well (Fig. 3). The samples were inserted forming a 90° angle (physiological position). The rotator cuff tendons were tested in parallel to the longitudinal axis of the supraspinatus tendon in its physiological position. During the test the traction speed rose to 1.8mm/min. The samples were subjected to load cycles of up to 10Newtons (N) for rats of 451–600g and 5N for rats weighing 300–450g; they were unloaded up to the initial point and the load was repeated with several new cycles up to the point of failure.
All the biomechanical tests were performed using a traction machine (Instron 5866®) which registered the maximum load values in N and the deformation in mm the rigidity of the tested tendon was then calculated. The definitions of the recorded values were: maximum load: strength necessary for permanent tissue rupture; deformation or deformity: displacement used to modify the initial form of the tendon; rigidity: the rigidity of the repaired tendon is defined as that pending maximum load divided by the displacement at the point of failure or maximum load.
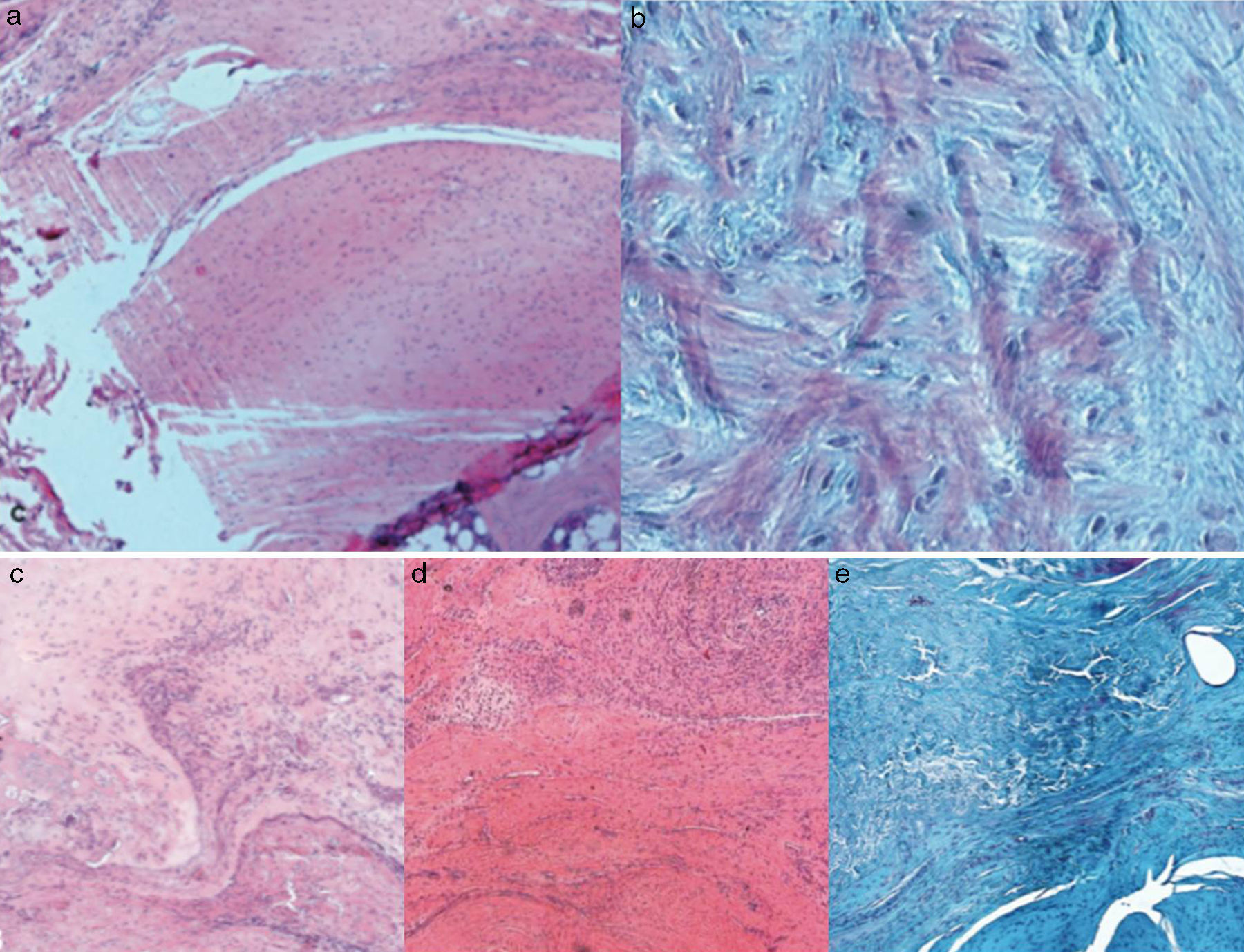
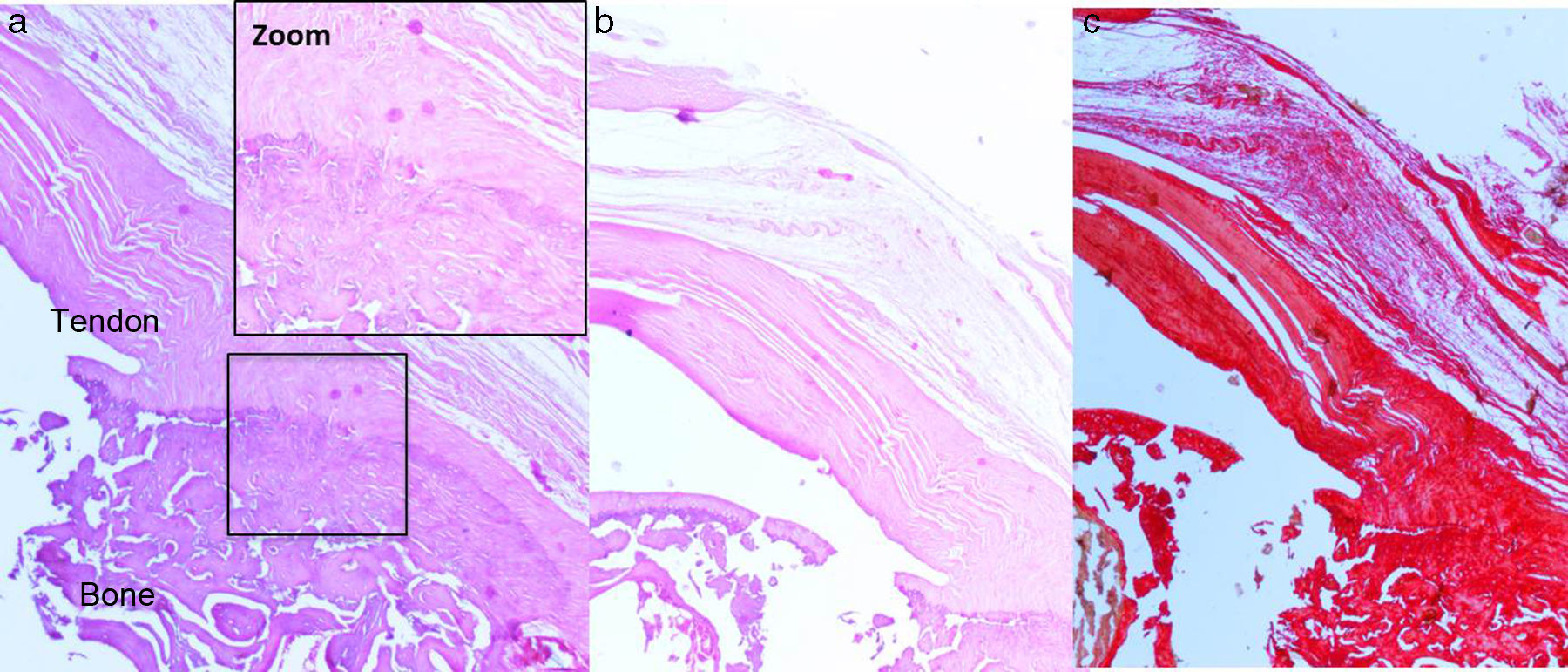
For the histological study, after fixing the samples in a 4% formaldehyde solution, decalcified with an EDTA solution in paraffin and cut with microtome, stains were performed with haematoxylin–eosin, Masson trichrome stain and syrian rue. Assessment of the specimens was made by a pathologist who was not familiar with the experimental group to which the sample belonged. To perform histological stain assessment both in the cases of chronic injury and the cases of repair, a semi-quantitative assessment scale was used based on those used in previous studies,25,26 for which the following parameters were assessed:
- 1.
Extracellular collagen matrix: orientation of the fibres which run parallel or oedema and disorganisation of fibres.
- 2.
Tendon transition area to bone: normal trabecular transition between bone and tendon, presence of collagen particles with less uniform direction or absence of transition area.
- 3.
Inflammation of bursa: presence or absence of inflammatory infiltration in the bursa which surrounds the sinewy matrix: lymphocytes, mastocytes, macrophages, etc.
- 4.
Vascular proliferation: presence of arterioles which run parallel to collagen fibres, irregular vascularisation or presence of thin wall blood vessels which run in a disorganised manner, even perpendicular to collagen fibres.
- 5.
Cellularity: presence of tenocytes (elongated cells with a small nucleus), presence of cells with rounded nuclei and increase of cellularity.
- 6.
Fat degeneration: presence or absence of adipocyes.
- 7.
Chondroid metaplasia: absence of chondrocytes or presence of chondrocytes inside the tendon matrix.
- 8.
A general scale or score of 4 points was established, quantifying each previously described parameter using 4 values which corresponded to: 0 normality, 1 mild, 2 moderate, 3 severe. Therefore, the normal tissue scored 0 and the worst result reached a score of 15 points. The parameters assessed are summarised in Table 1.
Table 1.Parameters of the general histological assessment scale.
Matrix Inflammation of the bursa 0 Parallel collagen fibres No 1 More diffuse fibres and with loops Yes 2 Presence of regular shaped waves Inflammatory infiltration in the tendon 3 Irregular fibres and presence of metachromatic substances Vascularity Transition 0 Arterioles parallel to collagen fibres Appearance of natural regions in bone-tendon transition 1 Irregular vascular pattern Areas with gaps in the transition region 2 Increased thin walled blood vessels Gaps and absence of transition 3 Blood vessels perpendicular to the fibres and nodular in appearance Cellularity Chondroid metaplasia 0 Elongated cells with thin nuclei Absence 1 Elongated cells with rounded nuclei Presence 2 Increase of cellularity 3 Regions without cells Each parameter is quantified as: 0 normal; 1 mild; 2 moderate, and 3 severe. The normal tissues scores 0 points and the worse results score 15 points.
During study design a calculation of statistical power was made using previously published data which detected differences of at least 25% in the load necessary up to failure of repair. In this way precisely 10 specimens by group for the biomechanical study were determined, which would provide a statistical power close to 80% with a type I error of 0.005.
The data analysis began with a normality study of distributions. For this reason, due to the population size, the Shapiro–Wilk test was made, thus confirming that the requirements of a normal distribution were not fulfilled. Following this, bearing in mind the characteristics and data obtained from the normality study, for the intergroup comparison the Kruskal–Wallis non-parametric test was carried out. Statistical significance was established at p<0.05.
ResultsTwo animals died as a result of anaesthesia complications and immediately after the operation. These were replaced so as to complete the predetermined number of test animals for conducting the study.
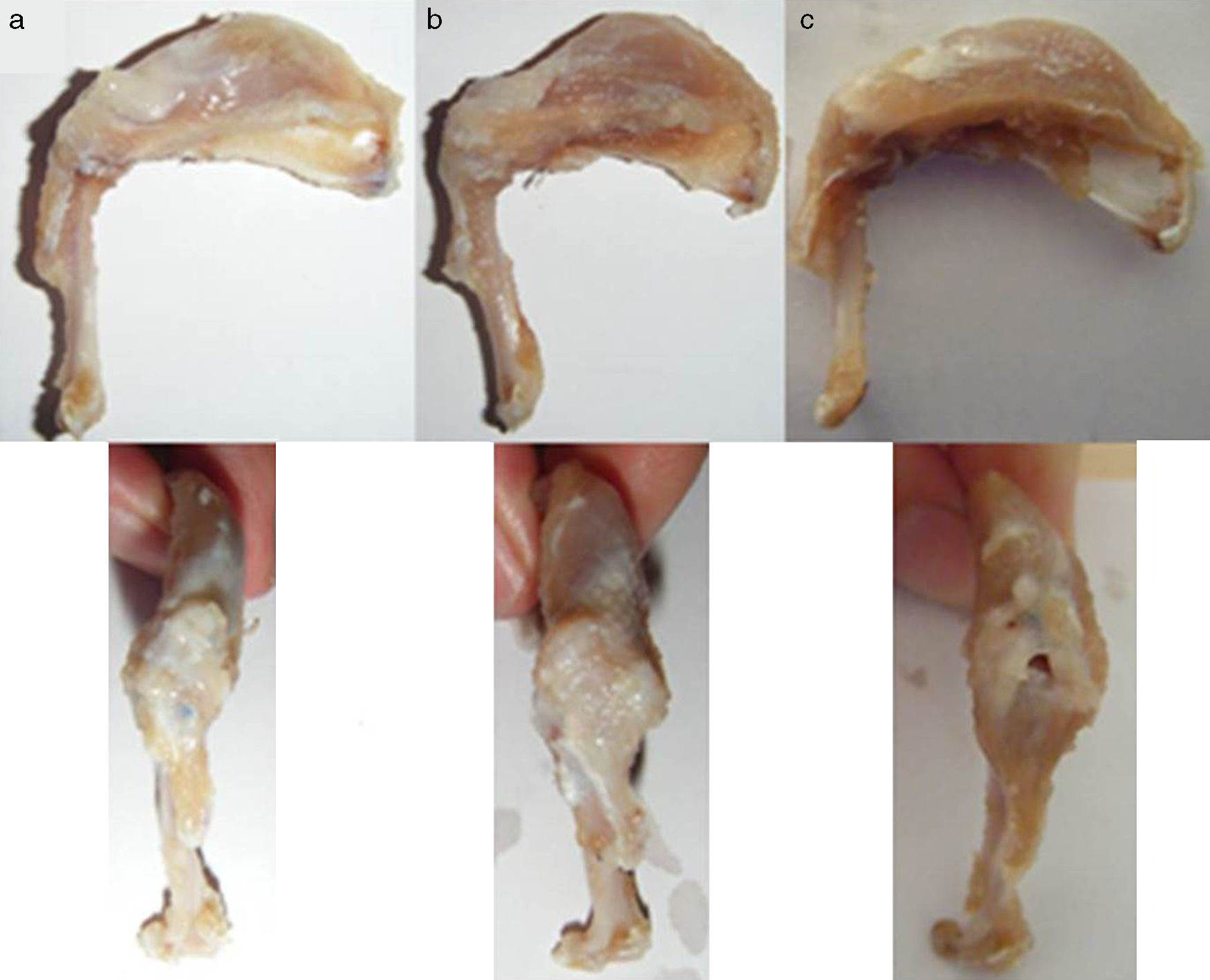
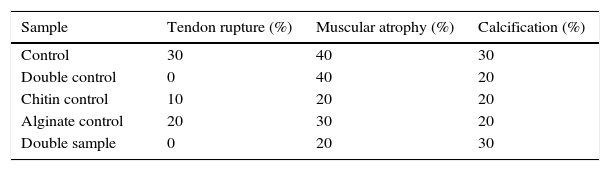
Macroscopic findingsAfter primary surgery the macroscopic assessment showed the presence of a rupture of the tendon in its insertion in the troquiter with tendon retraction in 100% of cases (Fig. 4). Repair of said injury led to the restoration of the tendon insertion into the troquiter in all cases but in 3 of them this suture was found to be excessively tense. Final macroscopic assessment, after the animals had been sacrificed, revealed the continuity of the tendon in all of the animals except in 3 cases, where there had been a re-rupture and all 3 cases coincided with those where the suture had been excessively tense. Furthermore, in 6 cases we discovered some type of macroscopic calcification (Fig. 5).
The macroscopic characteristics of the specimens under study are summarised in Table 2.
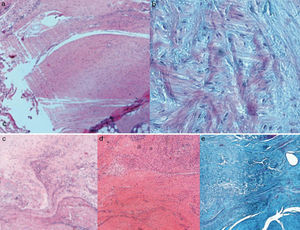
Histological findingsChondroid metaplasia and disorganisation of collagen fibres was observed in Group I as a consequence of the tendon degeneration and as an increase in vascularisation. Disorganisation of collagen fibres predominated in Group II, as did the appearance of fibroblasts and the absence of a correct bone-tendon transition with the absence of fibro cartilaginous regions, as occurs naturally in the tendon. In Groups III and IV, unlike the 2 previous groups, the fibres appeared more ordered and there was less vascularisation (Fig. 6). Finally, in Group V the inflammatory component was considerably lower and the tendon-to-bone transition was very similar in appearance to that found under physiological conditions.
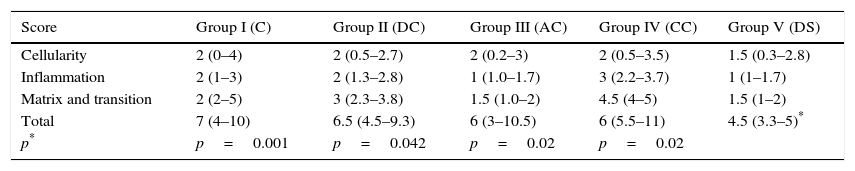
The histological score obtained by the groups with carrier with or without rhBMP-2 (Groups II, III, IV, V) was significantly higher (p<0.05) to that achieved by the Group with simple suture (Group I). In turn, comparing the total score obtained by the Groups which contained rhBMP-2, on the histological scale there was a significantly lower score statistically in Group V compared with Groups III and IV respectively. The results are summarised in Table 3.
Score obtained by the different groups on the histological assessment scale.
| Score | Group I (C) | Group II (DC) | Group III (AC) | Group IV (CC) | Group V (DS) |
|---|---|---|---|---|---|
| Cellularity | 2 (0–4) | 2 (0.5–2.7) | 2 (0.2–3) | 2 (0.5–3.5) | 1.5 (0.3–2.8) |
| Inflammation | 2 (1–3) | 2 (1.3–2.8) | 1 (1.0–1.7) | 3 (2.2–3.7) | 1 (1–1.7) |
| Matrix and transition | 2 (2–5) | 3 (2.3–3.8) | 1.5 (1.0–2) | 4.5 (4–5) | 1.5 (1–2) |
| Total | 7 (4–10) | 6.5 (4.5–9.3) | 6 (3–10.5) | 6 (5.5–11) | 4.5 (3.3–5)* |
| p* | p=0.001 | p=0.042 | p=0.02 | p=0.02 |
Median value (25–75 percentiles). The higher the score, the worse the result.
C, control; AC, alginate control; CC, chitin control; DC, double control; DS, double sample.
When comparing the 5 Groups it was observed that the shoulders in Group V had less rigidity than those in the other groups, with statistically significant differences only in Groups I and II (p=0.029, p=0.003). The mean maximum load up to failure was 48.3N for Group I, 62.6N for Group II, 56N for Group III, 49.4N for Group IV and 62.9N for Group V. The results of this assessment are contained in Table 4.
Values obtained by the different groups in the biomechanical assessment.
| Group | Maximum load (N) | Rigidity (N/mm) |
|---|---|---|
| I | 48.3 (32.2–62.2) | 16.8 (10.2–23.4) |
| II | 62.6 (33.3–87.8) | 15.8 (10.0–20.1) |
| III | 56.1 (52.0–65.1) | 15.1 (6.0–21.8) |
| IV | 49.4 (35.2–70.8) | 13.6 (7.2–18.8) |
| V | 62.9 (59.8–69.4) | 12.7 (9.7–15.9) |
Median value (25–75 percentiles).
The principal aim of rotator cuff surgery is repair of the tendon rupture to re-establish continuity between the muscle and the bone, in order to recover strength and function.
Acute rotator cuff tears must be immediately repaired, if possible within the first 3 weeks. Unfortunately, this situation is not standard in daily clinical practice. Patients usually present for consultation with chronic tears where the rotator cuff has retracted, is fibrous and with degeneration of the tendon edges and associated muscular atrophy. This is the type of tear which leads to a high rate of “failure” following its repair. In the light of all of this, the experimental model we used for the chronic injury is the model which is fairly similar to the real setting of daily clinical practice.
The majority of works published using animal models used acute injuries in their studies,27,28 without consideration of the fact that a restorative response depends on whether the injury is acute or chronic in its development. In chronic injuries the predominant characteristic is tendon degeneration and tendon quality is influential in the restorative response. Several authors, including Gimbel et al.,29 in chronic injury models observed that there was a natural tendon regeneration. In our study we did not find any tendon regeneration. Macroscopic findings confirmed the presence of a hole or gap in the region where the supraspinatus tendon should have been, and there was therefore no natural recuperation of the tendon on follow-up surgery, thus confirming the validity of the model used. The absence of this spontaneous regeneration activity could correspond to the age of the animals used. Studies exist30 which used young animals in the injury model; we used more mature animals (8 months old) resulting in a considerably reduced restorative response and thus mimicking more precisely that which occurs in humans.
Our initial hypothesis was that the use of the carrier selected in combination with rhBMP-2 improved the repair process, both histologically and biomechanically.
Histological findings indicated an improvement in the tendon quality of the shoulders which had a carrier, even without rhBMP-2, compared with those of Group I which were only repaired with Prolene®. This could be related to 2 events. On the one hand we believe that the carrier may provide resistance to the enzymes responsible for tendon degeneration, lowering to some extent the inflammatory process, and on the other, the carrier reinforces the tendon suture providing an additional biomechanical support to the suture. The combination of alginate and chitin is essential here. There are many materials which may be used in the processes of tissue engineering but few combine ideal characteristics. This has to consist of a biodegradable material but one which is capable of providing the appropriate mechanical support until tendon tissue regeneration has taken place.
The carrier we used here combines an extremely biocompatible material, alginate, with another, chitin, which has demonstrated its excellent capacity for cellular adhesion in previous studies.20 This capacity appears to be linked to the cationic nature of the biomaterial which leads to electrostatic interactions with anions such as glycosaminoglycans, proteoglycans, etc.21 The said interactions serve as mechanisms for the retention and uptake of cells, growth factors and cytokines, which convert this carrier into an ideal hybrid material in tissue engineering processes.31 Another additional advantage is that the fibroblasts embedded in this carrier mainly produce type I collagen, which is the collagen to be found mostly in health tendons and ligaments compared with type II collagen which is mainly found in injured tendons and ligaments and which is also biomechanically inferior to type I.
Furthermore, the results obtained by Group V (double sample group) were superior. In other words, when we added the bone morphogenetic protein (rhBMP-2) associated with 2 biomaterials (chitin and alginate) to constitute our hybrid transporter, the quality of the repair was greater, statistically significantly, than the other groups. Biomechanically, the rigidity of the tendons with rhBMP-2 was lower than in the other groups (albeit only significantly compared with the groups which did not contain rhBMP-2). This was justified by the presence of a higher elasticity in the tendons which contained rhBMP-2. As distortion increased due to greater elasticity, the result of the load/deformity ratio corresponding rigidity diminished. Between 2 materials with an equal maximum load at breaking point the one which presents lower deformity prior to breaking is more fragile and therefore is the one which has absorbed less energy before breaking. Thus, contrary to what may appear, on assessing the outcome, the repaired tendons with carrier and rhBMP-2 were more resistant.
The in vitro action of BMP-2 on tendon cells belonging to the rotator cuff has been analysed in previous studies.32 The application of the BMP-2 significantly increases production of type I collagen but not its expression.
Several authors support these results. Pelled et al.33 analysed the histology and biomechanics of the Achilles tendon in mice which had been treated with surgery, BMP-2 composites and SMAD8 intracellular protein. The histological analysis revealed better cellular distribution and greater functional recovery of the group treated with both composites. Similarly, Kim et al.34 were able to improve the repair at the bone-tendon level by injecting BMP-2 carried in collagen into the insertion site of the clamps used for patellar tendon repair in rabbits. With the addition of the protein, both the histological characteristics of repair were improved and the mechanical strength of them was raised considerably. Chen et al.35 used periosteum progenitor cells BMP-2 hydrogel (polyethylene glycol diacrylate), and found that after 8 weeks the formation of fibrocartilage and bone increased in the tendon-bone junction in rotator cuff tears in rabbits. Immunohistochemical techniques are able to prove the presence of aggrecans and type II collagen. Finally, biomechanical studies confirm an increase in the load the tendon is able to support prior to its failure at the said level. The only doubt we are left within this study is how much to attribute to each of the elements used in tissue engineering. Is improvement the result of using BMP-2, the cells or the combination of both elements? The contribution of cells to the restorative process, though not the aim of our study, continues to be a controversial issue. Whilst there are authors who have been able to prove how their use improved the maximum load the tendon can bear,36 others,37 report that the use of stem cells derived from lipoaspirate does not recreate the cellular organisation of entesis nor does it improve the biomechanical properties of it.
In our study we obtained better tendon regeneration in all groups with carriers compared with simple suture, and when the carrier was associated with rhBMP-2 these results improved. It appears that the greater the concentration of rhBMP-2, the better the regeneration tissue characteristics. No differences between the control alginate and the control chitin group were found which suggests the absence of biochemical differences in relation to the combining of the morphogenetic protein to one or other component of the hybrid carrier. The highest rate of re-ruptures found in the alginate control group were attributed to the presence of a larger number of sutures under tension compared with a higher retraction of the tendon on creating the chronic injury model and not to the carrier characteristics themselves.
We may therefore conclude from the results obtained that the use of an alginate-chitin hybrid carrier with rhBMP-2 appears to have a beneficial effect on tendon repair at the tendon-to-bone interface, and these changes may have an effect on the improvement of the tendon's biomechanical properties. Notwithstanding, due to the amount of factors involved in the restorative response, further studies would be necessary to optimise aspects such as the ideal dose of rhBMP-2 and the durability of the results obtained.
Level of evidenceLevel of evidence I.
Ethical disclosuresProtection of people and animalsThe authors declare that the procedures followed conform to the ethical guidelines of the Committee responsible for human experimentation and are in keeping with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FinancingThis article was financed by a grant provided by the SECOT Foundation for Basic Research, 2013.
Authors contributionYL and CA contributed in equal measure to the preparation of this document.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Lopiz Y, Arvinius C, García-Fernández C, Rodriguez-Bobada MC, González-López P, Civantos A, et al. Reparación de las lesiones crónicas del manguito rotador mediante diferentes compuestos. Rev Esp Cir Ortop Traumatol. 2017;61:51–62.