To assess whether a peripheral intravenous infusion of adipose tissue stem cells (ATSC), after an ischaemic spinal cord injury, can promote selective cell migration and cell survival in the damaged neural tissue.
Animals and methodAn ischaemic spinal cord injury was provoked by trapping the abdominal aorta for 20min in 11 male New Zealand rabbits (2.5±0.5kg). They were randomised into two groups: one group (n=5) received an intravenous transfusion of 10±2×106 ATSC at 24h from the injury, and the control group (n=6) were only given the vehicle. The functional status was assessed, using the Tarlov scale at 24h, and 7 and 14 days. The animals were sacrificed at 14 days and a histological and immunohistochemical study was performed.
ResultsComplete paraplegia was achieved in both groups. There were no significant differences as regards neurological recovery, which was nil in both cases. In the histological and immunohistochemical study, it was tested to see whether there was any bromodeoxyuridine-marked ATSC in the area of the lesion, but there was only a small amount.
ConclusionATSC are able to migrate and survive in the injured spinal cord after aortic ischaemia after they have been administered intravenously. Intravenous infusion is a harmless procedure with no side effect. No neurological recovery was achieved.
Valorar si la infusión intravenosa periférica de células madre procedentes del tejido adiposo (CMDG) después de una lesión medular isquémica, promueve la migración celular selectiva y la supervivencia celular en el tejido neural dañado.
Animales y métodoSe utilizaron once conejos macho Nueva Zelanda (2.5±0.5kg) a los que se les provocó una lesión medular isquémica mediante pinzamiento de la aorta abdominal durante 20min. Se aleatorizaron en dos grupos: un grupo (n=5) recibió una infusión intravenosa de 10±2x106 CMDG a las 24h de la lesión y el grupo control (n=6) sólo el vehículo. Se valoró el estado funcional a las 24h, 7 y 14 días mediante escala de Tarlov. A los 14 días los animales fueron sacrificados y se realizó el estudio histológico e inmunohistoquímico.
ResultadosEn todos los casos de ambos grupos se obtuvo una paraplejia completa. No hubo diferencias significativas en cuanto a la recuperación neurológica, que fue nula en ambos grupos. En el estudio histológico e inmunohistoquímico se comprobó la presencia de CMDG marcadas con bromodiuxerina en la zona de la lesión aunque en poca cantidad.
ConclusiónLas CMDG tienen capacidad de migrar y sobrevivir en la médula espinal lesionada tras isquemia aórtica una vez que se han administrado de forma intravenosa. La infusión intravenosa es un procedimiento inocuo y sin efectos secundarios. No hemos obtenido recuperación neurológica.
Implantation of mesenchymal stem cells (MSC) is considered as a promising treatment to achieve improvement in patients who have suffered spinal cord injury (SCI). An increasing number of experimental studies have demonstrated the positive effects of MSC implantation in SCI recovery.1 MSC can be isolated from tissues such as bone, periosteum, muscle, synovium or fat.2 The differentiation of stem cells in vivo is dependent on the environment into which they are implanted and the environment in which they develop.3,4 Recent studies have shown the capacity for expression of neural markers on the surface of MSC obtained from bone marrow.5,6 It has been shown that MSC can differentiate into astrocytes, microglia and neurons both in vivo and in vitro.7–9 Fatty or adipose tissue also contains certain stem cell populations.10,11 The genetic profile of these adipose tissue stem cells (ATSC) is similar to that of bone marrow tissue stem cells (BMTSC).12 ATSC can differentiate into adipocytes, chondrocytes, myocytes, osteoblasts and even neural lines.10
SCI can lead to cell death, particularly of neurons, oligodendrocytes, astrocytes and precursor cells.13 SCI culminates in the formation of a glial scar, a multifactorial process in which transmembrane molecular inhibitors of axonal growth are expressed.14 ATSC could have a therapeutic potential in neurological diseases, since functional recovery has been described following in vivo transplantation of ATSC into SCI areas in rodent and canine models with spinal lesions.15,16
There are very few published works which have demonstrated the potential usefulness of a peripheral infusion of these cells in the treatment of ischaemic lesions.17 Intrathecal or direct injection of cells entails a risk of damaging the spinal cord, so we consider that a safer means to use these treatments clinically should be sought. Injection into the peripheral venous system can be considered as a promising option as it is safer, faster and easier.
The objective of this study is to verify whether, following ischaemic spinal cord injury, peripheral venous injection of adipose tissue stem cells may selectively promote cell migration and cell survival in the damaged neural tissue. A secondary objective of this model of ischaemic spinal cord injury in rabbits is to assess a possible improvement in neurological function.
Materials and methodsAnimalsWe used 11 male, New Zealand rabbits, weighing 2.5±0.5kg. The animals were kept under perfect temperature conditions, with ample space and with free access to food and water. The experiment was designed according to the ethical guidelines of the International Association for the Study of Pain18 and was approved by the Animal Welfare and Ethics Committee of our hospital. It also followed the legal regulations regarding the use of experimental animals.
The rabbits were randomly assigned to 2 groups based on their treatment following spinal cord injury. The first group (n=5) received an intravenous infusion of 10±2×106 adipose tissue stem cells 24h after injury. The control group (n=6) only received the support vehicle for the stem cells (5ml of Dulbecco's modified eagle medium [DMEM] [Gibco®]). The cells were injected through a 14-G venous catheter into the marginal vein of the ear.
Cell isolation and cultureThe stem cells were obtained from adipose tissue from the groin of the rabbits under sterile conditions. The samples were cut and digested in PBS buffer at 37°C with 0.075% type I collagenase (Gibco® BRL, Paisley, UK) for 30min. Mature adipocytes were eliminated by centrifugation (300×g, 5min) and low density mononuclear cells were isolated by centrifugation through a density gradient (670×g, 30min) and grown in DMEM with 10% foetal bovine serum (FBS) and 0.1% of antibiotic-antimycotic (Gibco®) solution within a moist chamber at 37°C.
In order to extract nonadherent cells, the medium was changed at 24h and subsequently every 4 days. For subculture, the cells were resuspended in trypsin 0.05 (v/v) in Hank's balanced salt solution (HBSS) (BioWhittaker® Europe, Verviers, Belgium) when a confluence of 80% was reached. They were labelled with bromodeoxyuridine (BrdU), a thymidine analogue, for subsequent location within tissue, 48h prior to injection.
Ischaemic spinal cord injuryWe channelled the marginal vein of the ear of the animals using a 24-G intravenous catheter and performed anaesthetic inducement through a bolus of propofol (25mg/kg) (Diprivan® 1%, AstraZeneca), maintaining sedation through perfusion of 0.5mg/kg propofol and sevoflurane at 1.5% with 1% oxygen. Body temperature was maintained at 37°C with a heating pad. The animals breathed spontaneously. In addition, we applied 1% lidocaine in the area of incision as a local anaesthetic.
All surgical procedures were performed in the experimental operating room of our hospital by the same researcher (AB). Once the rabbits were anaesthetised and placed on the operating table in the supine position, the abdominal region was shaved. A midline laparotomy was performed, with removal and lateral displacement of the intestinal package, which was covered with gauzes soaked in hot serum to reduce heat loss. After careful dissection, the abdominal aorta and inferior vena cava were located and exposed. The abdominal aorta was clamped for 20min using a vascular clamp placed 1cm distally to the left renal artery and another upstream of the iliac bifurcation as an indirect means to produce spinal cord ischaemia. In order to decrease the risk of thrombosis, heparin (150U/kg) was administered intravenously immediately before occlusion. After 20min, the clamps were removed and the aorta was reviewed to verify its proper function. Lastly, we proceeded to close the incision. The animals received postoperative analgesia and gentamicin once daily for 3 days as prophylaxis against infection. Bladder expression was performed at least 3 times per day until the completion of the experiment.
Neurological assessmentThe motor function of the lower limbs was evaluated by a researcher blinded to the treatment performed, both preoperatively and postoperatively, at 24h and 7 and 14 days after injury, according to the modified Tarlov scale19 (Table 1).
Histological and immunohistochemical studyOn the 14th day of SCI, the animals were anaesthetised once again and, after undergoing a new laparotomy, perfused with 4% paraformaldehyde in phosphate buffer through the abdominal aorta. The spinal cord was extracted, maintained for 3 days in paraformaldehyde and subsequently embedded in paraffin. Lastly, the samples were placed in 30% sucrose in PB at 0.1M until they were sectioned. Spinal sections with a thickness of 12μm were obtained within a cryostat for further study.
Histological examination of the ischaemic area was performed by staining samples with haematoxylin–eosin.
The immunohistochemical study used InmunoCruz Mouse ABC Staining System (Santa Cruz Biotechnology, CA, USA). The deparaffinised spinal cord sections were incubated for 1 hour in blocking buffer (0.1M PBS containing 5% normal goat serum, 0.1% Triton X-100®) and then incubated overnight with 1:20 monoclonal anti-BrdU (DAB, Dako, Glostrup, Denmark). Previously, endogenous peroxidase was inactivated with hydrogen peroxide (0.03% in methanol). For immunodetection, the samples were incubated with the secondary antibody supplied in the Biotin-goat anti-mouse IgG kit (Zymed®, San Francisco, CA, USA), using 3,3-diaminobenzidine tetrahydrochloride (DAB, Dako, Glostrup, Denmark) as chromogen.
The specimens from the control group were processed into samples that were incubated without primary antibody and subsequently processed with the secondary antibody and chromogen.
Statistical analysisThe results of the Tarlov neurological test were expressed as mean±standard deviation. The statistical analysis was performed using the software package SPSS® v.21.0 (IBM®, USA). The Tarlov test was analysed using the Kruskal–Wallis test. Statistical significance was established at a value of P<.05.
ResultsNeurological functionAll animals obtained the maximum score (5) in the Tarlov scale prior to spinal cord injury, thus indicating full spinal function. Following ischaemic injury, all animals in the control group suffered paraplegia with a mean score of 0, 0.125±0.37 and 0.125±0.37 in the Tarlov scale on days 1, 7 and 14, respectively. Those animals receiving intravenous infusion of ATSC at a concentration of 10±2×106 also presented severe neurological impairment with very low mean scores in the Tarlov scale. These scores were 0.4±0.54, 0.2±0.44 and 0.4±0.54 on days 1, 7 and 14, respectively. No statistically significant differences in the Tarlov scale were found between the 2 study groups.
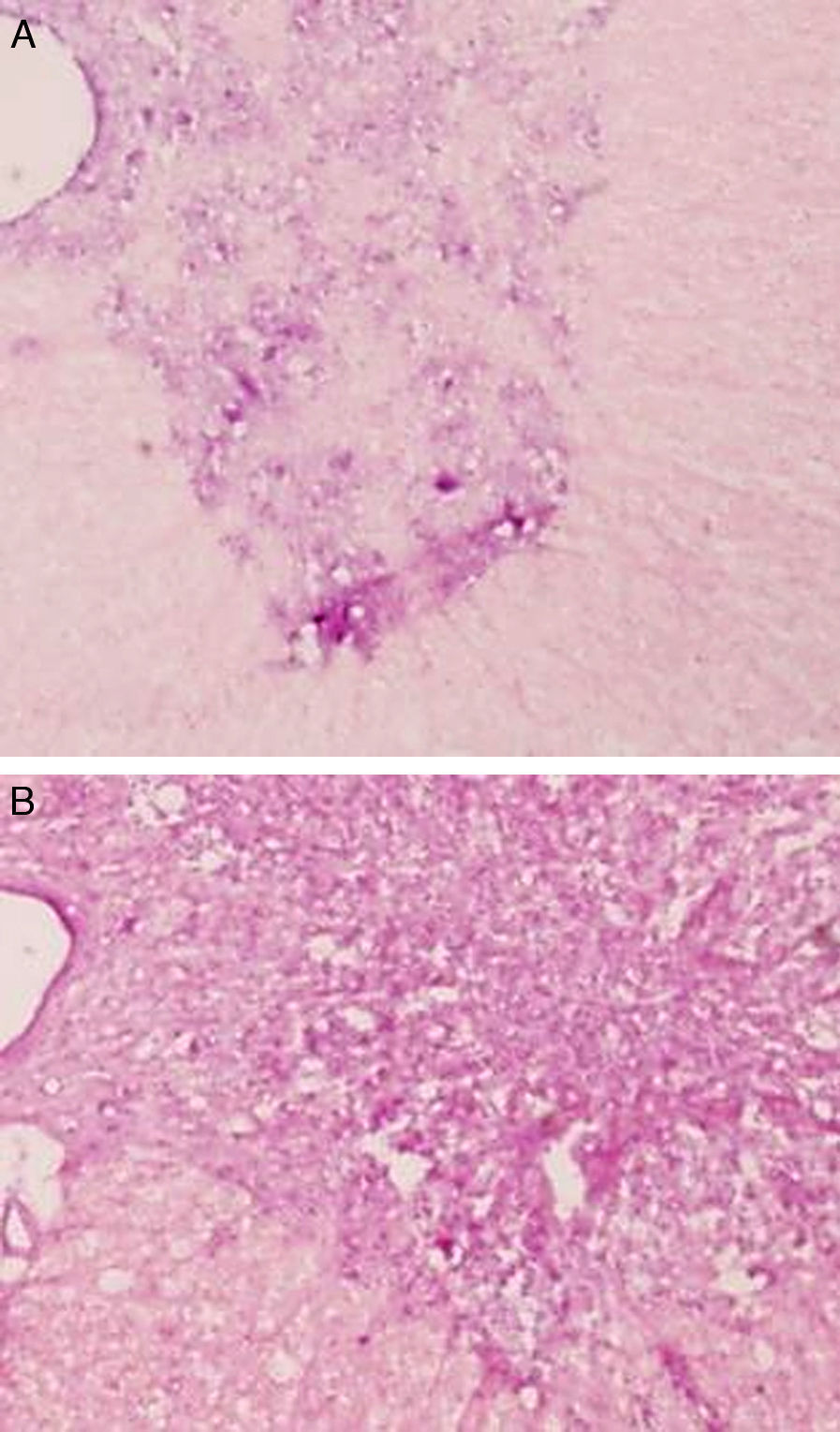
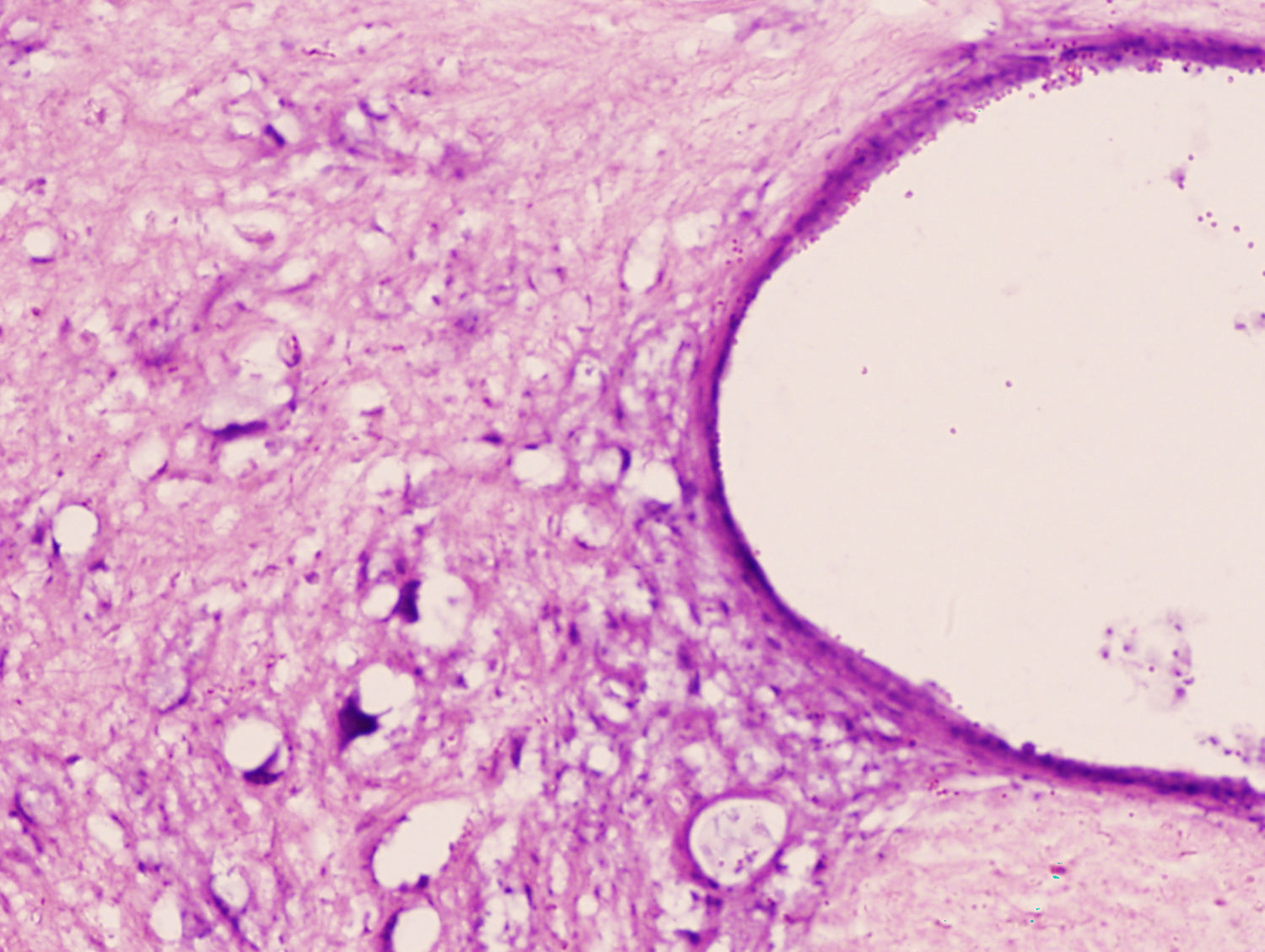
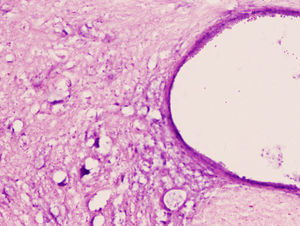
Microscopic and immunochemical studyThe histopathological study showed tissue damage with similar characteristics in both groups of rabbits. We observed a clear loss of the neuronal population at the level of the spinal grey matter. The cytoplasm of the remaining neurons was highly eosinophilic, with an absence of Nissl granules and considerable vacuolisation (Figs. 1 and 2). SCI was more notable and more extensive in the lumbar region than in the thoracic region.
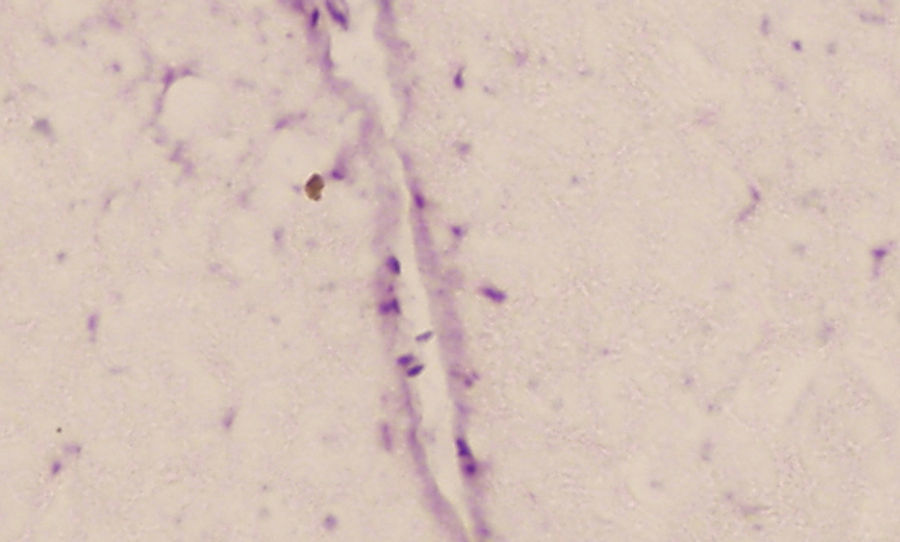
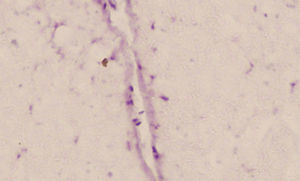
We observed migration of the ATSC infused at a dose of 10±2×106 in the injured area, although this was scarce. Nevertheless, isolated cells labelled with BrdU were found on day 14 after the lesion (Fig. 3).
DiscussionThrough the present work we have demonstrated that intravenous infusion of mesenchymal stem cells from adipose tissue promotes selective migration of cells towards damaged neural tissue following ischaemic spinal cord injury, although, at the doses used, the cells that would reach the target are few.
Previous studies in cerebral ischaemic lesions have shown the benefits of transplantation, through a local injection, of MSC from bone marrow into the damaged neural tissue.5,6 These benefits have also been demonstrated in traumatic spinal injuries.20,21 However, except in some traumatic lesions, the exact level of spinal cord injury is usually ample and not clearly identified in routine clinical practice. This is especially true when the lesion is secondary to spinal cord ischaemia, as is the case in some surgical complications such as correction of scoliotic curves or repair of abdominal aortic aneurysms. Since they have been shown to protect against such ischaemic damage, MSC can even be injected as prophylaxis before surgical procedures, which entail a risk of spinal cord injury, through intrathecal injection 2 days earlier.22
Furthermore, the use of local injections could cause added damage to the neural parenchyma. For this reason, we believe it is important to develop less invasive techniques for clinical use such as an intravenous infusion of stem cells, which our study has demonstrated to be safe and potentially useful in the treatment of spinal cord injury. This method seems to be easier, faster and safer than direct injection into the injured area.
ATSC are easily obtained and multiply rapidly in vitro, quickly yielding a sufficient number of cells for infusion.10,23 In addition, they retain their mesenchymal pluripotent capacity for a long period.10,11 Given that the time elapsed from spinal cord injury until the start of treatment is one of the main factors in the prognosis of patients with spinal cord injury, we believe that ATSC may be more useful than those obtained from bone marrow. Another factor that renders these cells very interesting is that they can secrete angiogenic and anti-apoptotic factors in vitro depending on existing hypoxia.24
In a recent study,16 ATSC inserted with lentiviral vectors were positive for different neural antigens (GFAP, NF160, Tuj-1) and oligodendrocyte markers in spinal cord injuries. This suggests that the implanted ATSC differentiated into astrocytes, oligodendrocytes and neural cells. Both the transplanted ATSC and the chemotactic factors induced by spinal cord injury play an important role in proliferation, migration and differentiation of endogenous neural progenitor cells derived from the spinal cord in the damaged area.15
The capacity of ATSC to migrate selectively towards an ischaemic spinal cord via the bloodstream has been confirmed by the present study. Previous studies had already demonstrated the ability of mesenchymal stem cells to move into damaged areas. Thus, after intravenously infusing MSC into rats with myocardial infarction, their presence was verified in the infarct area, along with an improvement in ventricular function. Meanwhile, in the control group without infarction, MSC migrated to the bone marrow.25 The mechanism by which this migration was promoted is still not known.
The intravenous application of ATSC for the treatment of cellular damage secondary to various diseases has recently increased, since they represent an attractive source of stem cells with no associated ethical problems. The toxicity and possible capacity of ATSC to generate tumours have recently been studied in connection with their clinical application.17 In order to test toxicity, different amounts of human ATSC were injected into immunodeficient mice, which were then monitored for 13 weeks. Even at the maximum dose (2.5×108cells/kg body weight) the mice were viable and showed no side effects. The capacity to produce tumours was also investigated in mice for 26 weeks. Even at the highest dose (2×108ATSC/kg) there was no evidence of tumour development. This research group conducted a clinical trial involving 8 male patients who had suffered SCI over 12 months earlier. The patients received an intravenous infusion of autologous ATSC (4×108cells) in a single dose. None of the patients in the study presented side effects during the 3 months of follow-up.17 However, practically no signs of neurological improvement in the ASIA (American Spinal Injury Association) classification of spinal cord injury were detected.
Our study has various limitations. Firstly, although cell migration existed, it was very poor. This could be improved by increasing the number of infused ATSC and is currently being tested experimentally. Moreover, there was no significant improvement in neurological function, perhaps due to the small number of cells, which reached the site of injury.
Another limitation arose from the injury model employed. Aortic occlusion requires an extensive midline laparotomy, as well as an abdominal injury towards which some of the infused cells might migrate. This could be minimised by endovascular occlusion with a surgical balloon inserted through the femoral artery.
Moreover, even if the cells migrated adequately to the injured area, the extent of the ischaemic injury may limit their potential usefulness in neurological recovery. This would not happen with more limited traumatic injuries.
Finally, the number of cells infused in our study was based on previous works which used a local injection of MSC.5,26 A working hypothesis could consider that the number of cells to be applied intravenously should be higher than the equivalent for local infusion. This hypothesis could justify the poor results obtained. However, a recent clinical trial17 used higher doses of ATSC whilst also monitoring any possible neurological recovery for a longer period, but the results were also disappointing.
In summary, following intravenous administration, ATSC have the ability to migrate and survive in an injured spinal cord after aortic ischaemia. Intravenous infusion is a safe procedure with no side effects. We did not obtain neurological recovery.
Level of evidenceLevel of evidence I.
Ethical responsibilitiesProtection of animalsThe authors declare that the experiment was designed according to the ethical guidelines of the International Association for the Study of Pain and approved by the Animal Welfare and Ethics Committee of our hospital.
FinancingThis work received support through a research grant from the Foundation for Health Research in Castilla-La Mancha (FISCAM), under the Government of Castilla-La Mancha, in 2008.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Barriga A, et al. Infusión intravenosa de células madre adultas procedentes del tejido adiposo para la reparación de lesiones medulares isquémicas. Estudio experimental en conejos. Rev Esp Cir Ortop Traumatol. 2013;57:89-94.